Anthropogenic Influence on Moth Populations: A Comparative Study in Southern Sweden
Abstract
:Simple Summary
Abstract
1. Introduction
- Do species richness, abundance, and diversity measurements differ between the provinces and local trapping sites?
- Does the community composition vary between the two trapping sites, and do the provincial colonisation rates differ between the two provinces?
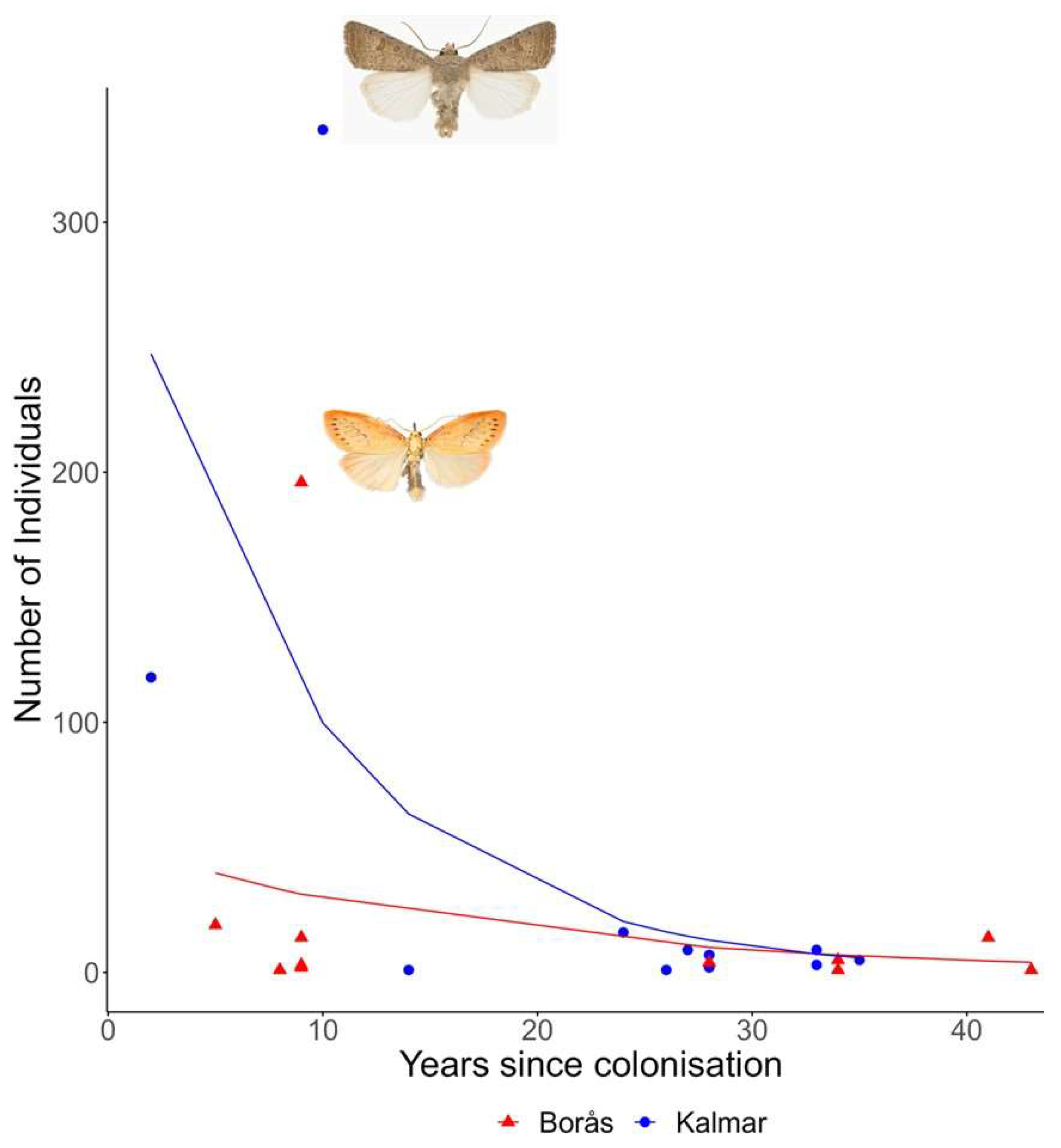
- Is there a positive or negative correlation between the years since provincial colonisation and the abundance of moth species found in the local traps? Such a correlation might be expected if species rapidly become abundant after colonisation and subsequently decrease or if species gradually increase in number over time. Are recently arriving species to the provinces rare, expansive, or potentially non-native invaders that have not previously reported in Sweden?
