Parasitism and Suitability of Trichogramma chilonis on Large Eggs of Two Factitious Hosts: Samia cynthia ricini and Antheraea pernyi
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Hosts
2.2. Parasitoids
2.3. Comparison of Basic Parameters of Two Host Eggs
2.4. Preferences of T. chilonis for Two Factitious Hosts
2.5. Assessment of Parasitoid Body Size
2.6. Data Analysis
3. Result
3.1. Egg Size and Eggshell Thickness of Two Factitious Hosts
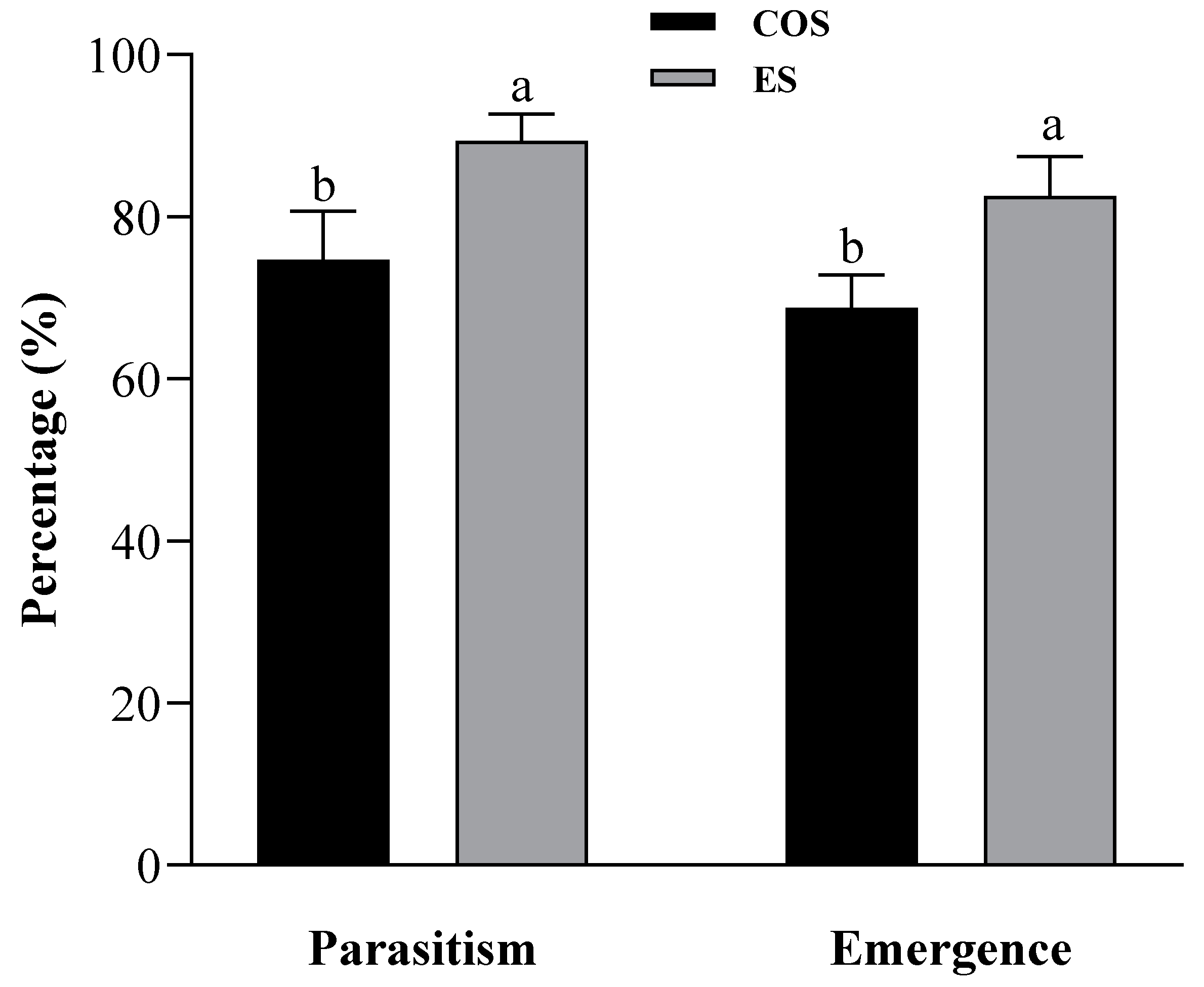
3.2. Effects of Different Factitious Hosts on Parasitism Preference and Offspring Performance of Trichogramma chilonis
3.3. Effects of Different Factitious Hosts on the Body Size of Parasitoid Offspring
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zang, L.-S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status, and perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhu, B.; Liang, P.; Gao, X. Identification of carboxylesterase genes contributing to multi-insecticide resistance in Plutella xylostella (L.). Entomol. Gen. 2022, 42, 967–976. [Google Scholar] [CrossRef]
- Smith, S.M. Biological Control with Trichogramma: Advances, Successes, and Potential of Their Use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef]
- Huang, N.-X.; Jaworski, C.C.; Desneux, N.; Zhang, F.; Yang, P.-Y.; Wang, S. Long-term and large-scale releases of Trichogramma promote pesticide decrease in maize in northeastern China. Entomol. Gen. 2020, 40, 331–335. [Google Scholar] [CrossRef]
- Jin, T.; Lin, Y.; Ma, G.; Liu, J.; Hao, Z.; Han, S.; Peng, Z. Biocontrol potential of Trichogramma species against Spodoptera Fru-giperda and their field efficacy in maize. Crop Prot. 2021, 150, 105790. [Google Scholar] [CrossRef]
- Wang, P.; Li, M.-J.; Bai, Q.-R.; Ali, A.; Desneux, N.; Dai, H.-J.; Zang, L.-S. Performance of Trichogramma japonicum as a vector of Beauveria bassiana for para-sitizing eggs of rice striped stem borer, Chilo suppressalis. Entomol. Gen. 2021, 41, 147–155. [Google Scholar] [CrossRef]
- Nadeem, S.; Hamed, M. Biological control of sugarcane borers with inundative release of Trichogramma chilonis (Ishii) (Hy-menoptera: Trichogrammatidae) in farmer fields. Pak. J. Agri. Sci. Vol. 2011, 48, 71–74. [Google Scholar]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.-A.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; Berg, J.v.D.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2023, 43, 187–241. [Google Scholar] [CrossRef]
- Hou, Y.-Y.; Ma, Y.; Xu, W.; Desneux, N.; Nkunika, P.O.; Bao, H.-P.; Zang, L.-S. Spodoptera frugiperda egg mass scale thickness modulates Trichogramma parasitoid performance. Entomol. Gen. 2022, 42, 589–596. [Google Scholar] [CrossRef]
