Atg2 Regulates Cellular and Humoral Immunity in Drosophila
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Strains and Culture Conditions
2.2. In Vivo Phagocytosis
2.3. Immunostaining of Melanotic Nodules
2.4. Hemocyte Assays
2.5. Quantitative Real-Time PCR (qRT—PCR)
2.6. Survival Rates upon Bacterial Infection
2.7. Statistical Analysis
3. Results
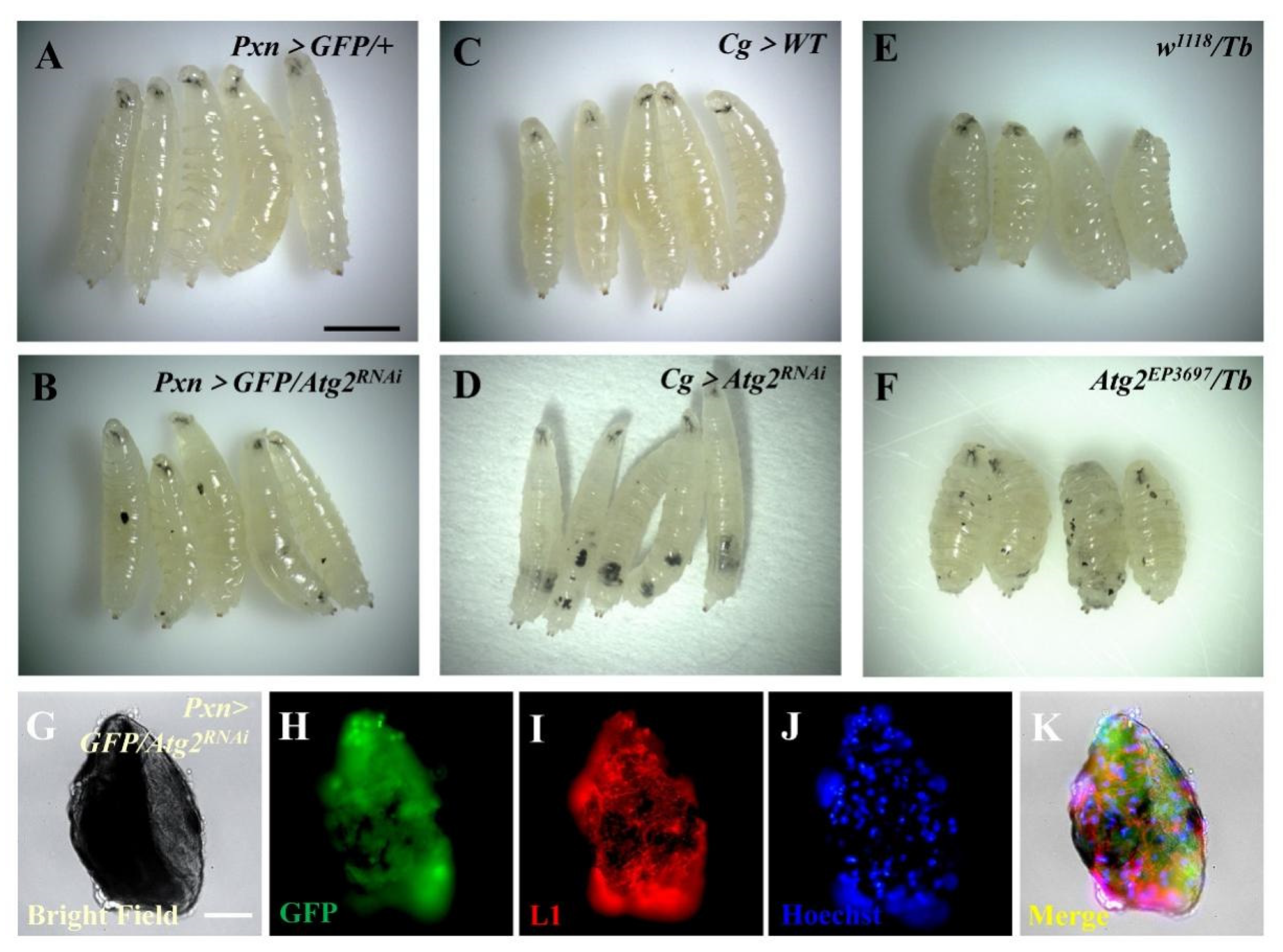
3.1. Inhibiting Atg2 Induced Massive Melanotic Nodule Formation
3.2. Inhibiting Atg2 Impaired In Vivo Phagocytosis
3.3. Inhibiting Atg2 Altered the Proportion of NimC1-Positive Hemocytes and the Actin Cytoskeleton
3.4. Inhibiting Atg2 Altered AMP-Encoding Gene Expression
3.5. Flies Were Susceptible to Bacterial Infection upon Inhibition of Atg2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Luo, F.; Xu, Y.; Zhang, Y.; Jin, L.H. Drosophila innate immunity involves multiple signaling pathways and coordinated communication between different tissues. Front. Immunol. 2022, 13, 905370. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.J.; Hartenstein, V.; Banerjee, U. Thicker than blood: Conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell. 2003, 5, 673–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanot, R.; Zachary, D.; Holder, F.; Meister, M. Postembryonic hematopoiesis in Drosophila. Dev. Biol. 2001, 230, 243–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, U.; Girard, J.R.; Goins, L.M.; Spratford, C.M. Drosophila as a genetic model for hematopoiesis. Genetics 2019, 211, 367–417. [Google Scholar] [CrossRef] [Green Version]
- Melcarne, C.; Lemaitre, B.; Kurant, E. Phagocytosis in Drosophila: From molecules and cellular machinery to physiology. Insect Biochem. Mol. Biol. 2019, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Krishnamoorthy, P.; Walker, E.C.; Joshi, H.; Morley, S.C. Expression of non-phosphorylatable S5A-L-plastin exerts phenotypes distinct from L-plastin deficiency during podosome formation and phagocytosis. Front. Cell Dev. Biol. 2023, 11, 1020091. [Google Scholar] [CrossRef]
- Ulvila, J.; Vanha-Aho, L.M.; Rämet, M. Drosophila phagocytosis—Still many unknowns under the surface. Apmis 2011, 119, 651–662. [Google Scholar] [CrossRef]
- Rizki, T.M.; Rizki, R.M. Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev. Comp. Immunol. 1992, 16, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Luo, F.; Jin, L.H. The Drosophila lymph gland is an ideal model for studying hematopoiesis. Dev. Comp. Immunol. 2018, 83, 60–69. [Google Scholar] [CrossRef]
- Levine, B. Eating oneself and uninvited guests: Autophagy-related pathways in cellular defense. Cell 2005, 120, 159–162. [Google Scholar] [PubMed] [Green Version]
