Spatial Patterns of Adelges tsugae Annand (Hemiptera: Adelgidae) in Eastern Hemlock Stands: Implications for Sampling and Management
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study 1: Determining Optimal Sampling Unit for A. tsugae Ovisac Sampling
2.1.1. Study Sites
2.1.2. Sampling
2.1.3. Characterizing the Within-Tree Distribution of A. tsugae
2.1.4. Characterizing the Within-Branch Distribution of A. tsugae
2.1.5. Determining Sampling Units for A. tsugae Ovisacs
2.1.6. Estimating Within-Branch Abundance of A. tsugae Ovisacs
2.2. Study 2: Determining Spatial Distribution Patterns of A. tsugae Ovisac
2.2.1. Study Sites and Sampling A. tsugae and Environmental Factors
2.2.2. Characterizing Spatial Distribution Patterns of A. tsugae
2.2.3. Spatial Associations between Generations of A. tsugae
2.2.4. Spatial Association of A. tsugae Ovisacs with Environmental Factors
3. Results
3.1. Study 1: Determining Optimal Sampling Unit for A. tsugae Ovisac Sampling
3.1.1. Within-Tree Distribution of A. tsugae Ovisacs
3.1.2. Within-Branch Distribution of A. tsugae Ovisac
3.1.3. Determination of Optimal Sampling Unit
3.1.4. Estimating Within-Branch Abundance of A. tsugae Ovisacs for the Recommended Sampling Unit
3.2. Study 2: Determining Spatial Distribution Patterns of A. tsugae Ovisac
3.2.1. Population Dynamics A. tsugae Ovisacs
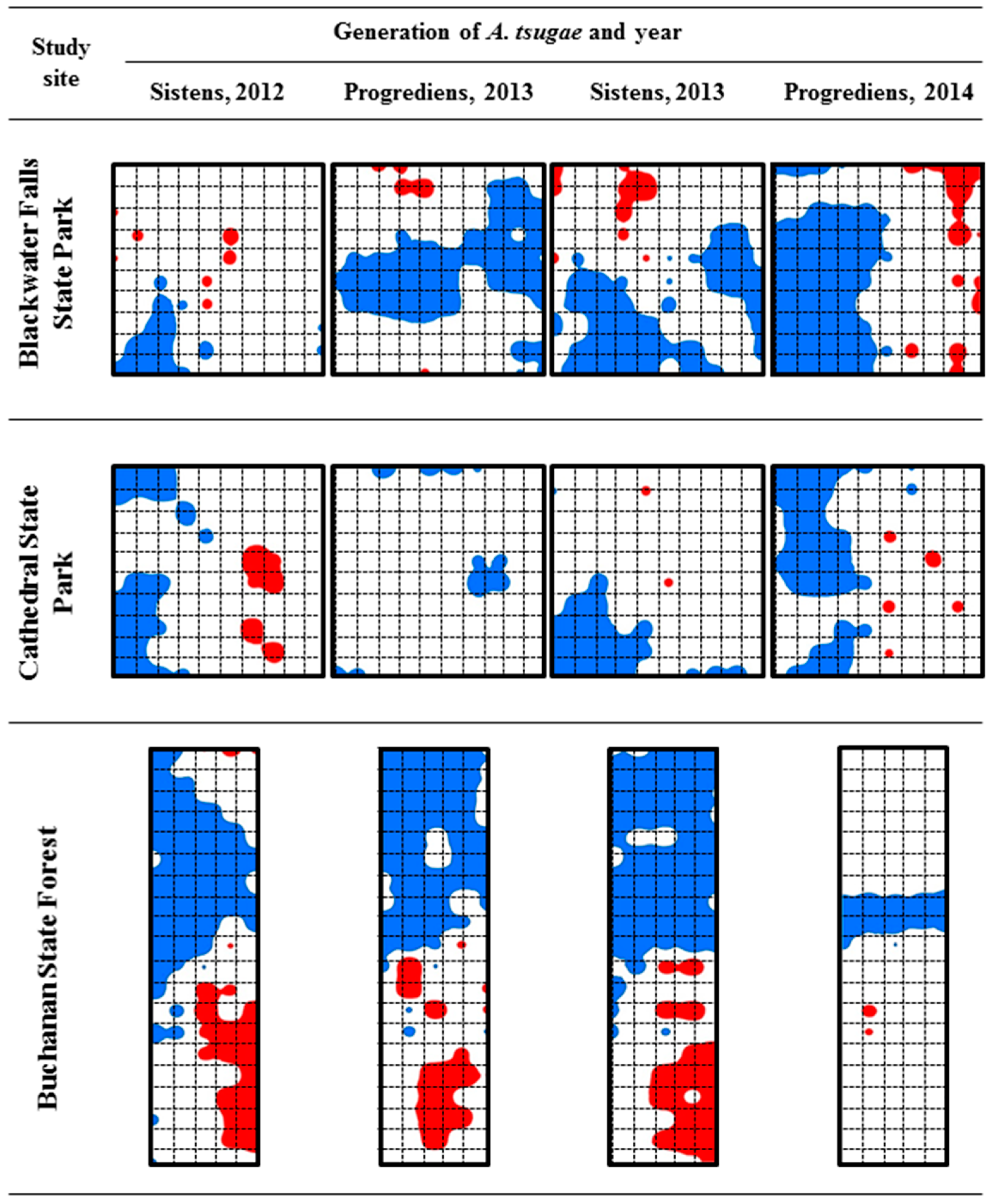
3.2.2. Spatial Distribution Patterns of A. tsugae
3.2.3. Spatial Associations between Generations of A. tsugae
3.2.4. Spatial Association of A. tsugae Ovisacs with Environmental Factors
4. Discussion
4.1. Study 1: Determining Optimal Sampling Unit for A. tsugae Ovisac Sampling
4.2. Study 2: Determining Optimal Sampling Unit for A. tsugae Ovisac Sampling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellison, A.M.; Orwig, D.A.; Fitzpatrick, M.C.; Presser, E.L. The past, present, and future of the hemlock woolly adelgid (Adelges tsugae) and its ecological interactions with eastern hemlock (Tsuga canadensis) forests. Insects 2018, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Flowers, R.W.; Salom, S.M.; Kok, L.T. Competitive interactions among two specialist predators and a generalist predator of hemlock woolly adelgid, Adelges tsugae (Hemiptera: Adelgidae) in south-western Virginia. Agric. For. Entomol. 2006, 8, 253–262. [Google Scholar] [CrossRef]
- Letheren, A.; Hill, S.; Salie, J.; Parkman, J.; Chen, J. A little bug with a big bite: Impact of hemlock woolly adelgid infestations on forest ecosystems in the eastern USA and potential control strategies. Int. J. Environ. Res. 2017, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Battles, J.J.; Cleavitt, N.; Fahey, T.J.; Evans, R.A. Vegetation composition and structure in two hemlock strands threatened by hemlock woolly adelgids. In Proceedings of the Symposium on the Sustainable Management of Hemlock Ecosystems in Eastern North America, Durham, NH, USA, 22–24 June 1999; McManus, K.A., Shields, K.S., Souto, D.R., Eds.; USDA Forest Service: Newtown Square, PA, USA, 2000; pp. 22–24. [Google Scholar]