- Li, T.-H.; Wang, S.; Ramirez-Romero, R.; Zang, L.-S. Protective scale variation on Spodoptera egg masses can potentially support the cost-effective use of Trichogramma parasitoids. Entomol. Gen. 2023, 43, 939–944. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Z.-P.; Hou, Y.-Y.; Yang, X.; Wang, S.; Dai, H.-J.; Xu, Y.-Y.; Zang, L.-S. Manually-extracted unfertilized eggs of Chinese oak silkworm, Antheraea pernyi, enhance mass production of Trichogramma parasitoids. Entomol. Gen. 2020, 40, 397–406. [Google Scholar] [CrossRef]
- Mandour, N.S.; Mahmoud, M.F.; Osman, M.A.-N.; Qiu, B. Efficiency, intrinsic competition and interspecific host discrimination of Copidosoma desantisi and Trichogramma evanescens, two parasitoids of Phthorimaea operculella. Biocontrol Sci. Technol. 2008, 18, 903–912. [Google Scholar] [CrossRef]
- Bertin, A.; Pavinato, V.A.C.; Parra, J.R.P. Fitness-related changes in laboratory populations of the egg parasitoid Trichogramma galloi and the implications of rearing on factitious hosts. BioControl 2017, 62, 435–444. [Google Scholar] [CrossRef]
- Li, T.-H.; Tian, C.-Y.; Zang, L.-S.; Hou, Y.-Y.; Ruan, C.-C.; Yang, X.; Monticelli, L.; Desneux, N. Multiparasitism with Trichogramma dendrolimi on egg of Chinese oak silkworm, Antheraea pernyi, enhances emergence of Trichogramma ostriniae. J. Pest Sci. 2018, 92, 707–713. [Google Scholar] [CrossRef]
- Li, X.-Y.; Lei, Q.; Hua, H.-Q.; Song, H.-F.; Wang, S.; Ramirez-Romero, R.; Dai, H.; Li, J.; Li, Y.-X. Impact of host suitability on oviposition preference toward fertilized and unfertilized host eggs in two Trichogramma parasitoid species. Entomol. Gen. 2019, 39, 313–323. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, Y.-F.; Song, Q.-T.; Zhang, F.; Li, Y.-X. The suitability of Ostrinia furnacalis (Lepidoptera: Crambidae) eggs for Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae) can be changed by T. ostriniae. Appl. Entomol. Zool. 2014, 49, 265–272. [Google Scholar] [CrossRef]
- Pehlivan, S. Role of host diet on the fitness of the egg parasitoid species, Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). Egypt. J. Biol. Pest Control. 2021, 31, 10. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Lin, L.; Terenius, O. Antheraea pernyi (Lepidoptera: Saturniidae) and Its Importance in Sericulture, Food Consumption, and Traditional Chinese Medicine. J. Econ. Entomol. 2017, 110, 1404–1411. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, J.S.; Kim, M.J.; Kang, P.D.; Kim, S.G.; Jin, B.R.; Han, Y.S.; Kim, I. Complete nucleotide sequence and organi-zation of the mitochondrial genome of Eri-silkworm, Samia cynthia ricini (Lepidoptera: Saturniidae). J. Asia-Pac. Entomol. 2012, 15, 162–173. [Google Scholar] [CrossRef]
