Factors to Male-Female Sex Approaches and the Identification of Volatiles and Compounds from the Terminalia of Proholopterus chilensis (Blanchard) (Coleoptera: Cerambycidae) Females in Nothofagus obliqua (Mirb.) Oerst. (Nothofagaceae) Forests in Chile
Abstract
Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Localities, Study Seasons and Insect ID
2.2. Emergence of P. chilensis from Trunks, Their Sex Ratio, and the Determination of the Attractive Sex and Attractiveness According to Age
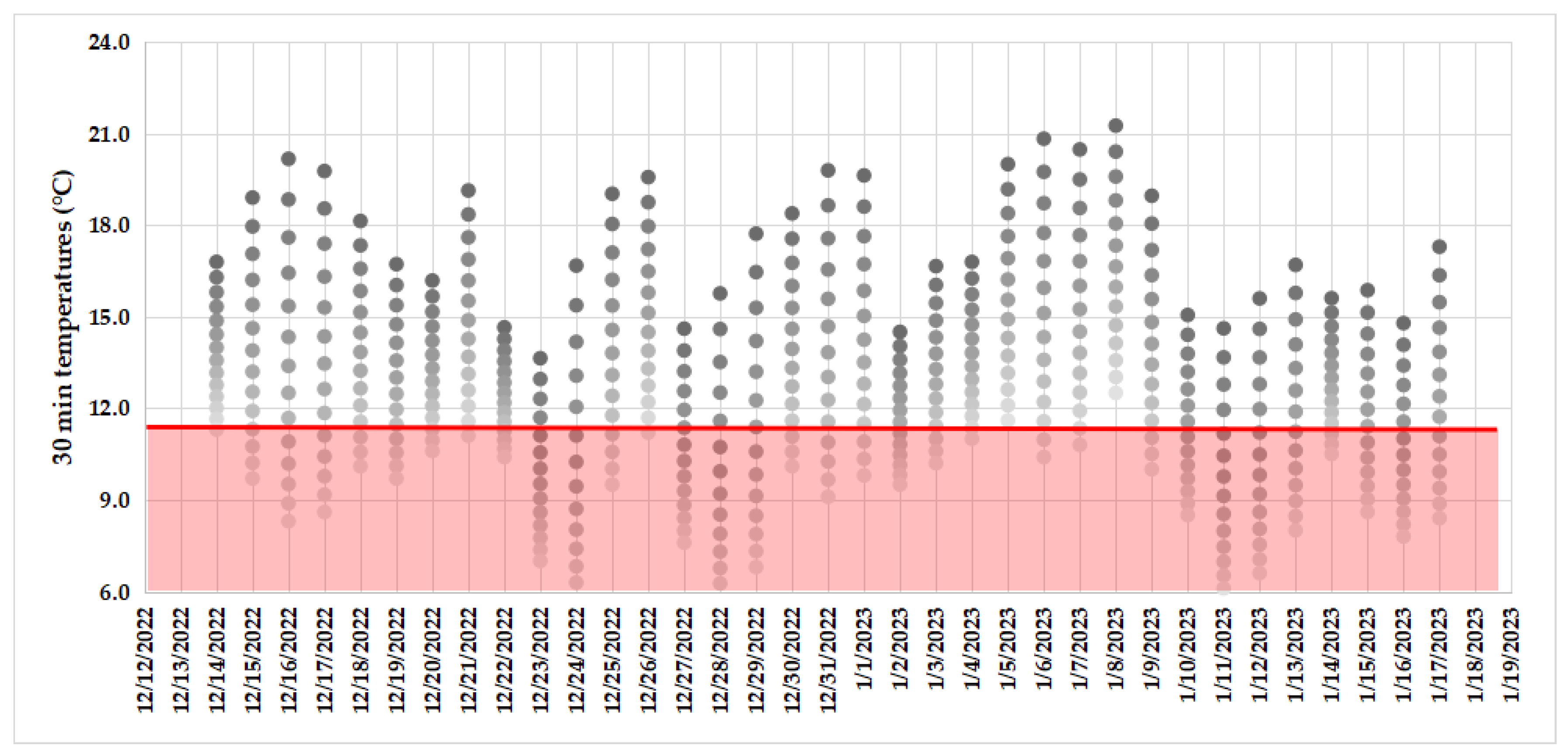
2.3. Successful Behavioral Observations, Environmental Conditions, and Hours during P. chilensis Female–Male Sex Approaches
2.4. Capture of Volatiles and Collection of Compounds from the P. chilensis Terminalia of Females
2.5. Identification of Volatiles and Terminalia Compounds from P. chilensis Females
3. Results
3.1. Emergence of Virgin P. chilensis from Trunks, Their Sex Ratio, and the Determination of the Attractive Sex and Attractiveness According to Age
3.2. Successful Behavioral Observations, Environmental Conditions, and Hours during P. chilensis Female–Male Sex Approaches
3.3. Identification of Volatiles and Terminalia Compounds from P. chilensis Females
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monne, M. Catalogue of the Cerambycidae (Coleoptera) of the Neotropical Region. Part I. Subfamily Cerambycinae. Zootaxa 2005, 946, 1–765. [Google Scholar] [CrossRef]
- Barriga, J.E.; Curkovic, T.; Fichet, T.; Henríquez, J.; Macaya, J. Nuevos antecedentes de coleópteros xilófagos y plantas hospederas en Chile, con una recopilación de citas previas. Rev. Chil. De Entomol. 1993, 20, 65–91. [Google Scholar]
- Artigas, J.N. Familia Cerambycidae. In Entomología Económica: Insectos de Interés Agrícola, Forestal, Médico y Veterinario, 1st ed.; Ediciones Universidad de Concepción: Concepción, Chile, 1994; Chapter 27.6; pp. 120–160. [Google Scholar]
- Gara, R.; Cerda, L.; Krahmer, E. Sobre la emergencia y vuelo de dos cerambícidos chilenos: Holopterus Chil. Y Cheloderus childreni. Turrialba 1978, 18, 117–121. [Google Scholar]
- Cabrera, P. Impacto de Holopterus chilensis (Coleoptera: Cerambycidae) en renovales de Nothofagus obliqua en la provincia de Valdivia, Chile: Avances hacia la evaluación del daño. Bosque 1997, 18, 9–19. [Google Scholar] [CrossRef]
- Baldini, A.; Alvarado, A. Manual de Plagas y Enfermedades del Bosque Nativo en Chile, 1st ed.; FAO: Santiago, Chile, 2008; 240p. [Google Scholar]
- Diaz, A. Gusanera del Roble: Holopterus chilensis. In Entomología Forestal en Chile, 1st ed.; Lanfranco, D., Ruiz, C., Eds.; Ediciones Universidad Austral de Chile: Valdivia, Chile, 2010; pp. 337–348. [Google Scholar]