- Mladenoff, D.J. Dynamics of nitrogen mineralization and nitrification in hemlock and hardwood treefall gaps. Ecology 1987, 68, 1171–1180. [Google Scholar] [CrossRef]
- Jenkins, J.C.; Aber, J.D.; Canham, C.D. Hemlock woolly adelgid impacts on community structure and N cycling rates in eastern hemlock forests. Can. J. For. Res. 1999, 29, 630–645. [Google Scholar] [CrossRef]
- Havill, N.; Vieira, L.C.; Salom, S.M. Biology and Control of Hemlock Woolly Adelgid; FHTET-2014-05; USDA Forest Service: Morgantown, WV, USA, 2016. [Google Scholar]
- Mayer, M.; Chianese, R.; Scudder, T.; White, J.; Vongpaseuth, K.; Ward, R. Thirteen years of monitoring the hemlock woolly adelgid in New Jersey forests. In Proceedings of the Symposium on the Hemlock Woolly Adelgid in the Eastern United States, East Brunswick, NJ, USA, 5–7 February 2002; Reardon, R.C., Onken, B.P., Lamshomb, J., Eds.; New Jersey Agricultural Experimental Station, Rutgers University: East Brunswick, NJ, USA, 2002; pp. 189–196. [Google Scholar]
- Havill, N.P.; Shiyake, S.; Lamb Gallowa, A.; Foottit, R.G.; Yu, G.; Paradis, A.; Elkinton, J.; Montgomery, M.E.; Sano, M.; Caccone, A. Ancient and modern colonization of North America by hemlock woolly adelgid, Adelges tsugae (Hemiptera: Adelgidae), an invasive insect from East Asia. Mol. Ecol. 2016, 25, 2065–2080. [Google Scholar] [CrossRef]
- Schmidt, T.L.; McWilliams, W.H. Status of eastern hemlock in the northern U.S. In Proceedings of the Regional Conference on Ecology and Management of Eastern Hemlock, Iron Mountain, MI, USA, 27–28 September 1995; Mroz, G., Martin, J., Eds.; Department of Forestry, University of Wisconsin: Madison, WI, USA, 1996; pp. 61–72. [Google Scholar]
- Limbu, S.; Keena, M.; Whitemore, M. Hemlock woolly adelgid (Hemiptera: Adelgidae): A non-native pest of hemlocks in eastern north America. J. Integr. Pest Manag. 2018, 9, 27. [Google Scholar] [CrossRef]
- Mayfield, A.E., III; Bittner, T.D.; Dietschler, N.J.; Elkinton, J.S.; Havill, N.P.; Keena, M.A.; Mausel, D.L.; Rhea, J.R.; Salom, S.M.; Whitmore, M.C. Biological control of hemlock woolly adelgid in North America: History, status, and outlook. Biol. Contr. 2023, 24, 105308. [Google Scholar] [CrossRef]
- McDonald, K.M.; Seiler, J.R.; Wang, B.; Salom, S.M.; Rhea, R.J. Effects of hemlock woolly adelgid control using imidacloprid on leaf-level physiology of eastern hemlock. Forests 2023, 14, 1228. [Google Scholar] [CrossRef]
- Salom, S.M.; Sharov, A.A.; Mays, W.T.; Neal, J.W. Evaluation of aestival diapause in hemlock woolly adelgid (Homoptera: Adelgidae). Environ. Entomol. 2001, 30, 877–882. [Google Scholar] [CrossRef]