- Are certain species traits more likely to be associated with moths in anthropogenic landscapes (species present in the traps) compared to those in the surrounding species pool (species known to occur in the provinces).
2. Material and Methods
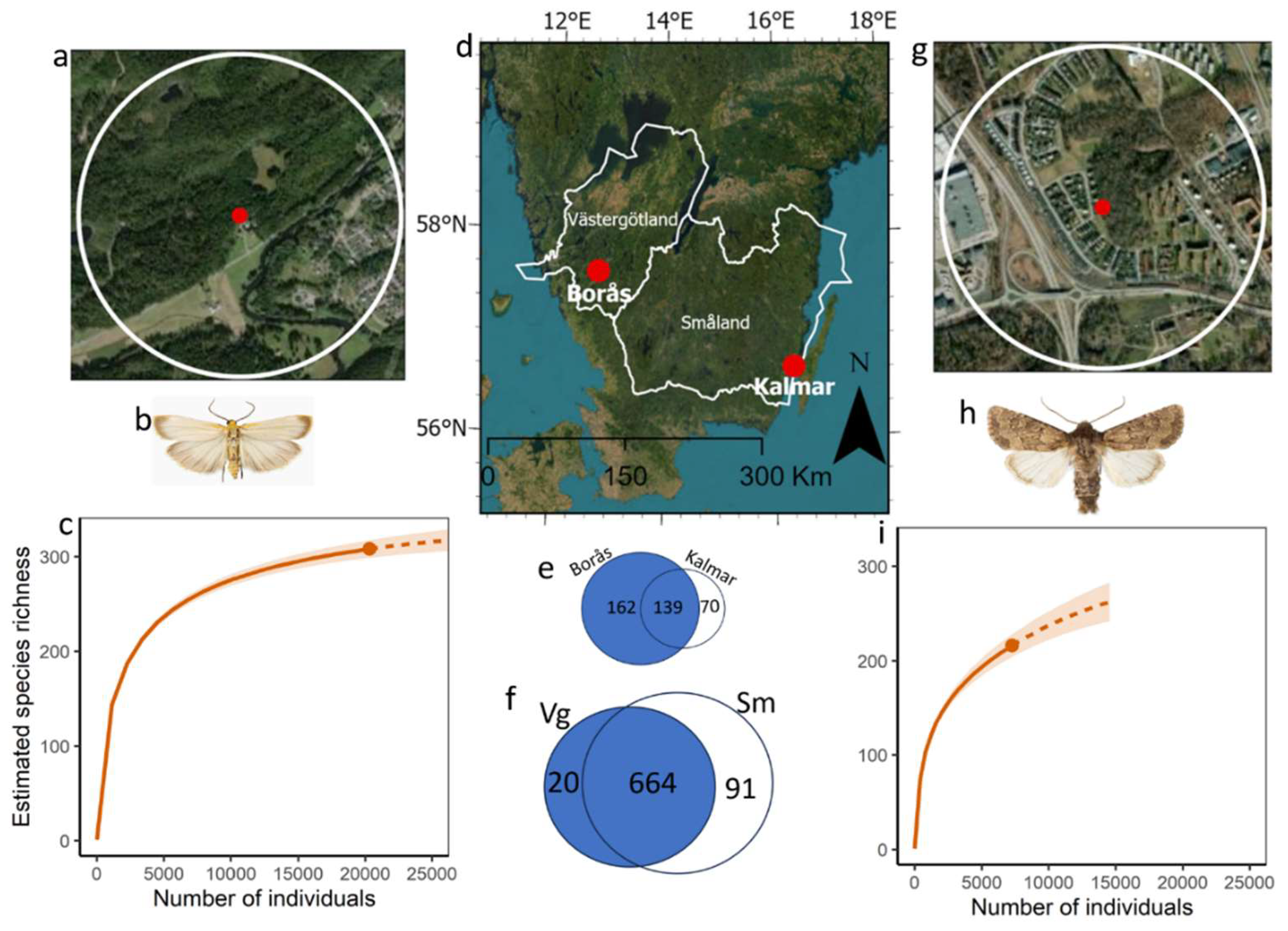
2.1. Study Sites
2.2. Characterisation of Species Traits
2.3. Data at the Provincial Level
2.4. Data Analysis
3. Results
3.1. Species Richness, Abundance, Rarefaction Curves, and Diversity
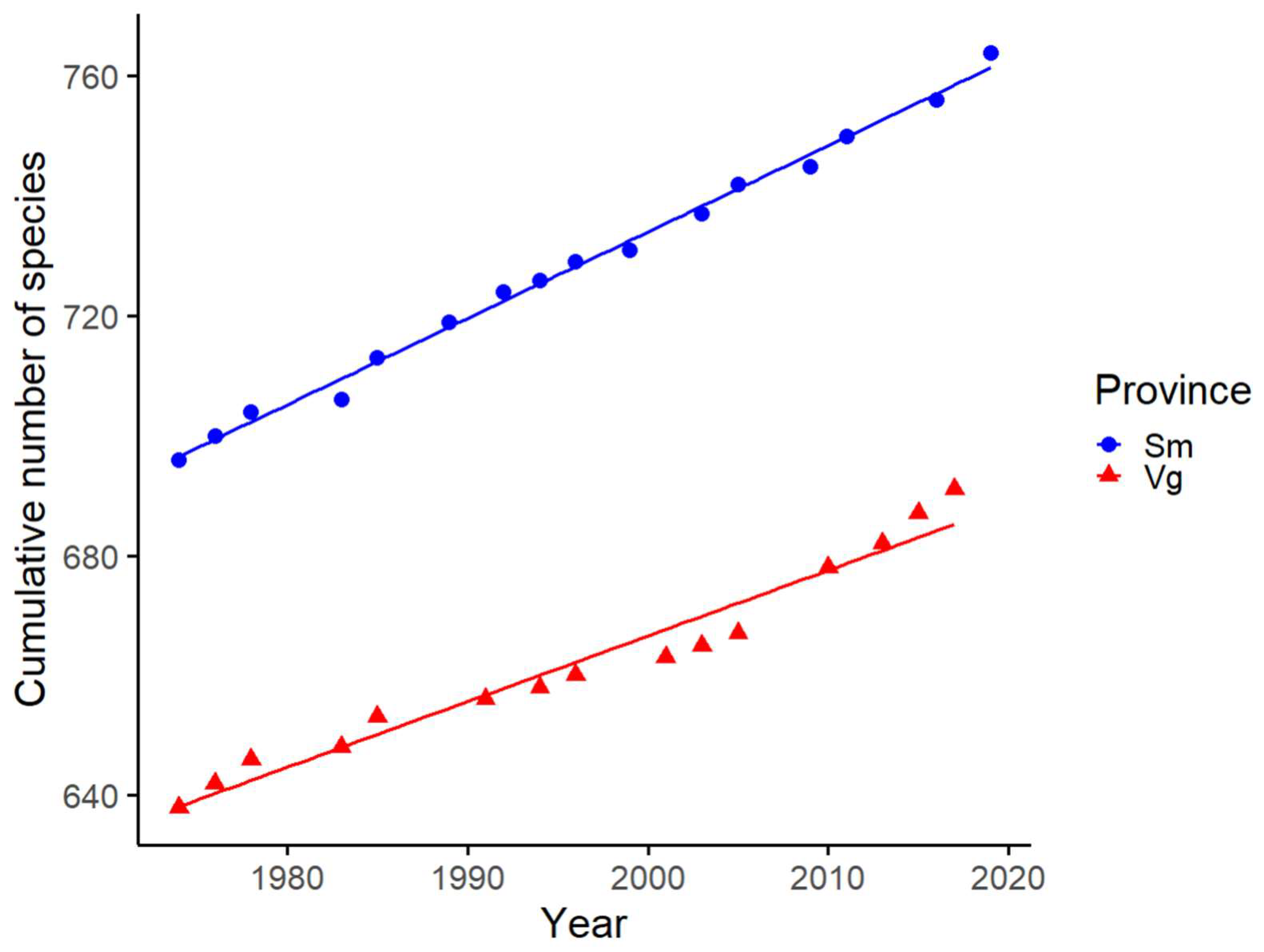
3.2. Provincial Colonisation Rates and Species Shared between Province Records and Light Traps
3.3. Comparisons of Species Characteristics in the Trapping Sites and the Surrounding Regional Species Pool
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunde, J.; Franzén, M.; Betzholtz, P.-E.; Francioli, Y.; Pettersson, L.B.; Pöyry, J.; Ryrholm, N.; Forsman, A. Century-long butterfly range expansions in northern Europe depend on climate, land use and species traits. Commun. Biol. 2023, 6, 601. [Google Scholar]
- Betzholtz, P.-E.; Forsman, A.; Franzén, M. Associations of 16-year population dynamics in range-expanding moths with temperature and years since establishment. Insects 2023, 14, 55. [Google Scholar] [CrossRef]
- Neff, F.; Korner-Nievergelt, F.; Rey, E.; Albrecht, M.; Bollmann, K.; Cahenzli, F.; Chittaro, Y.; Gossner, M.M.; Martínez-Núñez, C.; Meier, E.S. Different roles of concurring climate and regional land-use changes in past 40 years’ insect trends. Nat. Commun. 2022, 13, 7611. [Google Scholar]
- Thomas, J.A.; Telfer, M.G.; Roy, D.B.; Preston, C.D.; Greenwood, J.J.D.; Asher, J.; Fox, R.; Clarke, R.T.; Lawton, J.H. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 2004, 303, 1879–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerer, K.S. Biological diversity in agriculture and global change. Annu. Rev. Environ. Resour. 2010, 35, 137–166. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, N.P. Lepidoptera: Moths and Butterflies. 1. Evolution, Systematics, and Biogeography. Handbook of Zoology Vol. IV, Part 35; De Gruyter: Berlin, Germany; New York, NY, USA, 1999. [Google Scholar]
- Tilman, D. The ecological consequences of changes in biodiversity: A search for general principles. Ecology 1999, 80, 1455–1474. [Google Scholar] [CrossRef]