- Baldini, A.; Pancel, L. Agentes de Daño en el Bosque Nativo; Editorial Universitaria: Santiago, Chile, 2002; 412p. [Google Scholar]
- Ruiz, C.; Montalva, C.; González, M. Native Forest Health in Chile: Toward a Strategy of Sustainable Management. In Forest Pest and Disease Management in Latin America; Estay, S., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Chapter 7; pp. 89–103. [Google Scholar] [CrossRef]
- Alzamora, R.M.; Apiolaza, L.A.; Ruiz, C.; Lanfranco, D. Site, Tree and Silvicultural Factors Influencing the Infestation of Xylophagous Insects on Nothofagus Forests. J. For. Res. 2020, 9, 226. [Google Scholar] [CrossRef]
- Alvarado, T. Análisis de Factores de Predisposición de Proholopterus chilensis (Coleoptera: Cerambycidae) en el Tipo Forestal Roble-Raulí-coihue, Región de la Araucanía. Bachelor Thesis, Universidad de Chile, Santiago, Chile, 2020; 48p. [Google Scholar]
- Curkovic, T. La sierra del manzano: Antecedentes biológicos y bases para el desarrollo del control de adultos. Aconex 2008, 98, 10–14. [Google Scholar]
- Rodríguez-González, A.; Pelaez, H.J.; González-Nuñez, M.; Casquero, P.A. Control of egg and neonate larvae of Xylotrechus arvicola (Coleoptera: Cerambycidae), a new vineyard pest, under laboratory conditions. Aust. J. Grape Wine Res. 2017, 23, 112–119. [Google Scholar] [CrossRef]
- Sánchez-Husillos, E.; Etxebeste, I.; Pajares, J. Effectiveness of mass trapping in the reduction of Monochamus galloprovincialis Olivier (Col.: Cerambycidae) populations. J. Appl. Entomol. 2015, 139, 747–758. [Google Scholar] [CrossRef]
- Sweeney, J.; Silk, P.J.; Rhainds, M.; MacKay, W.; Hughes, C.; Van Rooyen, K.; MacKinnon, W.; Leclair, G.; Holmes, S.; Kettela, E.G. First report of mating disruption with an aggregation pheromone: A case study with Tetropium fuscum (Coleoptera. Cerambycidae). J. Econ. Entomol. 2017, 110, 1078–1086. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Graham, E.E.; Wong, J.C.; Reagel, P.F.; Striman, B.L.; Hughes, G.P.; Hanks, L.M. Fuscumol and fuscumol acetate are general attractants for many species of cerambycid beetles in the subfamily Lamiinae. Entomol. Exp. Appl. 2011, 141, 71–77. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Reagel, P.F.; Wong, J.H.C.; Meier, L.R.; Diaz Silva, W.; Mongold-Diers, J.; Millar, J.G.; Hanks, L.M. Cerambycid beetle species with similar pheromones are segregated by phenology and minor pheromone components. J. Chem. Ecol. 2015, 41, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Hanks, L.M.; Millar, J.G. Sex and aggregation-sex pheromones of cerambycid beetles: Basic science and practical applications. J. Chem. Ecol. 2016, 42, 631–654. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.G.; Haynes, K.F. Methods in Chemical Ecology: Chemical Methods; Springer: New York, NY, USA, 1998; Volume 1, p. 390. [Google Scholar]