- McClure, M.S. Density-dependent feedback and population cycles in Adelges tsugae (Homoptera: Adelgidae) on Tsuga canadensis. Environ. Entomol. 1991, 20, 258–264. [Google Scholar] [CrossRef]
- Pedigo, L.P.; Buntin, G.D. Handbook of Sampling Methods for Arthropods in Agriculture; CRC Press: Boca Raton, FL, USA, 1994; pp. 127–129. [Google Scholar]
- Wu, C.; Thompson, M.E. Sampling Theory and Practice; Springer International Publishing: New York, NY, USA, 2020. [Google Scholar]
- Choi, Y.-S.; Kim, M.-J.; Baek, S. Within-plant distribution of two-spotted spider mites, Tetranychus urticae Koch (Acari: Tetranychidae), on strawberries: Decision of an optimal sampling unit. Insects 2022, 13, 55. [Google Scholar] [CrossRef]
- Baek, S.; Seo, H.-Y.; Park, C.-G. Determination of an optimum sampling unit of Ricania shantungensis (Hemiptera: Ricaniidae) eggs in persimmons. PLoS ONE 2022, 17, e0265083. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; Gregoire, T.G. The tree crown distribution of hemlock woolly adelgid, Adelges tsugae (Hem.; Adelgidae) from randomized branch sampling. J. Appl. Entomol. 2007, 131, 26–33. [Google Scholar] [CrossRef]
- Whitmore, M.C. Early detection of the hemlock woolly adelgid (Adelges tsugae) in small northeastern hemlock (Tsuga canadensis) woodlots. In Forest Connect Fact Sheet; Smallidge, P., Ed.; Cornell University Cooperative Extension: Ithaca, NY, USA, 2009; pp. 1–4. [Google Scholar]
- Joseph, S.V.; Hanula, J.L.; Braman, S.K. Distribution and abundance of Adelges tsugae (Hemiptera: Adelgidae) within hemlock trees. J. Econ. Entomol. 2011, 104, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Fidgen, J.G.; Legg, D.E.; Salom, S.M. A sequential sampling plan for counts of Adelges tsugae on individual eastern hemlock trees. South. J. Appl. For. 2013, 37, 75–80. [Google Scholar] [CrossRef]
- Evans, A.M.; Gregoire, T.G. A geographically variable model of hemlock woolly adelgid spread. Biol. Invasions 2007, 9, 369–382. [Google Scholar] [CrossRef]
- Koch, F.H.; Cheshire, H.M.; Devine, H.A. Landscape-scale prediction of hemlock woolly adelgid, Adelges tsugae (Homoptera: Adelgidae), infestation in the southern Appalachian mountains. Environ. Entomol. 2006, 35, 1313–1323. [Google Scholar] [CrossRef]
- Orwig, D.A.; Foster, D.R.; Mausel, D.L. Landscape patterns of hemlock decline in New England due to the hemlock woolly adelgid. J. Insect Physiol. 2002, 19, 1475–1487. [Google Scholar] [CrossRef]
- Rentch, J.; Fajvan, M.A.; Evans, R.; Onken, B. Using dendrochronology to model hemlock woolly adelgid effects on eastern hemlock growth and vulnerability. Biol. Invasions 2009, 11, 551–563. [Google Scholar] [CrossRef]
- Costa, S.; Onken, B. Standardizing Sampling for Detection and Monitoring of Hemlock Woolly Adelgid in Eastern Hemlock Forests; FHTET-2006-16; USDA Forest Service: Morgantown, WV, USA, 2006. [Google Scholar]