- Das, G.; Shravage, B.V.; Baehrecke, E.H. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb. Perspect. Biol. 2012, 4, a008813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhang, L.; Zhang, G.; Shangguan, G.; Hou, X.; Duan, W.; Xi, Y.; Xu, N.; Zhang, B.; Dong, J.; et al. A nonautophagic role of ATG5 in regulating cell growth by targeting c-Myc for proteasome-mediated degradation. iScience 2021, 24, 103296. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, J.; Zhao, J.; Ma, N.; Kim, S.W.; Qiao, S.; Ma, X. Autophagy: The last defense against cellular nutritional stress. Adv. Nutr. 2018, 9, 493–504. [Google Scholar] [PubMed] [Green Version]
- King, J.S.; Veltman, D.M.; Insall, R.H. The induction of autophagy by mechanical stress. Autophagy 2011, 7, 1490–1499. [Google Scholar] [CrossRef] [Green Version]
- Chun, Y.; Kim, J. Autophagy: An essential degradation program for cellular homeostasis and life. Cells 2018, 7, 278. [Google Scholar]
- Mariño, G.; Madeo, F.; Kroemer, G. Autophagy for tissue homeostasis and neuroprotection. Curr. Opin. Cell Biol. 2011, 23, 198–206. [Google Scholar] [CrossRef]
- Allen, E.A.; Baehrecke, E.H. Autophagy in animal development. Cell Death Differ. 2020, 27, 903–918. [Google Scholar] [CrossRef]
- Jiang, P.; Mizushima, N. Autophagy and human diseases. Cell Res. 2014, 24, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.Y.; Neufeld, T.P. Autophagy takes flight in Drosophila. FEBS Lett. 2010, 584, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- McPhee, C.K.; Baehrecke, E.H. Autophagy in Drosophila melanogaster. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 1452–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.C.; Juhász, G.; Neufeld, T.P. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr. Biol. 2007, 17, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayachit, M.S.; Shravage, B.V. Atg1 modulates mitochondrial dynamics to promote germline stem cell maintenance in Drosophila. Biochem. Biophys. Res. Commun. 2023, 643, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sandford, E.; Gatica, D.; Qiu, Y.; Liu, X.; Zheng, Y.; Schulman, B.A.; Xu, J.; Semple, I.; Ro, S.H.; et al. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. Elife 2016, 5, e12245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Nicolson, S.; Denton, D.; Kumar, S. Distinct requirements of Autophagy-related genes in programmed cell death. Cell Death. Differ. 2015, 22, 1792–1802. [Google Scholar] [CrossRef] [Green Version]