- Millar, J.G.; Hanks, L.M. Chemical Ecology of cerambycids. In Cerambycidae of the World; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 161–208. [Google Scholar]
- Matthews, R.W.; Matthews, J.R. Insect Behavior; Springer: New York, NY, USA, 2010; 520p. [Google Scholar]
- Lacey, E.S.; Ray, A.M.; Hanks, L.M. Calling behavior of the cerambycid beetle Neoclytus acuminatus acuminatus (F.). J. Insect Behav. 2007, 20, 117–128. [Google Scholar] [CrossRef]
- Lemay, M.A.; Silk, P.J.; Sweeney, J. Calling behavior of Tetropium fuscum (Coleoptera: Cerambycidae: Spondylidinae). Can. Entomol. 2010, 142, 256–260. [Google Scholar] [CrossRef]
- Barbour, J.; Cervantes, E.; Lacey, E.; Hanks, L. Calling behavior in the primitive longhorned beetle Prionus californicus Mots. J. Insect Behav. 2006, 19, 623–629. [Google Scholar] [CrossRef]
- Curkovic, T.; Ferrera, C. Female calling and male flight orientation and searching behaviors in Callisphyris apicicornis: Evidence for a female-produced sex attractant pheromone. Int. J. Agric. Nat. Resour. 2012, 39, 147–158. [Google Scholar] [CrossRef][Green Version]
- Curkovic, T.; Arraztio, D.; Huerta, A.; Rebolledo, R.; Cheuquel, A.; Contreras, A.; Millar, J.G. Generic Pheromones Identified from Northern Hemisphere Cerambycidae (Coleoptera) Are Attractive to Native Longhorn Beetles from Central-Southern Chile. Insects 2022, 13, 1067. [Google Scholar] [CrossRef]
- Conover, W.J. Practical Nonparametric Statistics; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1999; 608p. [Google Scholar]
- Parra, L.; Mutis, A.; Ceballos, R.; Lizama, M.; Pardo, F.; Perich, F.; Quiroz, A. Volatiles Released from Vaccinium corymbosum Were Attractive to Aegorhinus superciliosus (Coleoptera: Curculionidae) in an Olfactometric Bioassay. Environ. Entomol. 2009, 38, 781–789. [Google Scholar] [CrossRef]
- Mutis, A.; Parra, L.; Manosalva, L.; Palma, R.; Candia, O.; Lizama, M.; Pardo, F.; Perich, F.; Quiroz, A. Electroantennographic and Behavioral Responses of Adults of Raspberry Weevil Aegorhinus superciliosus (Coleoptera: Curculionidae) to Odors Released from Conspecific Females. Environ. Entomol. 2010, 39, 1276–1282. [Google Scholar] [CrossRef]
- Monné, M.L.; Monne, M.A.; Wang, Q. General Morphology, Classification and Biology of Cerambycidae. In Cerambycidae of the World; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 2–70. [Google Scholar]
- Kovats, E.; Keuleman, A. The Kovats retention index system. Rep. Anal. Chem. 1964, 36, 31–41. [Google Scholar]