- SAS Institute. SAS OnlineDoc®, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2017. [Google Scholar]
- Perry, J.N. Spatial analysis by distance indices. J. Anim. Ecol. 1995, 64, 303–314. [Google Scholar] [CrossRef]
- Perry, J.N.; Winder, L.; Holland, J.M.; Alston, R.D. Red-blue plots for detecting clusters in count data. Ecol. Lett. 1999, 2, 106–113. [Google Scholar] [CrossRef]
- Perry, J.N.; Dixon, P. A new method for measuring spatial association in ecological count data. Ecoscience 2002, 9, 133–141. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, J.-H.; Yiem, M.-S. Spring emergence pattern of Carposina sasakii (Lepidoptera: Carposinidae) in apple orchards in Korea and its forecasting models based on degree-days. Environ. Entomol. 2000, 29, 1188–1198. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Issaks, E.H.; Srivastava, R.M. An Introduction to Applied Geostatistics; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Journel, A.G.; Huijbregts, C.J. Mining Geostatistics; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Park, Y.-L.; Krell, R.K. Generation of prescription maps for preventative and curative site-specific management of bean leaf beetles (Coleoptera: Chrysomelidae). J. Asia-Pacific Entomol. 2005, 8, 375–380. [Google Scholar] [CrossRef]
- He, Z.; Chen, L.; Yang, Y.; Zhao, F.; Zhou, C.; Zhang, D. Geostatistical analysis of the spatial variation of Chrysolina aeruginosa larvae at different stages in desert ecosystems. Insects 2023, 14, 379. [Google Scholar] [CrossRef]
- Trangmar, B.B.; Yost, R.S.; Uehara, G. Application of geostatistics to spatial studies of soil properties. In Advances in Agronomy; Norman, A.G., Ed.; Academic Press, Inc.: New York, NY, USA, 1985; Volume 38, pp. 45–94. [Google Scholar]
- Park, Y.-L.; Tollefson, J.J. Spatio-temporal dynamics of corn rootworm, Diabrotica spp.; adults and their spatial association with environment. Entomol. Exper. Appl. 2005, 120, 105–112. [Google Scholar] [CrossRef]
- Atkinson, P.M.; Lloyd, C.D. Geostatistical models and spatial interpolation. In Handbook of Regional Science; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1813–1827. [Google Scholar]
- Park, Y.-L.; Perring, T.M.; Krell, R.K.; Farrar, C.A.; Gispert, C. Spatial distribution of Pierce’s disease in the Coachella Valley: Implications for sampling. Am. J. Enol. Viticult. 2006, 57, 221–226. [Google Scholar] [CrossRef]
- Park, Y.-L.; Perring, T.M.; Krell, R.K.; Hashim-Buckey, J.M.; Hill, B.L. Spatial distribution of Pierce’s disease related to incidence, vineyard characteristics, and surrounding land uses. Am. J. Enol. Viticult. 2011, 62, 229–238. [Google Scholar] [CrossRef]
- Park, Y.L.; Tollefson, J.J. Spatial prediction of corn rootworm (Coleoptera: Chrysomelidae) adult emergence in Iowa cornfields. J. Econ. Entomol. 2005, 98, 121–128. [Google Scholar] [CrossRef]
