Multi-Species Host Use by the Parasitoid Fly Ormia lineifrons
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Collection and Animal Care
2.2. Parasitism Measurements
2.3. Fly Care and Measurements
2.4. Host Measurements
2.5. Data Analysis
3. Results
3.1. Parasitism Rates and Superparasitism
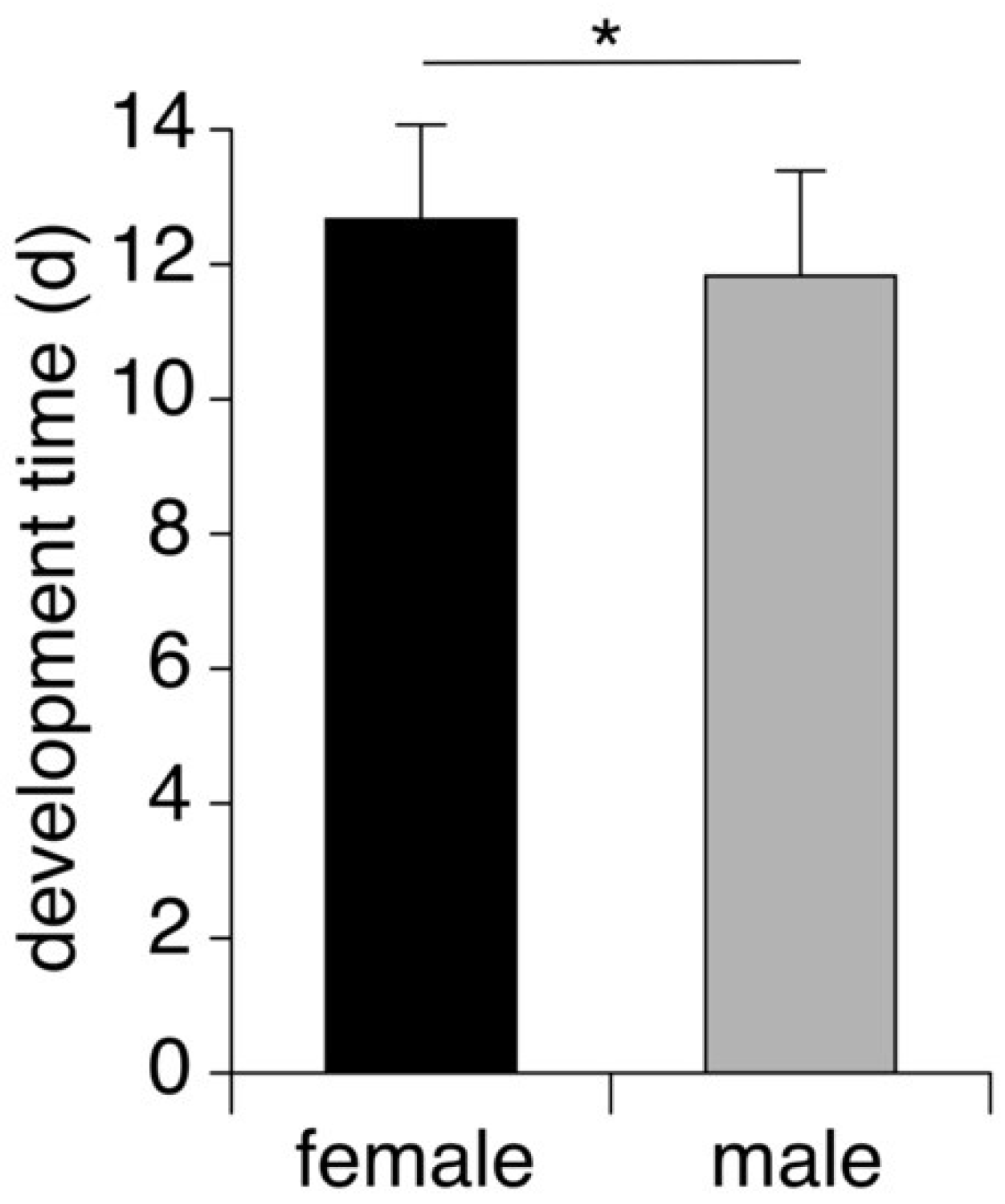
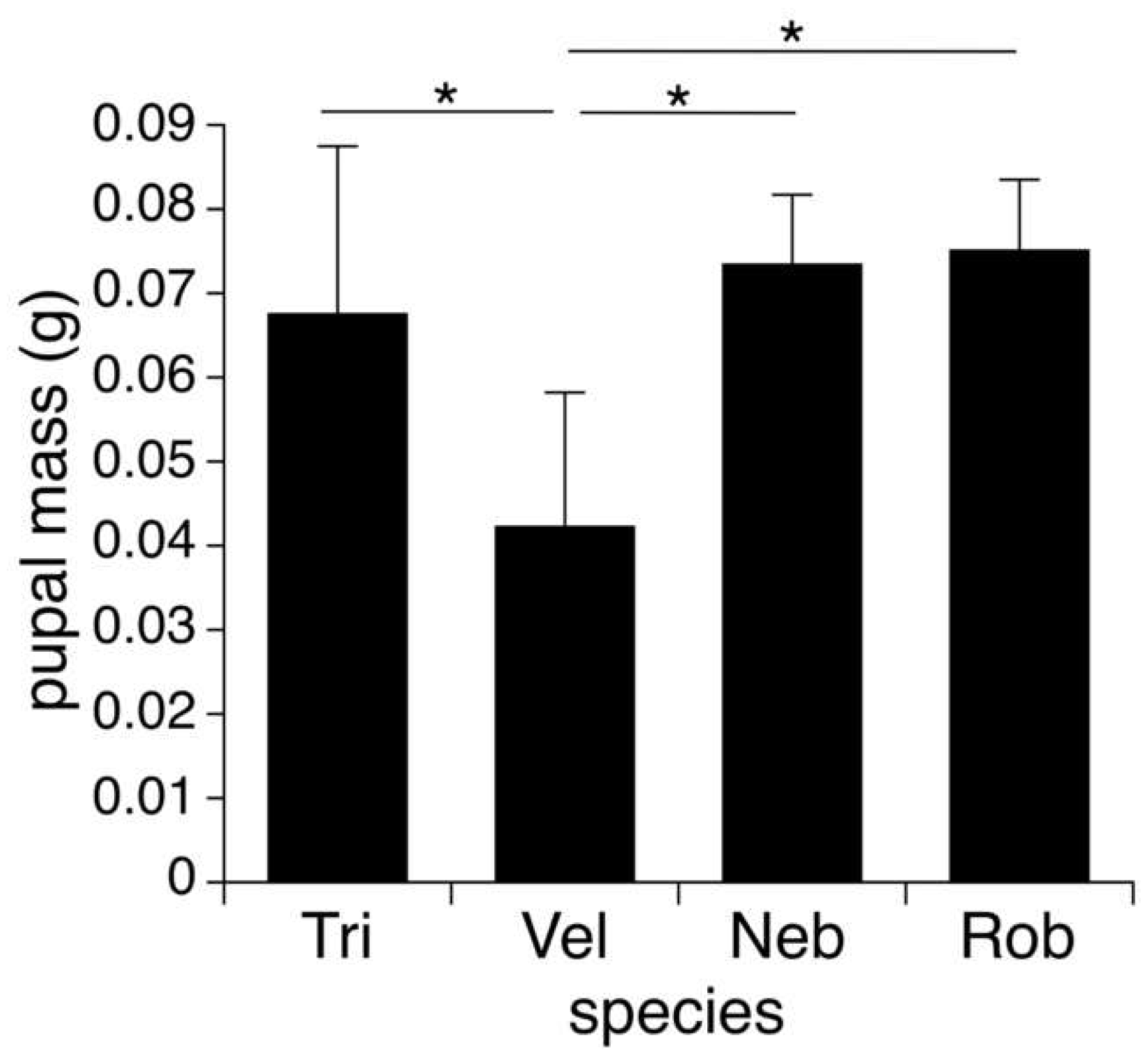
3.2. Pupal Development
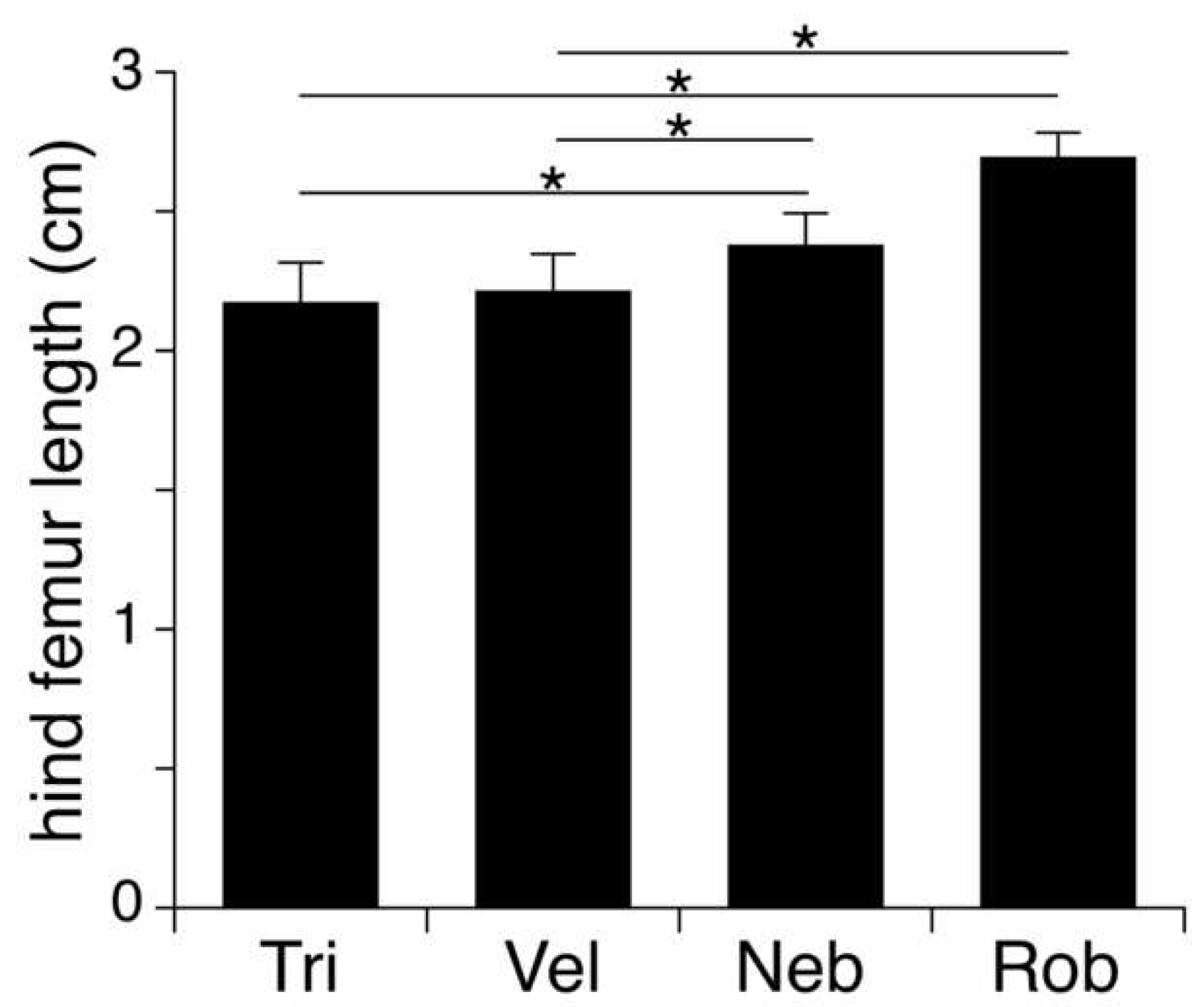
3.3. Host Size
4. Discussion
4.1. Differences in Fly Development and Possible Explanations
4.2. Similarities in Host Use
4.3. Co-Evolution
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doebeli, M.; Dieckmann, U. Evolutionary branching and sympatric speciation caused by different types of ecological interactions. Am. Nat. 2000, 156, S77–S101. [Google Scholar] [CrossRef]
- Yoder, J.B.; Nuismer, S.L. When does coevolution promote diversification? Am. Nat. 2010, 176, 802–817. [Google Scholar] [CrossRef]
- Arnqvist, G.; Rowe, L. Sexual conflict and arms races between the sexes: A morphological adaptation for control of mating in a female insect. Proc. R. Soc. Lond. B Biol. Sci. 1995, 261, 123–127. [Google Scholar]
- Leisler, B.; Winkler, H. The role of female investment in a sexual arms race. J. Avian Biol. 2020, 51. [Google Scholar] [CrossRef]
- Bucciarelli, G.M.; Alsalek, F.; Kats, L.B.; Green, D.B.; Shaffer, H.B. Toxic relationships and arms-race coevolution revisited. Annu. Rev. Anim. Biosci. 2022, 10, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Hague, M.T.; Miller, L.E.; Stokes, A.N.; Feldman, C.R.; Brodie, E.D., Jr.; Brodie, E.D., III. Conspicuous coloration of toxin-resistant predators implicates additional trophic interactions in a predator–prey arms race. Mol. Ecol. 2022, 32, 4482–4496. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; Volume 67. [Google Scholar]