- Baruah, M. Studies on larval weight and shell ratio of Eri silkworm (Philosamia ricini) on castor, kesseru and treated kesseru by foliar spray. Int. J. Comput. Appl. Eng. Sci. 2012, 2, 133–137. [Google Scholar]
- Shappirio, D.G. Comparative studies of oxidative enzyme systems in epidermis and fat body of diapausing and non-diapausing silkmoths. J. Insect Physiol. 1974, 20, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Ichida, M.; Kusakabe, T.; Koga, K. Chorion morphology of the Eri-silkworm, Samia cynthia ricini (Donovan) (Lepidoptera: Saturniidae). Appl. Entomol. Zool. 2000, 35, 427–434. [Google Scholar] [CrossRef]
- Manisha, B.L. Resource efficient and cost reduction technology for Trichogramma chilonis Ishii (Hymenoptera: Trichogram-matidae) production. J. Biol. Control 2020, 34, 43–46. [Google Scholar] [CrossRef]
- Lalitha, Y.; Navik, O.; Varshney, R.; Patel, V.; Ballal, C. Field efficacy of Trichogramma chilonis reared on different factitious hosts for the management of sugarcane stem borers. Bull. Insectology 2023, 76, 1–7. [Google Scholar]
- Chen, Y.; Iqbal, A.; Lv, R.; Wang, X.; Desneux, N.; Zang, L. Chinese oak silkworm Antherae pernyi egg, a suitable factitious host for rearing eupelmid egg parasitoids. Pest Manag. Sci. 2022, 78, 1789–1799. [Google Scholar] [CrossRef]
- Stouthamer, R.; Hu, J.; van Kan, F.J.; Platner, G.R.; Pinto, J.D. The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for distinguishing sibling species of Trichogramma. BioControl 1999, 43, 421–440. [Google Scholar] [CrossRef]
- Nii, T.; Isobe, N.; Yoshimura, Y. Effects of avian infectious bronchitis virus antigen on eggshell formation and immunoreaction in hen oviduct. Theriogenology 2014, 81, 1129–1138. [Google Scholar] [CrossRef]
- McFarlane, J.E. Structure and Function of the Egg Shell as Related to Water Absorption by the Eggs of Acheta domesticus (L.). Can. J. Zool. 1960, 38, 231–241. [Google Scholar] [CrossRef]
- Renou, M.; Nagnan, P.; Berthier, A.; Durier, C. Identification of compounds from the eggs of Ostrinia nubilalis and Mamestra brassicae having kairomone activity on Trichogramma brassicae. Entomol. Exp. Appl. 1992, 63, 291–303. [Google Scholar] [CrossRef]
- Foerster, M.R.; Foerster, L.A. Effects of temperature on the immature development and emergence of five species of Trichogramma. BioControl 2008, 54, 445–450. [Google Scholar] [CrossRef]
- Rahimi-Kaldeh, S.; Ashouri, A.; Bandani, A. Long-term storage of sexual and asexual Trichogramma brassicae (Hymenoptera: Trichogrammatidae). Biocontrol Sci. Technol. 2017, 27, 1339–1347. [Google Scholar] [CrossRef]