- Akessé, E.N.; Ouali-N’goran, S.W.M. Reproductive Parameters of Diastocera trifasciata (Fabricius, 1775) (Coleoptera: Cerambycidae: Lamiinae), Cashew Branches Girdler in Côte d’Ivoire, Under Semi-Natural Conditions. Int. J. Sci. Res. Publ. 2018, 8, 686–698. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Mendiola-Díaz, F.J.; Sánchez-González, Á. Adult size and sex ratio variation of Cerambyx welensii (Coleoptera: Cerambycidae) in Mediterranean oak (Fagaceae) woodlands. Can. Entomol. 2018, 150, 334–346. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.Y. Mating behavior of a flower-visiting longhorn beetle Zorion guttigerum (Westwood) (Coleoptera: Cerambycidae: Cerambycinae). Naturwissenschaften 2005, 92, 237–241. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G.; Paine, T.D. Mating behavior of the eucalyptus longhorned borer (Coleoptera: Cerambycidae) and the adaptive significance of long “horns”. J. Insect Behav. 1996, 9, 383–393. [Google Scholar] [CrossRef]
- Curkovic, T.; Muñoz, J. Caracterización del cortejo y cópula en Callisphyris apicicornis: Herramienta para definir la viabilidad para desarrollar estrategias de manejo. Agrociencia 2011, 45, 453–464. [Google Scholar]
- Cerda, M. Lista sistemática de los cerambícidos chilenos. Rev. Chil. Entomol. 1986, 14, 29–39. [Google Scholar]
- Vitali, F. Versetzung der Tribus Holopterini Lacordaire, 1869 zur Unterfamilie Lepturinae (Coleoptera, Cerambycidae). Entomofauna 2002, 23, 29–33. [Google Scholar]
- Monne, M. Catalogue of the type-species of the genera of the Cerambycidae, Disteniidae, Oxypeltidae and Vesperidae (Coleoptera) of the Neotropical Region. Zootaxa 2012, 3213, 1–183. [Google Scholar] [CrossRef]
- Švácha, P.; Lawrence, J.F. Chapter 2.4. Cerambycidae Latreille, 1802. In Handbook of Zoology: Arthropoda: Insecta: Coleoptera, Beetles; Volume 3: Morphology and Systematics (Phytophaga); Leschen, R.A.B., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany, 2014; pp. 77–177. [Google Scholar]
- Mangalgikar, P.; Madhura Bhanu, K.R.; Belavadi, V.; Muniyappa, C.; Ammagarahalli, B. 1-octadecene, A Female Produced Aggregation Pheromone of the Coffee White Stem Borer (Xylotrechus quadripes). Horticulturae 2023, 9, 173. [Google Scholar] [CrossRef]
- Cohen, C.; Liltved, W.R.; Colville, J.F.; Shuttleworth, A.; Weissflog, J.; Svatoš, A.; Bytebier, B.; Johnson, S.D. Sexual deception of a beetle pollinator through floral mimicry. Curr. Biol. 2021, 31, 1962–1969. [Google Scholar] [CrossRef]
- Wang, W.C.; Cao, D.D.; Men, J.; Wei, J.R. (R)-(+)-citronellal identified as a female-produced sex pheromone of Aromia bungii Faldermann (Coleoptera: Cerambycidae). Egypt. J. Biol. Pest Control. 2018, 28, 1–6. [Google Scholar] [CrossRef]
- Sabri, M.S.; Abdullah, F. Mating behaviour and evidence of a female sex pheromone in Rytidodera simulans White (Coleoptera: Cerambycidae). J. Entomol. Res. 2016, 40, 313–326. [Google Scholar] [CrossRef]