- Askew, R.R.; Shaw, M.R. Parasitoid communities: Their size, structure and development. In Insect Parasitoids, Proceedings of the 13th Symposium of Royal Entomological Society of London, London, UK, 18–19 September 1985; Waage, J., Greathead, D., Eds.; Academic Press: London, UK; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Shaw, S.R. Euphorine phylogeny: The evolution of diversity in host-utilization by parasitoid wasps (Hymenoptera: Braconidae). Ecol. Entom. 1988, 13, 323–335. [Google Scholar] [CrossRef]
- Vinson, B.S. The behavior of parasitoids. Compr. Insect Physiol. Biochem. Pharmacol. 1985, 9, 417–469. [Google Scholar]
- Lehmann, G.U. Review of biogeography, host range and evolution of acoustic hunting in Ormiini (Insecta, Diptera, Tachinidae), parasitoids of night-calling bushcrickets and crickets (Insecta, Orthoptera, Ensifera). Zool. Anz. 2003, 242, 107–120. [Google Scholar] [CrossRef]
- Adamo, S.A.; Robert, D.; Hoy, R.R. Effects of a tachinid parasitoid, Ormia ochracea, on the behaviour and reproduction of its male and female field cricket hosts (Gryllus spp.). J. Insect Physiol. 1995, 41, 269–277. [Google Scholar] [CrossRef]
- Burk, T. Evolutionary significance of predation on sexually signaling males. Fla. Entomol. 1982, 65, 90–104. [Google Scholar] [CrossRef]
- Wineriter, S.A.; Walker, T.J. Rearing phonotactic parasitoid flies [Diptera: Tachinidae, Ormiini, Ormia spp.]. Entomophaga 1990, 35, 621–632. [Google Scholar] [CrossRef]
- Rogers, K.J.; Beckers, O.M. Parasitism of Neoconocephalus katydids by the parasitoid fly, Ormia lineifrons. Ethology 2022, 128, 111–118. [Google Scholar] [CrossRef]
- van Baaren, J.; Le Lann, C.; van Alphen, J.J.M. Consequences of climate change for aphid-based multi-trophic systems. In Aphid Biodiversity under Environmental Change: Patterns and Processes; Springer: Berlin/Heidelberg, Germany, 2010; pp. 55–68. [Google Scholar]