- Pak, G.A.; van Dalen, A.; Kaashoek, N.; Dijkman, H. Host egg chorion structure influencing host suitability for the egg para-sitoid Trichogramma Westwood. J. Insect. Physiol. 1990, 36, 869–875. [Google Scholar] [CrossRef]
- Cônsoli, F.; Kitajima, E.; Parra, J. Ultrastructure of the natural and factitious host eggs of Trichogramma galloi Zucchi and Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Int. J. Insect Morphol. Embryol. 1999, 28, 211–231. [Google Scholar] [CrossRef]
- Dahlan, A.N.; Gordh, G. Development of Trichogramma australicum (Hym.: Trichogrammatidae) at low and high population density in artificial diet. BioControl 1997, 42, 525–536. [Google Scholar] [CrossRef]
- Subandi, M.; Setiati, Y.; Mutmainah, N.H.; Setiati, Y.; Mutmainah, N.H. Suitability of Corcyra cephalonica eggs parasitized with Trichogramma japonicum as intermediate host against sugarcane borer Chilo auricilius. Bulg. J. Agric. Sci. 2017, 23, 779–786. [Google Scholar]
- Wang, J.-J.; Liu, X.-B.; Zhang, Y.-A.; Wen, C.; Wei, J.-R. The reproductive capability of Ooencyrtus kuvanae reared on eggs of the factitious host Antheraea pernyi. J. Appl. Entomol. 2014, 138, 267–272. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Wang, Q.; Ning, S.; Che, W.; Dong, H. Optimal clutch size for quality control of bisexual and Wolbachia -infected thelytokous lines of Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) mass reared on eggs of a substitutive host, Antheraea pernyi Guérin-Méneville (Lepidoptera: Saturniidae). Pest. Manag. Sci. 2020, 76, 2635–2644. [Google Scholar]
- Wang, X.; Biondi, A.; Nance, A.H.; Zappalà, L.; Hoelmer, K.A.; Daane, K.M. Assessment of Asobara japonica as a potential biological control agent for the spotted wing drosophila, Drosophila suzukii. Entomol. Gen. 2021, 41, 1–12. [Google Scholar] [CrossRef]
- Harvey, J.A. Factors affecting the evolution of development strategies in parasitoid wasps: The importance of functional con-straints and incorporating complexity. Entomol. Exp. Appl. 2005, 117, 1–13. [Google Scholar] [CrossRef]
- Stoepler, T.M.; Lill, J.T.; Murphy, S.M. Cascading effects of host size and host plant species on parasitoid resource allocation. Ecol. Entomol. 2011, 36, 724–735. [Google Scholar] [CrossRef]
- Häckermann, J.; Rott, A.S.; Dorn, S. How two different host species influence the performance of a gregarious parasitoid: Host size is not equal to host quality. J. Anim. Ecol. 2007, 76, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Van Alphen, J.J.; Visser, M.E. Superparasitism as an adaptive strategy for insect parasitoids. Annu. Rev. Entomol. 1990, 35, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.S.; Serrão, J.E.; Zanuncio, J.C.; Zanuncio, T.V.; Leite, G.L.D.; Polanczyk, R.A. Immunity of an Alternative Host Can Be Overcome by Higher Densities of Its Parasitoids Palmistichus elaeisis and Trichospilus diatraeae. PLoS ONE 2010, 5, e13231. [Google Scholar] [CrossRef] [PubMed]