- Fay, P.A.; Whitham, T.G. Within-plant distribution of a galling adelgid (Homoptera: Adelgidae): The consequences of conflicting survivorship, growth, and reproduction. Ecol. Entomol. 1990, 15, 245–254. [Google Scholar] [CrossRef]
- McClure, M.S. Effects of implanted and injected pesticides and fertilizers on the survival of Adelges tsugae (Homoptera: Adelgidae) and on the growth of Tsuga canadensis. J. Econ. Entomol. 1992, 85, 468–472. [Google Scholar] [CrossRef]
- Vose, J.M.; Wear, D.N.; Mayfield, A.E., III; Nelson, C.D. Hemlock woolly adelgid in the southern Appalachians: Control strategies, ecological impacts, and potential management responses. For. Ecol. Manag. 2013, 291, 209–219. [Google Scholar] [CrossRef]
- Oten, K.L.; Cohen, A.C.; Hain, F.P. Stylet bundle morphology and trophically related enzymes of the hemlock woolly adelgid (Hemiptera: Adelgidae). Ann. Entomol. Soc. Am. 2014, 107, 680–690. [Google Scholar] [CrossRef]
- Fay, P.A.; Preszler, R.W.; Whitham, T.G. The functional resource of a gall-forming adelgid. Oecologia 1996, 105, 199–204. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Salom, S.M. Volatile emissions of eastern hemlock, Tsuga Canadensis, and the influence of hemlock woolly adelgid. Phytochemistry 2003, 62, 175–180. [Google Scholar] [CrossRef]
- Pedigo, L.P.; Rice, M.E.; Krell, R.K. Entomology and Pest Management; Waveland Press: Long Grove, IL, USA, 2021. [Google Scholar]
- Skinner, M.; Parker, B.L.; Gouli, S.; Ashikaga, T. Regional responses of hemlock woolly adelgid (Homoptera: Adelgidae) to low temperature. Environ. Entomol. 2003, 32, 523–528. [Google Scholar] [CrossRef]
- McClure, M.S. Role of wind, birds, deer, and humans in the dispersal of hemlock woolly adelgid (Homoptera: Adelgidae). Environ. Entomol. 1990, 19, 36–43. [Google Scholar] [CrossRef]
- Park, Y.L.; Krell, R.K.; Carroll, M. Theory, technology and practice of site-specific insect pest management. J. Asia-Pac. Entomol. 2007, 10, 81–101. [Google Scholar] [CrossRef]




| Sites and Sample Variables | Length of Sampled Branch | |||||
|---|---|---|---|---|---|---|
| 100-cm Branch | Whole Branch | |||||
| F | df | p | F | df | p | |
| Cathedral State Park | ||||||
| Cardinal direction | ||||||
| No. of ovisacs per branch | 1.51 | 3, 236 | 0.22 | 0.08 | 3, 236 | 0.97 |
| Density of ovisacs per branch | 1.06 | 3, 236 | 0.37 | 0.06 | 3, 236 | 0.98 |
| Branch height | ||||||
| No. of ovisacs per branch | 6.95 | 5, 234 | <0.01 | 1.35 | 5, 234 | 0.25 |
| Density of ovisacs per branch | 6.60 | 5, 234 | <0.01 | 2.84 | 5, 234 | 0.02 |
| Blackwater Falls State Park | ||||||
| Cardinal direction | ||||||
| No. of ovisacs per branch | 0.32 | 3, 620 | 0.81 | 0.02 | 3, 620 | 0.99 |
| Density of ovisacs per branch | 0.14 | 3, 620 | 0.94 | 0.13 | 3, 620 | 0.94 |