- Tinghitella, R.M.; Broder, E.D.; Gallagher, J.H.; Wikle, A.W.; Zonana, D.M. Responses of intended and unintended receivers to a novel sexual signal suggest clandestine communication. Nat. Commun. 2021, 12, 797. [Google Scholar] [CrossRef] [PubMed]
- Zuk, M.; Rotenberry, J.T.; Tinghitella, R.M. Silent night: Adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2006, 2, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.A.; Banuelos, C.; Walker, S.E.; Cade, W.H.; Zuk, M. Behavioural specialization among populations of the acoustically orienting parasitoid fly Ormia ochracea utilizing different cricket species as hosts. Anim. Behav. 2007, 73, 99–104. [Google Scholar] [CrossRef]
- Aluja, M.; J Sivinski, R.; Van Driesche, A.; Anzures-Dadda, A.; Guilen, L. Pest management through tropical tree conservation. Biodivers. Conserv. 2014, 24, 831–853. [Google Scholar] [CrossRef]
- Beckers, O.M. Parasitism of the Katydid Neoconocephalus triops (Orthoptera: Tettigoniidae) by the tachinid flies Ormia lineifrons and Neomintho sp. (Diptera: Tachinidae). Fla. Entomol. 2022, 105, 133–136. [Google Scholar] [CrossRef]
- Dorn, S.; Beckage, N.E. Superparasitism in gregarious hymenopteran parasitoids: Ecological, behavioural and physiological perspectives. Physiol. Entomol. 2007, 32, 199–211. [Google Scholar] [CrossRef]
- Lehmann, G.U.; Lehmann, A.W. Potential lifetime reproductive success of male bushcrickets parasitized by a phonotactic fly. Anim. Behav. 2006, 71, 1103–1110. [Google Scholar] [CrossRef]
- Lehmann, G.U. How different host species influence parasitism patterns and larval competition of acoustically-orienting parasitoid flies (Tachinidae: Ormiini). In Animal Behavior: New Research; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2008; pp. 93–132. [Google Scholar]