- Fox, R. The Decline of Moths in Great Britain: A Review of Possible Causes. Insect Conserv. Divers. 2013, 6, 5–19. [Google Scholar]
- Franzén, M.; Betzholtz, P.-E.; Pettersson, L.B.; Forsman, A.J. Urban moth communities suggest that life in the city favours thermophilic multi-dimensional generalists. Proc. R. Soc. B Biol. Sci. 2020, 287, 20193014. [Google Scholar] [CrossRef]
- Foden, W.B.; Young, B.E.; Akçakaya, H.R.; Garcia, R.A.; Hoffmann, A.A.; Stein, B.A.; Thomas, C.D.; Wheatley, C.J.; Bickford, D.; Carr, J.A. Climate change vulnerability assessment of species. Wiley Interdiscip. Rev. Clim. Chang. 2019, 10, e551. [Google Scholar]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.B.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401. [Google Scholar] [CrossRef]
- Forsman, A.; Polic, D.; Sunde, J.; Betzholtz, P.-E.; Franzén, M. Variable colour patterns indicate multidimensional, intraspecific trait variation and ecological generalization in moths. Ecography 2020, 43, 823–833. [Google Scholar] [CrossRef]
- Franzén, M.; Francioli, Y.; Sjöberg, G.; Forsman, A. Positive shifts in species richness and abundance of moths over five decades coincide with community-wide phenotypic trait homogenisation. J. Insect Conserv. 2023, 27, 323–333. [Google Scholar] [CrossRef]
- Burner, R.C.; Selås, V.; Kobro, S.; Jacobsen, R.M.; Sverdrup-Thygeson, A. Moth species richness and diversity decline in a 30-year time series in Norway, irrespective of species’ latitudinal range extent and habitat. J. Insect Conserv. 2021, 25, 887–896. [Google Scholar]
- Hällfors, M.H.; Heikkinen, R.K.; Kuussaari, M.; Lehikoinen, A.; Luoto, M.; Pöyry, J.; Virkkala, R.; Saastamoinen, M.; Kujala, H. Recent range shifts of moths, butterflies, and birds are driven by the breadth of their climatic niche. Evol. Lett. 2023, qrad004. [Google Scholar] [CrossRef]
- Hällfors, M.H.; Pöyry, J.; Heliölä, J.; Kohonen, I.; Kuussaari, M.; Leinonen, R.; Schmucki, R.; Sihvonen, P.; Saastamoinen, M. Combining range and phenology shifts offers a winning strategy for boreal Lepidoptera. Ecol. Lett. 2021, 24, 1619–1632. [Google Scholar] [CrossRef]