| Branch height | ||||||
| No. of ovisacs per branch | 17.01 | 5, 618 | <0.01 | 24.71 | 5, 618 | <0.01 |
| Density of ovisacs per branch | 14.10 | 5, 618 | <0.01 | 41.67 | 5, 618 | <0.01 |
| Buchanan State Forest | ||||||
| Cardinal direction | ||||||
| No. of ovisacs per branch | 0.61 | 3, 236 | 0.61 | 1.39 | 3, 236 | 0.25 |
| Density of ovisacs per branch | 0.94 | 3, 236 | 0.42 | 0.61 | 3, 236 | 0.61 |
| Branch height | ||||||
| No. of ovisacs per branch | 2.84 | 5, 234 | 0.02 | 1.65 | 5, 234 | 0.15 |
| Density of ovisacs per branch | 2.94 | 5, 234 | 0.02 | 13.33 | 5, 234 | <0.01 |
| Location of Sampled Branch | Study Site | ||
|---|---|---|---|
| Cathedral State Park | Blackwater Falls State Park | Buchanan State Forest | |
| Upper crown | 3.27 (0.01) | 7.05 (0.01) | 1.35 (0.03) |
| Middle crown | 4.11 (0.01) | 10.88 (0.01) | 1.22 (0.13) |
| Lower crown | 1.92 (0.01) | 2.02(0.01) | 1.40 (0.06) |
| Site | Crown Location | Branch Length a | ||
|---|---|---|---|---|
| 25 cm | 50 cm | 100 cm | ||
| Cathedral State Park | Lower crown, bottom half | 1.82 | 1.75 | 2.03 |
| Lower crown, top half | 1.17 | 1.64 | 2.36 | |
| Middle crown, bottom half | 1.49 | 1.57 | 1.62 | |
| Middle crown, top half | 1.10 | 1.14 | 1.11 | |
| Upper crown, bottom half | 1.00 | 1.17 | 1.12 | |
| Upper crown, top half | 1.12 | 0.95 | 0.93 | |
| Blackwater Falls State Park | Lower crown, bottom half | 1.47 | 1.30 | 1.22 |
| Lower crown, top half | 1.24 | 1.24 | 1.16 | |
| Middle crown, bottom half | 1.48 | 1.32 | 1.21 | |
| Middle crown, top half | 1.24 | 1.17 | 1.22 | |
| Upper crown, bottom half | 1.21 | 1.23 | 1.23 | |
| Upper crown, top half | 1.29 | 1.37 | 1.38 | |
| Buchanan State Forest | Lower crown, bottom half | 1.28 | 1.01 | 1.03 |
| Lower crown, top half | 1.77 | 1.34 | 1.27 | |
| Middle crown, bottom half | 2.01 | 1.78 | 1.51 | |
| Middle crown, top half | 1.40 | 1.49 | 1.44 | |
| Upper crown, bottom half | 1.22 | 1.09 | 1.24 | |
| Upper crown, top half | 1.35 | 1.16 | 1.34 | |
| Site | A. tsugae Generation and Year | Density a | Model | Nugget | Sill | Range (m) | r2 |
|---|---|---|---|---|---|---|---|
| Blackwater Falls State Park | Sistens, 2012 b | 156.8 | Nugget | 9257 | 9257 | N/A c | <0.01 |
| Progrediens, 2013 b | 35.2 | Nugget | 3681 | 3681 | N/A | <0.01 | |
| Sistens, 2013 b | 14.8 | Nugget | 638.6 | 638.6 | N/A | <0.01 | |
| Progrediens, 2014 b | 7.0 | Nugget | 42.3 | 42.3 | N/A | <0.01 | |
| Cathedral State Park | Sistens, 2012 b | 68.0 | Nugget | 26431 | 26,431 | N/A | <0.01 |
| Progrediens, 2013 | 74.0 | Nugget | 1.930 | 1.930 | N/A | <0.01 | |
| Sistens, 2013 b | 34.2 | Nugget | 3982 | 3982 | N/A | <0.01 | |
| Progrediens, 2014 b | 6.4 | Nugget | 81.93 | 81.93 | N/A | <0.01 | |
| Buchannan State Forest | Sistens, 2012 b | 98.7 | Spherical | 4500 | 19,680 | 41.3 | 0.897 |
| Progrediens, 2013 b | 32.8 | Nugget | 2848 | 2848 | N/A | <0.01 | |