- Curkovic, T.; Rodríguez, D.; Huerta, A.; Bergmann, J.; Ceballos, R. Behavioral and physiological response of male Callisphyris apicicornis (Coleoptera: Cerambycidae) to virgin con-specific females’ extracts. Chil. J. Agric. Res. 2018, 78, 470–477. [Google Scholar] [CrossRef]
- Nakayama, Y.; Jikumaru, S.; Togashi, K. Reproductive traits and diel activity of adult Monochamus saltuarius (Coleoptera: Cerambycidae) at two different temperatures. J. For. Res. 1998, 3, 55–61. [Google Scholar] [CrossRef]
- Cervantes, D.E.; Hanks, L.M.; Lacey, E.S.; Barbour, J.D. First documentation of a volatile sex pheromone in a longhorned beetle (Coleoptera: Cerambycidae) of the primitive subfamily Prioninae. Ann. Entomol. Soc. Am. 2006, 99, 718–722. [Google Scholar] [CrossRef]
- Xu, T.; Hansen, L.; Teale, S.A. Female calling behaviour in the Asian longhorned beetle (Coleoptera: Cerambycidae). Can. Entomol. 2019, 151, 600–607. [Google Scholar] [CrossRef]
- Adams, K.B. The Bionomics of a Monophagous, Native Wood Borer, Glycobius speciosus (Say) (Coleoptera: Cerambycidae). Ph.D. Thesis, State University of New York College of Environmental Science and Forestry, New York, NY, USA, 2017; 142p. [Google Scholar]
- Hoover, K.; Keena, M.; Nehme, M.; Wang, S.; Meng, P.; Zhang, A. Sex-specific trail pheromone mediates complex mate finding behavior in Anoplophora glabripennis. J. Chem. Ecol. 2014, 40, 169–180. [Google Scholar] [CrossRef]
- Fukaya, M.; Yasui, H. Pheromones in Longhorn Beetles with a Special Focus on Contact Pheromones. In Insect Sex Pheromone Research and Beyond: From Molecules to Robots; Ishikawa, Y., Ed.; Springer: Singapore, 2020; pp. 77–107. [Google Scholar]
- Kiriyama, S.; Iwata, R.; Fukaya, M.; Hoshino, Y.; Yamanaka, Y. Mating behavior of Rosalia batesi (Coleoptera: Cerambycidae) is mediated by male-produced sex pheromones. Insects 2018, 9, 48. [Google Scholar] [CrossRef]
- Fonseca, M.G.; Vidal, D.M.; Zarbin, P.H. Male-produced sex pheromone of the cerambycid beetle Hedypathes betulinus: Chemical identification and biological activity. J. Chem. Ecol. 2010, 36, 1132–1139. [Google Scholar] [CrossRef]
- Xu, T.; Hansen, L.; Teale, S.A. Mating and adult feeding behaviour influence pheromone production in female Asian longhorn beetle Anoplophora glabripennis (Coleoptera: Cerambycidae). Agric. For. Entomol. 2021, 23, 276–286. [Google Scholar] [CrossRef]