- Forsman, A.; Betzholtz, P.-E.; Franzén, M. Faster poleward range shifts in moths with more variable colour patterns. Sci. Rep. 2016, 6, 36265. [Google Scholar] [CrossRef]
- Harvey, J.A.; Tougeron, K.; Gols, R.; Heinen, R.; Abarca, M.; Abram, P.K.; Basset, Y.; Berg, M.; Boggs, C.; Brodeur, J.; et al. Scientists’ warning on climate change and insects. Ecol. Monogr. 2023, 93, e1553. [Google Scholar] [CrossRef]
- Betzholtz, P.-E.; Pettersson, L.B.; Ryrholm, N.; Franzén, M. With that diet, you will go far: Trait-based analysis reveals a link between rapid range expansion and a nitrogen-favoured diet. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122305. [Google Scholar] [CrossRef]
- Thomas, C.D.; Bodsworth, E.J.; Wilson, R.J.; Simmons, A.D.; Davies, Z.G.; Musche, M.; Conradt, L. Ecological and evolutionary processes at expanding range margins. Nature 2001, 411, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Fristoe, T.S.; Chytrý, M.; Dawson, W.; Essl, F.; Heleno, R.; Kreft, H.; Maurel, N.; Pergl, J.; Pyšek, P.; Seebens, H. Dimensions of invasiveness: Links between local abundance, geographic range size, and habitat breadth in Europe’s alien and native floras. Proc. Natl. Acad. Sci. USA 2021, 118, e2021173118. [Google Scholar]
- Fox, R.; Dennis, E.; Harrower, C.; Blumgart, D.; Bell, J.; Cook, P.; Davis, A.; Evans-Hill, L.; Haynes, F.; Hill, D. The State of Britain’s Larger Moths 2021; Natural Environment Research Council (NERC): Swindon, UK, 2021. [Google Scholar]
- Angert, A.L.; Crozier, L.G.; Rissler, L.J.; Gilman, S.E.; Tewksbury, J.J.; Chunco, A.J. Do species’ traits predict recent shifts at expanding range edges? Ecol. Lett. 2011, 14, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Franzén, M.; Forsman, A.; Betzholtz, P.-E. Variable color patterns influence continental range size and species–area relationships on islands. Ecosphere 2019, 10, e02577. [Google Scholar] [CrossRef]
- Forsman, A.; Betzholtz, P.E.; Franzén, M. Variable coloration is associated with dampened population fluctuations in noctuid moths. Proc. R. Soc. B 2015, 282, 20142922. [Google Scholar] [CrossRef] [PubMed]
- Alexandersson, H. Temperatur och Nederbörd i Sverige 1860–2001; SMHI: Norrköping, Sweden, 2002. [Google Scholar]