- Vet, L.E.; Datema, A.; Janssen, A.; Snellen, H. Clutch size in a larval-pupal endoparasitoid: Consequences for fitness. J. Anim. Ecol. 1994, 63, 807–815. [Google Scholar] [CrossRef]
- Häckermann, J.; Rott, A.S.; Dorn, S. How two different host species influence the performance of a gregarious parasitoid: Host size is not equal to host quality. J. Anim. Ecol. 2007, 76, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Hardy, I.C.W.; Griffiths, N.T.; Godfray, H.C.J. Clutch size in a parasitoid wasp: A manipulation experiment. J. Anim. Ecol. 1992, 61, 121–129. [Google Scholar] [CrossRef]
- Zaviezo, T.; Mills, N. Factors influencing the evolution of clutch size in a gregarious insect parasitoid. J. Anim. Ecol. 2000, 69, 1047–1057. [Google Scholar] [CrossRef]
- Bell, H.A.; Marris, G.C.; Prickett, A.J.; Edwards, J.P. Influence of host size on the clutch size and developmental success of the gregarious ectoparasitoid Eulophus pennicornis (Nees) (Hymenoptera: Braconidae) attacking larvae of the tomato moth Lacanobia oleracea (L.) (Lepidoptera: Noctuidae). J. Exp. Biol. 2005, 208, 3199–3209. [Google Scholar] [CrossRef] [PubMed]
- Cade, W. Acoustically orienting parasitoids: Fly phonotaxis to cricket song. Science 1975, 190, 1312–1313. [Google Scholar] [CrossRef]
- Whitesell, J.J. Geographic Variation and Dimorphisms in Song, Development, and Color in a Katydid; Field and Laboratory Studies (Tettigoniidae, Orthoptera). Doctoral Dissertation, University of Florida-Gainesville, Gainesville, FL, USA, 1974. [Google Scholar]
- Thomson, I.R.; Vincent, C.M.; Bertram, S.M. Success of the parasitoid fly Ormia ochracea (Diptera: Tachinidae) on natural and unnatural cricket hosts. Fla. Entomol. 2012, 95, 43–48. [Google Scholar] [CrossRef]