- Lacey, E.S.; Moreira, J.A.; Millar, J.G.; Hanks, L.M. A male-produced aggregation pheromone blend consisting of alkanediols, terpenoids, and an aromatic alcohol from the cerambycid beetle Megacyllene caryae. J. Chem. Ecol. 2008, 34, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.F.; Ray, A.M.; Hanks, L.M.; Millar, J.G. The common natural products (S)-α-terpineol and (E)-2-hexenol are important pheromone components of Megacyllene antennata (Coleoptera: Cerambycidae). Environ. Entomol. 2018, 47, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Yasuda, T.; Fukaya, M.; Akino, T.; Wakamura, S.; Hirai, Y.; Fukuda, T. Host plant chemicals serve intraspecific communication in the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2007, 42, 255–268. [Google Scholar] [CrossRef]
- Pherobase. 2024. Available online: www.pherobase.com (accessed on 20 July 2024).
- Renthal, R.; Lohmeyer, K.; Borges, L.M.F.; Pérez de León, A.A. Surface lipidome of the lone star tick, Amblyomma americanum, provides leads on semiochemicals and lipid metabolism. Ticks Tick-Borne Diseases 2019, 10, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; Martín-Ramos, P.; Niño-Sánchez, J.; Diez-Hermano, S.; Álvarez-Taboada, F.; Pérez-García, R.; Santiago-Aliste, A.; Martín-Gil, J.; Diez-Casero, J.J. Characterization of Leptoglossus occidentalis Eggs and Egg Glue. Insects 2023, 14, 396. [Google Scholar] [CrossRef]
- Boyer, F.D.; Malosse, C.; Zagatti, P.; Einhorn, J. Identification and synthesis of vesperal, the female sex pheromone of the longhorn beetle Vesperus xatarti. Bull. Société Chim. Fr. 1997, 134, 757–764. [Google Scholar] [CrossRef]
- Tanigaki, T.; Yamaoka, R.; Sota, T. The role of cuticular hydrocarbons in mating and conspecific recognition in the closely related longicorn beetles Pidonia grallatrix and P. takechii. Zool. Sci. 2007, 24, 39–45. [Google Scholar] [CrossRef]
- Khannoon, E.R. Secretions of pre-anal glands of house-dwelling geckos (Family: Gekkonidae) contain monoglycerides and 1,3-alkanediol. A comparative chemical ecology study. Biochem. Syst. Ecol. 2012, 44, 341–346. [Google Scholar] [CrossRef]
- Wang, H.M.; Bai, P.H.; Zhang, J.; Zhang, X.M.; Qin, H.U.I.; Zheng, H.X.; Zhang, X.H. Attraction of bruchid beetles Callosobruchus chinensis (L.) (Coleoptera: Bruchidae) to host plant volatiles. J. Integr. Agric. 2020, 19, 3035–3044. [Google Scholar] [CrossRef]
- Boevé, J.L.; Giot, R. Chemical composition: Hearing insect defensive volatiles. Patterns 2011, 2, 100352. [Google Scholar] [CrossRef]
- Koli, P.; Agarwal, M.; Kessell, D.; Mahawar, S.; Du, X.; Ren, Y.; McKirdy, S.J. Metabolite Variation between Nematode and Bacterial Seed Galls in Comparison to Healthy Seeds of Ryegrass Using Direct Immersion Solid-Phase Microextraction (DI-SPME) Coupled with GC-MS. Molecules 2023, 28, 828. [Google Scholar] [CrossRef] [PubMed]