- Anonymous. Miljö och Väder. Statistisk Årsbok 2010; Statistiska Centralbyrån: Solna, Sweden, 2010. [Google Scholar]
- Leinonen, R.; Söderman, G.; Itämies, J.; Rytkonen, S.; Rutanen, I. Intercalibration of different light-traps and bulbs used in moth monitoring in northern Europe. Entomol. Fenn. 1998, 9, 37–51. [Google Scholar] [CrossRef]
- Jonason, D.; Franzen, M.; Ranius, T. Surveying moths using light traps: Effects of weather and time of year. PLoS ONE 2014, 9, e92453. [Google Scholar] [CrossRef]
- Franzén, M.; Betzholtz, P.E. Species traits predict island occupancy in noctuid moths. J. Insect Conserv. 2012, 16, 155–163. [Google Scholar] [CrossRef]
- Elmquist, H.; Liljeberg, G.; Top-Jensen, M.; Fibiger, M. Sveriges Fjärilar: En Fälthandbok över Sveriges Samtliga Dag-och Nattfjärilar; Bugbook Publishing: Østermarie, Denmark, 2019. [Google Scholar]
- Aarvik, L.; Bengtsson, B.Å.; Elven, H.; Ivinskis, P.; Jürivete, U.; Karsholt, O.; Mutanen, M.; Savenkov, N. Nordic-Baltic Checklist of Lepidoptera. Nor. J. Entomol. 2017, (Suppl. S3), 1–237. [Google Scholar]
- Eide, W.; Ahrné, K.; Bjelke, U.; Nordström, S.; Ottosson, E.; Sandström, J.; Sundberg, S. Tillstånd och Trender för Arter och Deras Livsmiljöer—Rödlistade Arter i Sverige 2020; SLU Artdatabanken: Uppsala, Sweden, 2020. [Google Scholar]
- Anonymous. Nationella Marktäckedata 2018 Basskikt: Produktbeskrivning [National Land-Cover Data 2018 Basic Layer: Product Description]; Version 2.2; Swedish Environmental Protection Agency: Stockholm, Sweden, 2020; Available online: https://gpt.vic-metria.nu/data/land/NMD/NMD_Produktbeskrivning_NMD2018Basskikt_v2_2.pdf (accessed on 20 October 2020). (In Swedish)
- Svensson, I. Lepidoptera-Calender; Hans Hellberg: Stockholm, Sweden, 1993; pp. 1–124. [Google Scholar]
- Huldén, L.; Albrecht, A.; Itämies, J.; Malinen, P.; Wettenhovi, J. Atlas of Finnish Macrolepidoptera; Lepidopterologiska Sällskapet i Finland: Helsingfors, Finland, 2000. [Google Scholar]
- Hydén, N. Nationalnyckeln till Sveriges Flora och Fauna. Fjärilar. Ädelspinnare—Tofsspinnare: Lepidoptera: Lasiocampidae—Lymantriidae; Artdatabanken, SLU: Uppsala, Sweden, 2007. [Google Scholar]