- Simonsen, A.; Cumming, R.C.; Lindmo, K.; Galaviz, V.; Cheng, S.; Rusten, T.E.; Finley, K.D. Genetic modifiers of the Drosophila blue cheese gene link defects in lysosomal transport with decreased life span and altered ubiquitinated-protein profiles. Genetics 2007, 176, 1283–1297. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kuo, C.J.; Hansen, M.; Troemel, E. Autophagy and innate immunity: Insights from invertebrate model organisms. Autophagy 2018, 14, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Moy, R.H.; Cherry, S. Antimicrobial autophagy: A conserved innate immune response in Drosophila. J. Innate Immun. 2013, 5, 444–455. [Google Scholar] [CrossRef]
- Shelly, S.; Lukinova, N.; Bambina, S.; Berman, A.; Cherry, S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 2009, 30, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Deretic, V.; Levine, B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009, 5, 527–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laczkó-Dobos, H.; Maddali, A.K.; Jipa, A.; Bhattacharjee, A.; Végh, A.G.; Juhász, G. Lipid profiles of autophagic structures isolated from wild type and Atg2 mutant Drosophila. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158868. [Google Scholar] [CrossRef]
- Harrison, D.A.; Binari, R.; Nahreini, T.S.; Gilman, M.; Perrimon, N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995, 14, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Pan, P.C.; Govind, S. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development 1998, 125, 1909–1920. [Google Scholar] [CrossRef]
- Zettervall, C.J.; Anderl, I.; Williams, M.J.; Palmer, R.; Kurucz, E.; Ando, I.; Hultmark, D. A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 2004, 101, 14192–14197. [Google Scholar] [CrossRef] [PubMed]
- Minakhina, S.; Steward, R. Melanotic mutants in Drosophila: Pathways and phenotypes. Genetics 2006, 174, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindner, B.; Burkard, T.; Schuler, M. Phagocytosis assays with different pH-sensitive fluorescent particles and various readouts. Biotechniques 2020, 68, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Péan, C.B.; Schiebler, M.; Tan, S.W.; Sharrock, J.A.; Kierdorf, K.; Brown, K.P.; Maserumule, M.C.; Menezes, S.; Pilátová, M.; Bronda, K.; et al. Regulation of phagocyte triglyceride by a STAT-ATG2 pathway controls mycobacterial infection. Nat. Commun. 2017, 8, 14642. [Google Scholar] [CrossRef] [Green Version]
- Horsthemke, M.; Bachg, A.C.; Groll, K.; Moyzio, S.; Müther, B.; Hemkemeyer, S.A.; Wedlich-Söldner, R.; Sixt, M.; Tacke, S.; Bähler, M.; et al. Multiple roles of filopodial dynamics in particle capture and phagocytosis and phenotypes of Cdc42 and Myo10 deletion. J. Biol. Chem. 2017, 292, 7258–7273. [Google Scholar] [CrossRef] [Green Version]
- Kress, H.; Stelzer, E.H.; Holzer, D.; Buss, F.; Griffiths, G.; Rohrbach, A. Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc. Natl. Acad. Sci. USA 2007, 104, 11633–11638. [Google Scholar] [CrossRef]