- Coppée, A.; Terzo, M.; Valterova, I.; Rasmont, P. Intraspecific variation of the cephalic labial gland secretions in Bombus terrestris (L.) (Hymenoptera: Apidae). Chem. Biodivers. 2008, 5, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Attygalle, A.B.; Cai-Hong, W.U.; Schwarz, J.; Vostrowsky, O.; Hasenfuss, I.; Bestmann, H.J. Sex pheromone of female Myelois cribrella Hübner (Lepidoptera: Pyralidae) Chemical identification, electrophysiological evaluation, and field attractancy tests. J. Chem. Ecol. 1998, 14, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Osorio, I.; López-Pantoja, G.; Domínguez, L.; López-Manzano, M.R.; Rosell, G.; Guerrero, Á. Contact chemoreceptive mate recognition in Cerambyx welensii Küster (Coleoptera: Cerambycidae). Agric. For. Entomol. 2023, 25, 622–636. [Google Scholar] [CrossRef]
- Silk, P.J.; Sweeney, J.; Wu, J.; Sopow, S.; Mayo, P.D.; Magee, D. Contact Sex Pheromones Identified for Two Species of Longhorned Beetles (Coleoptera: Cerambycidae) Tetropium fuscum and T. cinnamopterum in the Subfamily Spondylidinae. Environ. Entomol. 2011, 40, 714–726. [Google Scholar] [CrossRef]
- Howse, P.; Stevens, I.; Jones, O. Insect Pheromones and Their Use in Pest Management; Chapman & Hall: London, UK, 1998; 369p. [Google Scholar]
- Wang, Q.; Zeng, W.; Chen, L.; Li, J.; Yin, X. Circadian reproductive rhythms, pair-bonding, and evidence for sex-specific pheromones in Nadezhdiella cantori (Coleoptera: Cerambycidae). J. Insect Behav. 2002, 15, 527–539. [Google Scholar] [CrossRef]
- Graves, F.; Baker, T.C.; Zhang, A.; Keena, M.; Hoover, K. Sensory aspects of trail-following behaviors in the Asian longhorned beetle, Anoplophora glabripennis. J. Insect Behav. 2016, 29, 615–628. [Google Scholar] [CrossRef]
- Shimomura, K.; Ohsawa, K. Hybrid Sex Pheromone Communication Systems in Seed Beetles. In Insect Sex Pheromone Research and Beyond: From Molecules to Robots; Ishikawa, Y., Ed.; Springer: Singapore, 2020; pp. 61–76. [Google Scholar]


| 2019–2020 n = 4 | 2021–2022 n = 3 | 2022–2023 n = 4 | Total Captures | |
|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 |
| Male | 0 | 0 | 0 | 0 |
| Female | 58 | 80 | 46 | 184 |
| Retention Time (min) | Family Compound Name 1 | Area 2 (%) | Main Mass Fragments (m/z) |
|---|---|---|---|
| 24.1 | Not identified | 6.23 | 57 (100), 93 (76), 81 (70), 96 (63), 73 (56), 95 (53), 107 (48), 79 (46), 41 (46), 91 (40) |
| 24.87 | Oxigenated sesquiterpene 1 | 53.41 | 57 (100), 161 (79), 71 (59), 45 (54), 41 (39), 203 (37), 85 (36), 133 (32), 77 (31) |
| 26.57 | Oxigenated sesquiterpene 2 | 4.30 | 57 (100), 69 (89), 205 (83), 71 (57), 83 (40), 111 (37), 85 (100), 70 (35), 41 (30) |
| 41.21 | Nitrogenated compound (C20) | 33.17 | 210 (100), 211 (17), 281 (94) |
| 43.77 | Long-chain hydrocarbon (C26) | 2.88 | 43 (100), 57 (700), 71 (680), 85 (600), 41 (418), 71 (144) |