- de S Pereira, K.; Guedes, N.M.P.; Serrão, J.E.; Zanuncio, J.C.; Guedes, R.N.C. Superparasitism, immune response and optimum progeny yield in the gregarious parasitoid Palmistichus elaeisis. Pest. Manag. Sci. 2017, 73, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, E.; Roitberg, B.D.; Boivin, G. Using optimality models to improve the efficacy of parasitoids in biological control programmes. Entomol. Exp. Appl. 2015, 158, 2–16. [Google Scholar] [CrossRef]
- Bai, B.; Luck, R.F.; Forster, L.; Stephens, B.; Janssen, J.A.M. The effect of host size on quality attributes of the egg parasitoid, Trichogramma pretiosum. Entomol. Exp. Appl. 1992, 64, 37–48. [Google Scholar] [CrossRef]
- Hassan, S.A.; Liscsinszky, H.; Zhang, G. The Oak-silkworm Egg Antheraea pernyi (Lepidoptera: Anthelidae) as a Mass Rearing Host for Parasitoids of the Genus Trichogramma (Hymenoptera: Trichogrammatidae). Biocontrol Sci. Technol. 2004, 14, 269–279. [Google Scholar] [CrossRef]
- Hoffmann, M.P.; Ode, P.R.; Walker, D.L.; Gardner, J.; van Nouhuys, S.; Shelton, A.M. Performance of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) Reared on Factitious Hosts, Including the Target Host, Ostrinia nubilalis (Lepidoptera: Crambidae). Biol. Control. 2001, 21, 1–10. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Q.-Q.; Huo, Y.-J.; Zhao, X.; Shang, D.; Yang, Y.; Zhang, L.-S.; Dong, H.; Zhou, J.-C. Decreased Wolbachia titers cause gradual change in masculinization of intersex individuals of thelytokous Trichogramma dendrolimi. Entomol. Gen. 2022, 42, 751–759. [Google Scholar] [CrossRef]
- Roriz, V.; Oliveira, L.; Garcia, P. Host suitability and preference studies of Trichogramma cordubensis (Hymenoptera: Trichogrammatidae). Biol. Control 2006, 36, 331–336. [Google Scholar] [CrossRef]
- Casas, J.; Pincebourde, S.; Mandon, N.; Vannier, F.; Poujol, R.; Giron, D. Lifetime Nutrient Dynamics Reveal Simultaneous Capital and Income Breeding in a Parasitoid. Ecology 2005, 86, 545–554. [Google Scholar] [CrossRef]
- Sequeira, R.; Mackauer, M. Nutritional Ecology of an Insect Host-Parasitoid Association: The Pea Aphid-Aphidius Ervi System. Ecology 1992, 73, 183–189. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Collier, T.R. The Evolution of Host-Feeding Behaviour in Insect Parasitoids. Biol. Rev. 1996, 71, 373–400. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ferns, P.N. The timing of egg maturation in insects: Ovigeny index and initial egg load as measures of fitness and of resource allocation. Oikos 2004, 107, 449–461. [Google Scholar] [CrossRef]