- Emmet, A.M. Life history and habits of the British Lepidoptera. In The Moths and Butterflies of Great Britain and Ireland; Emmet, A.M., Heath, J., Eds.; Harley Books: Colchester, UK, 1991; pp. 61–203. [Google Scholar]
- Skou, P. Nordens Ugler; Apollo Books: Stenstrup, Denmark, 1991. [Google Scholar]
- Skou, P. Nordens Målare. Danmarks Dyreliv. Bind 2; Apollo Books: Stenstrup, Denmark, 1984. [Google Scholar]
- Forsman, A.; Ahnesjö, J.; Caesar, S.; Karlsson, M. A model of ecological and evolutionary consequences of color polymorphism. Ecology 2008, 89, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Forsman, A. Is colour polymorphism advantageous to populations and species? Mol. Ecol. 2016, 25, 2693–2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpestam, E.; Merilaita, S.; Forsman, A. Colour polymorphism protects prey individuals and populations against predation. Sci. Rep. 2016, 6, 22122. [Google Scholar] [CrossRef] [Green Version]
- Betzholtz, P.E.; Franzén, M.; Forsman, A. Colour pattern variation can inform about extinction risk in moths. Anim. Conserv. 2017, 20, 72–79. [Google Scholar] [CrossRef]
- Bengtsson, B.Å.; Gustafsson, B.; Palmqvist, G. Katalog över Svenska Fjärilar; Entomologiska Föreningen i Stockholm: Stockholm, Sweden, 2016. [Google Scholar]
- Douwes, P. Intressantare fynd av Macrolepidoptera i Sverige 1973. Entomol. Tidskr. 1974, 95, 190–191. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical. R Version 4.3.0; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Yue, J.C.; Clayton, M.K. A similarity measure based on species proportions. Commun. Stat. Theory Methods 2005, 34, 2123–2131. [Google Scholar] [CrossRef] [Green Version]
- Chen, H. VennDiagram: Generate High-Resolution Venn and Euler Plots R Package Version 1.7.3; Available online: https://cran.r-project.org/web/packages/VennDiagram/VennDiagram.pdf (accessed on 10 July 2023).
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. The vegan package. Vegan Community Ecol. Package 2019, 10, 631–637. [Google Scholar]
- Roswell, M.; Dushoff, J.; Winfree, R. A conceptual guide to measuring species diversity. Oikos 2021, 130, 321–338. [Google Scholar] [CrossRef]
- Hsieh, T.; Ma, K.; Chao, A.; Hsieh, M. Package ‘iNEXT’. 2016. Available online: http://chao.stat.nthu.edu.tw/blog/software-download/ (accessed on 22 August 2017).