| Retention Time (min) | Compound Name | Area 1 (%) | MW 2 (g/mol) | Chemical Composition | RI 3 | Main Mass Fragments (m/z) |
|---|---|---|---|---|---|---|
| 40.03 | Heneicosyl acetate | 14.12 | 354 | C23H46O2 | 2515 | 43 (100), 83 (88), 57 (86), 97 (81), 55 (10), 69 (62), 71 (54), 61 (49), 41 (41), 70 (39) |
| 43.29 | Docosyl acetate | 8.78 | 368 | C24H48O2 | 2618 | 43 (100), 57 (64), 97 (59), 83 (58), 55 (57), 61 (50), 69 (46), 111 (34), 41 (32), 71 (31) |
| 44.21 | Not identified | 2.90 | - | - | - | 130 (100), 117 (59), 131 (54), 95 (36), 55 (30), 57 (29), 43 (28), 81 (27), 71 (26) |
| 44.82 | Heptacosane | 22.69 | 380 | C27H56 | 2700 | 57 (100), 71 (85), 85 (63), 43 (50), 99 (26), 55 (24), 41 (19), 69 (19), 113 (16), 83 (15) |
| 48.21 | Heptacosyl acetate | 6.71 | 438 | C29H58O2 | 3071 | 97 (100), 57 (94), 83 (91), 69 (77), 55 (75), 43 (61), 71 (59), 111 (54), 41 (40), 85 (39) |
| 48.87 | Hentriacontane | 44.79 | 436 | C31H64 | 3100 | 57 (100), 71 (86), 85 (64), 43 (48), 99 (27), 55 (23), 69 (18), 41 (17), 113 (15), 83 (13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arraztio, D.; Huerta, A.; Quiroz, A.; Aniñir, W.; Rebolledo, R.; Curkovic, T. Factors to Male-Female Sex Approaches and the Identification of Volatiles and Compounds from the Terminalia of Proholopterus chilensis (Blanchard) (Coleoptera: Cerambycidae) Females in Nothofagus obliqua (Mirb.) Oerst. (Nothofagaceae) Forests in Chile. Insects 2024, 15, 741. https://doi.org/10.3390/insects15100741
Arraztio D, Huerta A, Quiroz A, Aniñir W, Rebolledo R, Curkovic T. Factors to Male-Female Sex Approaches and the Identification of Volatiles and Compounds from the Terminalia of Proholopterus chilensis (Blanchard) (Coleoptera: Cerambycidae) Females in Nothofagus obliqua (Mirb.) Oerst. (Nothofagaceae) Forests in Chile. Insects. 2024; 15(10):741. https://doi.org/10.3390/insects15100741
Chicago/Turabian StyleArraztio, Diego, Amanda Huerta, Andrés Quiroz, Washington Aniñir, Ramón Rebolledo, and Tomislav Curkovic. 2024. "Factors to Male-Female Sex Approaches and the Identification of Volatiles and Compounds from the Terminalia of Proholopterus chilensis (Blanchard) (Coleoptera: Cerambycidae) Females in Nothofagus obliqua (Mirb.) Oerst. (Nothofagaceae) Forests in Chile" Insects 15, no. 10: 741. https://doi.org/10.3390/insects15100741
APA StyleArraztio, D., Huerta, A., Quiroz, A., Aniñir, W., Rebolledo, R., & Curkovic, T. (2024). Factors to Male-Female Sex Approaches and the Identification of Volatiles and Compounds from the Terminalia of Proholopterus chilensis (Blanchard) (Coleoptera: Cerambycidae) Females in Nothofagus obliqua (Mirb.) Oerst. (Nothofagaceae) Forests in Chile. Insects, 15(10), 741. https://doi.org/10.3390/insects15100741