- Vet, L.E.M.; Jong, R.D.; Giessen, W.A.V.; Visser, J.H. A learning-related variation in electroantennogram responses of a parasitic wasp. Physiol. Entomol. 1990, 15, 243–247. [Google Scholar] [CrossRef]
- Bell, H.A.; Marris, G.C.; Prickett, A.J.; Edwards, J.P. Influence of host size on the clutch size and developmental success of the gregarious ectoparasitoid Eulophus pennicornis (Nees) (Hymenoptera: Braconidae) attacking larvae of the tomato moth Lacanobia oleracea (L.) (Lepidoptera: Noctuidae). J. Exp. Biol. 2005, 208 Pt 16, 3199–3209. [Google Scholar] [CrossRef]
| Parameters | Host | |
|---|---|---|
| COS | ES | |
| Egg length (μm) | 2908.80 ± 22.66 a | 1721.48 ± 9.31 b |
| Egg width (μm) | 2574.39 ± 8.41 a | 1178.85 ± 6.42 b |
| Eggshell thickness (μm) | 47.30 ± 1.64 a | 33.77 ± 0.62 b |
| Parameters | Parasitoids Reared on Different Factitious Hosts | |
|---|---|---|
| ES-Tc | COS-Tc | |
| Number of adults that emerged | 29.03 ± 1.79 b | 65.37 ± 3.60 a |
| Dead wasps in host egg | 0.77 ± 0.25 b | 7.27 ± 1.62 a |
| Female rate (%) | 86.53 ± 1.23 a | 83.68 ± 1.49 a |
| Emergence holes | 1.63 ± 0.09 a | 1.20 ± 0.07 b |
| Pre-emergence time (day) | 10.89 ± 0.13 b | 12.59 ± 0.14 a |
| Sex | Parameters | COS-Tc | ES-Tc |
|---|---|---|---|
| Female | Body length | 626.92 ± 6.50 a | 578.83 ± 12.69 b |
| Head width | 282.33 ± 8.82 a | 247.50 ± 4.80 b | |
| Hind tibia length | 219.79 ± 3.51 a | 194.92 ± 8.24 b | |
| Male | Body length | 556.69 ± 7.28 a | 438.43 ± 5.99 b |
| Head width | 242.57 ± 4.73 a | 210.33 ± 3.07 b | |
| Hind tibia length | 178.37 ± 3.46 a | 155.46 ± 2.91 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-H.; Xue, J.-Z.; Tariq, T.; Li, T.-H.; Qian, H.-Y.; Cui, W.-H.; Tian, H.; Monticelli, L.S.; Desneux, N.; Zang, L.-S. Parasitism and Suitability of Trichogramma chilonis on Large Eggs of Two Factitious Hosts: Samia cynthia ricini and Antheraea pernyi. Insects 2024, 15, 2. https://doi.org/10.3390/insects15010002
Zhang Y-H, Xue J-Z, Tariq T, Li T-H, Qian H-Y, Cui W-H, Tian H, Monticelli LS, Desneux N, Zang L-S. Parasitism and Suitability of Trichogramma chilonis on Large Eggs of Two Factitious Hosts: Samia cynthia ricini and Antheraea pernyi. Insects. 2024; 15(1):2. https://doi.org/10.3390/insects15010002
Chicago/Turabian StyleZhang, Yue-Hua, Ji-Zhi Xue, Talha Tariq, Tian-Hao Li, He-Ying Qian, Wen-Hui Cui, Hao Tian, Lucie S. Monticelli, Nicolas Desneux, and Lian-Sheng Zang. 2024. "Parasitism and Suitability of Trichogramma chilonis on Large Eggs of Two Factitious Hosts: Samia cynthia ricini and Antheraea pernyi" Insects 15, no. 1: 2. https://doi.org/10.3390/insects15010002