- Wilson, K.; Cotter, S.C. Host–parasite interactions and the evolution of immune defense. In Advances in the Study of Behavior; Academic Press: Cambridge, MA, USA, 2013; Volume 45, pp. 81–174. [Google Scholar]
- Lack, D. The significance of clutch size. Ibis 1947, 89, 309–352. [Google Scholar] [CrossRef]
- Stireman, J.O., III; O’Hara, J.E.; Wood, D.M. Tachinidae: Evolution, behavior, and ecology. Annu. Rev. Entomol. 2006, 51, 525–555. [Google Scholar] [CrossRef]
- Otto, M.; Mackauer, M. The developmental strategy of an idiobiont ectoparasitoid, Dendrocerus carpenteri: Influence of variations in host quality on offspring growth and fitness. Oecologia 1998, 117, 353–364. [Google Scholar] [CrossRef]
- Bertschy, C.; Turlings, T.C.; Bellotti, A.; Dorn, S. Host stage preference and sex allocation in Aenasius vexans, an encyrtid parasitoid of the cassava mealybug. Entomol. Exp. Appl. 2000, 95, 283–291. [Google Scholar] [CrossRef]
- Pedersen, A.B.; Altizer, S.; Poss, M.; Cunningham, A.A.; Nunn, C.L. Patterns of host specificity and transmission among parasites of wild primates. Int. J. Parasitol. 2005, 35, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Cleaveland, S.; Laurenson, M.K.; Taylor, L.H. Diseases of humans and their domestic mammals: Pathogen characteristics, host range and the risk of emergence. Philos. Trans. R. Soc. Lond. 2001, 356, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Williams, H. Parasitic Worms of Fish; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Walker, T.J.; Whitesell, J.J.; Alexander, R.D. The robust conehead: Two widespread sibling species (Orhtoptera: Tettigoniidae: Neoconocephalus ‘robustus’). Ohio J. Sci. 1973, 73, 321–330. [Google Scholar]
- O’Hara, J.E.; Henderson, S.J.; Wood, D.M. Preliminary Checklist of the Tachinidae (Diptera) of the World. Version 2.0. Available online: http://www.nadsdiptera.org/Tach/WorldTachs/Checklist/Worldchecklist.html (accessed on 5 May 2022).