- Chao, A.; Gotelli, N.J.; Hsieh, T.; Sander, E.L.; Ma, K.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Brehm, G.; Fiedler, K. Ordinating tropical moth ensembles from an elevational gradient: A comparison of common methods. J. Trop. Ecol. 2004, 20, 165–172. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S.; Price, B.; Adler, D.; Bates, D.; Baud-Bovy, G.; Bolker, B. Car: Companion to Applied Regression. R Package Version 3.0–2. 2019. Available online: https://cran.r-project.org/web/packages/car/index.html (accessed on 1 June 2019).
- Brooks, M.E.; Kristensen, K.; Van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Rita, H.; Komonen, A. Odds ratio: An ecologically sound tool to compare proportions. In Annales Zoologici Fennici; Finnish Zoological and Botanical Publishing Board: Washington, DC, USA, 2008; pp. 66–72. [Google Scholar]
- Wickham, H.; Wickham, M.H. The Ggplot Package. Google Scholar. 2007. Available online: http://Ftp.Uni-Bayreuth.De/Math/Statlib/R/CRAN/Doc/Packages/Ggplot.Pdf (accessed on 10 July 2023).
- Warnes, G.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S. Gplots: Various R Programming Tools for Plotting Data; R Package Version 3.1.3. 2022; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Steffen, W.; Grinevald, J.; Crutzen, P.; McNeill, J. The Anthropocene: Conceptual and historical perspectives. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 842–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, E.C. Evolution: Biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R831–R833. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [PubMed]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Borger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [PubMed] [Green Version]
- Antão, L.H.; Pöyry, J.; Leinonen, R.; Roslin, T. Contrasting latitudinal patterns in diversity and stability in a high-latitude species-rich moth community. Glob. Ecol. Biogeogr. 2020, 29, 896–907. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.J.; Fox, R. Insect responses to global change offer signposts for biodiversity and conservation. Ecol. Entomol. 2021, 46, 699–717. [Google Scholar] [CrossRef]
- Lindeborg, M.; Franzén, M.; Ryrholm, N. Intressanta fynd av storfjärilar (Macrolepidoptera) i Sverige 2022. Entomol. Tidskr. 2023, 144, 55–72. [Google Scholar]
- Audusseau, H.; Ryrholm, N.; Stefanescu, C.; Tharel, S.; Jansson, C.; Champeaux, L.; Shaw, M.R.; Raper, C.; Lewis, O.T.; Janz, N. Rewiring of interactions in a changing environment: Nettle-feeding butterflies and their parasitoids. Oikos 2021, 130, 624–636. [Google Scholar] [CrossRef]
- De Boer, J.G.; Harvey, J.A. Range-expansion in processionary moths and biological control. Insects 2020, 11, 267. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef]
- Nordström, F.; Wahlgren, E.; Tullgren, A. Svenska Fjärilar; Nordisk Familjeboks Förlag: Stockholm, Sweden, 1941. [Google Scholar]
- Valtonen, A.; Hirka, A.; Szőcs, L.; Ayres, M.P.; Roininen, H.; Csóka, G. Long-term species loss and homogenization of moth communities in Central Europe. J. Anim. Ecol. 2017, 86, 730–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenendijk, D.; Ellis, W. The state of the Dutch larger moth fauna. J. Insect Conserv. 2010, 15, 95–101. [Google Scholar] [CrossRef]
- Brown, J.H. Species diversity. In Analytical Biogeography: An Integrated Approach to the Study of Animal and Plant Distributions; Springer: Berlin/Heidelberg, Germany, 1988; pp. 57–89. [Google Scholar]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: New York, NY, USA, 1995. [Google Scholar]
- Cinzano, P.; Falchi, F.; Elvidge, C.D. The first world atlas of the artificial night sky brightness. Mon. Not. R. Astron. Soc. 2001, 328, 689–707. [Google Scholar] [CrossRef] [Green Version]