- Kadandale, P.; Stender, J.D.; Glass, C.K.; Kiger, A.A. Conserved role for autophagy in Rho1-mediated cortical remodeling and blood cell recruitment. Proc. Natl. Acad. Sci. USA 2010, 107, 10502–10507. [Google Scholar] [CrossRef]
- Hao, Y.; Yu, S.; Luo, F.; Jin, L.H. Jumu is required for circulating hemocyte differentiation and phagocytosis in Drosophila. Cell Commun. Signal 2018, 16, 95. [Google Scholar]
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila melanogaster--from microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014, 14, 796–810. [Google Scholar] [CrossRef]
- Kim, L.K.; Choi, U.Y.; Cho, H.S.; Lee, J.S.; Lee, W.B.; Kim, J.; Jeong, K.; Shim, J.; Kim-Ha, J.; Kim, Y.J. Down-regulation of NF-kappaB target genes by the AP-1 and STAT complex during the innate immune response in Drosophila. PLoS Biol. 2007, 5, e238. [Google Scholar] [CrossRef] [Green Version]
- Irving, P.; Ubeda, J.M.; Doucet, D.; Troxler, L.; Lagueux, M.; Zachary, D.; Hoffmann, J.A.; Hetru, C.; Meister, M. New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol. 2005, 7, 335–350. [Google Scholar] [CrossRef]
- Charroux, B.; Royet, J. Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proc. Natl. Acad. Sci. USA 2009, 106, 9797–9802. [Google Scholar] [CrossRef]
- Defaye, A.; Evans, I.; Crozatier, M.; Wood, W.; Lemaitre, B.; Leulier, F. Genetic ablation of Drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J. Innate Immun. 2009, 1, 322–334. [Google Scholar] [CrossRef] [Green Version]





| Target Gene | Forward (5’ to 3’) | Reverse (5’ to 3’) |
|---|---|---|
| ribosomal protein 49 | AGTCGGATCGATATGCTAAGCTGT | TAACCGATGTTGGGCATCAGATACT |
| Attacin-A | AGGTTCCTTAACCTCCAATC | CATGACCAGCATTGTTGTAG |
| Defensin | CGCTTTTGCTCTGCTTGCTTGC | TAGGTCGCATGTGGCTCGCTTC |
| Diptericin | ATGCAGTTCACCATTGCCGTC | TCCAGCTCGGTTCTGAGTTG |
| Drosomycin | CTCTTCGCTGTCCTGATGCT | ATCCTTCGCACCAGCACTT |
| Metchnikowin | GCATCAATCAATTCCCGCCACC | CGGCCTCGTATCGAAAATGGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, B.; Yu, S.; Chen, Q.; Jin, L.H. Atg2 Regulates Cellular and Humoral Immunity in Drosophila. Insects 2023, 14, 706. https://doi.org/10.3390/insects14080706
Qin B, Yu S, Chen Q, Jin LH. Atg2 Regulates Cellular and Humoral Immunity in Drosophila. Insects. 2023; 14(8):706. https://doi.org/10.3390/insects14080706
Chicago/Turabian StyleQin, Bo, Shichao Yu, Qiming Chen, and Li Hua Jin. 2023. "Atg2 Regulates Cellular and Humoral Immunity in Drosophila" Insects 14, no. 8: 706. https://doi.org/10.3390/insects14080706
APA StyleQin, B., Yu, S., Chen, Q., & Jin, L. H. (2023). Atg2 Regulates Cellular and Humoral Immunity in Drosophila. Insects, 14(8), 706. https://doi.org/10.3390/insects14080706