- Greenfield, M.D. Evolution of acoustic communication in the genus Neoconocephalus: Discontinuous songs, synchrony, and interspecific interactions. In The Tettigoniidae: Biology, Systematics and Evolution; Springer: Berlin/Heidelberg, Germany, 1990; pp. 71–97. [Google Scholar]
- Walker, T.J.; Greenfield, M.D. Songs and systematics of Caribbean Neoconocephalus (Orthoptera: Tettigoniidae). Trans. Am. Entomol. Soc. 1983, 109, 357–389. [Google Scholar]
- Jeffs, C.T.; Lewis, O.T. Effects of climate warming on host–parasitoid interactions. Ecol. Entomol. 2013, 38, 209–218. [Google Scholar] [CrossRef]


| Species | Development Time (mean ± S.D.) | Clutch Size (mean ± S.D.) | Cumulative Parasitism Rate (%) |
|---|---|---|---|
| N. triops | 12.33 ± 1.1 (25) | 1.93 ± 0.97 (44) | 31.8% (154) |
| N. velox | 12.51 ± 3.0 (21) | 2.30 ± 1.18 (23) | 73.0% (33) |
| N. nebrascensis | 12.50 ± 1.9 (4) | 1.17 ± 0.41 (6) | 17.5% (40) |
| N. robustus | 12.10 ± 1.0 (10) | 1.42 ± 0.67 (12) | 14.3% (84) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, K.J.; Beckers, O.M. Multi-Species Host Use by the Parasitoid Fly Ormia lineifrons. Insects 2023, 14, 744. https://doi.org/10.3390/insects14090744
Rogers KJ, Beckers OM. Multi-Species Host Use by the Parasitoid Fly Ormia lineifrons. Insects. 2023; 14(9):744. https://doi.org/10.3390/insects14090744
Chicago/Turabian StyleRogers, Kyler J., and Oliver M. Beckers. 2023. "Multi-Species Host Use by the Parasitoid Fly Ormia lineifrons" Insects 14, no. 9: 744. https://doi.org/10.3390/insects14090744
APA StyleRogers, K. J., & Beckers, O. M. (2023). Multi-Species Host Use by the Parasitoid Fly Ormia lineifrons. Insects, 14(9), 744. https://doi.org/10.3390/insects14090744