- Vellend, M.; Baeten, L.; Myers-Smith, I.H.; Elmendorf, S.C.; Beauséjour, R.; Brown, C.D.; De Frenne, P.; Verheyen, K.; Wipf, S. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl. Acad. Sci. USA 2013, 110, 19456–19459. [Google Scholar] [CrossRef]
- Wiens, J.A. Spatial scaling in ecology. Funct. Ecol. 1989, 3, 385–397. [Google Scholar] [CrossRef]
- Franzén, M.; Schweiger, O.; Betzholtz, P.-E. Species-Area Relationships Are Controlled by Species Traits. PLoS ONE 2012, 7, e37359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangels, J.; Fiedler, K.; Schneider, F.D.; Blüthgen, N. Diversity and trait composition of moths respond to land-use intensification in grasslands: Generalists replace specialists. Biodivers. Conserv. 2017, 26, 3385–3405. [Google Scholar] [CrossRef]
- Teder, T. Phenological responses to climate warming in temperate moths and butterflies: Species traits predict future changes in voltinism. Oikos 2020, 129, 1051–1060. [Google Scholar] [CrossRef]
- Mattila, N.; Kotiaho, J.S.; Kaitala, V.; Komonen, A.; Päivinen, J. Interactions between ecological traits and host plant type explain distribution change in noctuid moths. Conserv. Biol. 2009, 23, 703–709. [Google Scholar]
- van Langevelde, F.; Braamburg-Annegarn, M.; Huigens, M.E.; Groendijk, R.; Poitevin, O.; van Deijk, J.R.; Ellis, W.N.; van Grunsven, R.H.A.; de Vos, R.; Vos, R.A.; et al. Declines in moth populations stress the need for conserving dark nights. Glob. Chang. Biol. 2018, 24, 925–932. [Google Scholar] [CrossRef]
- Jonason, D.; Andersson, G.K.S.; Öckinger, E.; Rundlöf, M.; Smith, H.G.; Bengtsson, J. Assessing the effect of the time since transition to organic farming on plants and butterflies. J. Appl. Ecol. 2011, 48, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buxton, M.N.; Gaskett, A.C.; Lord, J.M.; Pattemore, D.E. A global review demonstrating the importance of nocturnal pollinators for crop plants. J. Appl. Ecol. 2022, 59, 2890–2901. [Google Scholar] [CrossRef]
- Alison, J.; Alexander, J.M.; Diaz Zeugin, N.; Dupont, Y.L.; Iseli, E.; Mann, H.M.; Høye, T.T. Moths complement bumblebee pollination of red clover: A case for day-and-night insect surveillance. Biol. Lett. 2022, 18, 20220187. [Google Scholar] [CrossRef] [PubMed]


| Variable | Df | LR Chisq | p-Value |
|---|---|---|---|
| Year | 1 | 1361.4 | <0.001 |
| Province | 1 | 4740.8 | <0.001 |
| Year × Province | 1 | 25.3 | <0.001 |
| Variable | Df | LR Chisq | p-Value |
|---|---|---|---|
| Years since colonisation | 1 | 857.37 | <0.001 |
| Site | 1 | 266.64 | <0.001 |
| Years since colonisation × site | 1 | 34.37 | <0.001 |
| Variable | Df | LR Chisq | p-Value |
|---|---|---|---|
| Colour pattern variation | 1 | 10.455 | <0.001 |
| Habitat preference | 2 | 30.443 | <0.001 |
| Length of flight period | 1 | 8.3084 | <0.003 |
| Host plant specificity | 1 | 49.089 | <0.001 |
| Overwintering stage | 3 | 22.741 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzén, M.; Forsman, A.; Karimi, B. Anthropogenic Influence on Moth Populations: A Comparative Study in Southern Sweden. Insects 2023, 14, 702. https://doi.org/10.3390/insects14080702
Franzén M, Forsman A, Karimi B. Anthropogenic Influence on Moth Populations: A Comparative Study in Southern Sweden. Insects. 2023; 14(8):702. https://doi.org/10.3390/insects14080702
Chicago/Turabian StyleFranzén, Markus, Anders Forsman, and Bafraw Karimi. 2023. "Anthropogenic Influence on Moth Populations: A Comparative Study in Southern Sweden" Insects 14, no. 8: 702. https://doi.org/10.3390/insects14080702
APA StyleFranzén, M., Forsman, A., & Karimi, B. (2023). Anthropogenic Influence on Moth Populations: A Comparative Study in Southern Sweden. Insects, 14(8), 702. https://doi.org/10.3390/insects14080702