| Sistens, 2013 b | 45.3 | Nugget | 4601 | 4601 | N/A | <0.01 | |
| Progrediens, 2014 | 34.7 | Nugget | 3824.7 | 3827.7 | N/A | <0.01 |
| Study Site | A. tsugae Generation and Year | ||||
|---|---|---|---|---|---|
| Sistens, 2012 | Progrediens, 2013 | Sistens, 2013 | Progrediens, 2014 | Pooled | |
| Blackwater Falls State Park | 1.35 (0.03) | 1.49 (0.01) | 1.73 (0.01) | 1.91 (0.01) | 1.56 (0.01) |
| Cathedral State Park | 1.55 (0.01) | 0.99 (0.46) | 1.32 (0.04) | 1.61 (0.01) | 1.35 (0.03) |
| Buchanan State Forest | 2.59 (0.01) | 2.33 (0.01) | 2.61 (0.01) | 0.95 (0.46) | 2.97 (0.01) |
| Study Site | A. tsugae Generations Compared | ||
|---|---|---|---|
| Sistens, 2012 vs. Progrediens, 2013 | Sistens, 2013 vs. Progrediens, 2013 | Sistens, 2013 vs. Progrediens, 2014 | |
| Blackwater Falls State Park | −0.09 (0.79) | 0.36 (< 0.01) | −0.02 (0.59) |
| Cathedral State Park | 0.06 (0.28) | 0.12 (0.13) | 0.13 (0.11) |
| Buchanan State Forest | 0.36 (<0.01) | 0.49 (<0.01) | 0.18 (0.04) |
| Study Site | A. tsugae Generation and Year | DBH a | Tree Height | Basal Area | Diversity b | Elevation | Aspect |
|---|---|---|---|---|---|---|---|
| Blackwater Falls State Park | Sistens, 2012 | −0.03 (0.61) | 0.05 (0.30) | 0.02 (0.41) | 0.09 (0.19) | 0.28 (<0.01) | 0.14 (0.09) |
| Progrediens, 2013 | −0.11 (0.85) | 0.22 (0.02) | 0.19 (0.03) | 0.18 (0.04) | −0.04 (0.66) | −0.28 (0.99) | |
| Sistens, 2013 | −0.18 (0.96) | 0.12 (0.12) | 0.01 (0.47) | 0.32 (<0.01) | −0.03 (0.62) | −0.08 (0.74) | |
| Progrediens, 2014 | −0.01 (0.52) | −0.02 (0.56) | −0.25 (0.99) | −0.24 (0.99) | 0.11 (0.14) | 0.72 (<0.01) | |
| Cathedral State Park | Sistens, 2012 | 0.29 (<0.01) | 0.25 (<0.01) | −0.07 (0.77) | −0.37 (1.00) | 0.18 (0.04) | 0.46 (<0.01) |
| Progrediens, 2013 | −0.07 (0.76) | 0.01 (0.47) | 0.08 (0.21) | −0.03 (0.61) | 0.12 (0.13) | 0.29 (0.01) | |
| Sistens, 2013 | 0.30 (<0.01) | 0.34 (<0.01) | 0.12 (0.13) | −0.27 (0.99) | 0.07 (0.24) | 0.18 (0.05) | |
| Progrediens, 2014 | 0.35 (<0.01) | 0.31 (<0.01) | 0.05 (0.68) | −0.05 (0.67) | 0.05 (0.31) | 0.37 (<0.01) | |
| Buchanan State Forest | Sistens, 2012 | 0.28 (<0.01) | 0.21 (0.01) | −0.11 (0.85) | 0.21 (0.02) | - | - |
| Progrediens, 2013 | 0.09 (0.17) | 0.08 (0.20) | 0.09 (0.17) | 0.35 (< 0.01) | - | - | |
| Sistens, 2013 | 0.20 (0.04) | 0.18 (0.05) | 0.20 (0.04) | 0.09 (0.19) | - | - | |
| Progrediens, 2014 | −0.03 (0.63) | −0.10 (0.84) | −0.18 (0.93) | −0.03 (0.61) | - | - |
| Site | Sampling Unit | CV Value |
|---|---|---|
| Cathedral State Park | Current | 1.85 |
| As suggested by this study | 1.14 | |
| Blackwater Falls State Park | Current | 1.17 |
| As suggested by this study | 1.17 | |
| Buchanan State Forest | Current | 1.03 |
| As suggested by this study | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.; Park, Y.-L. Spatial Patterns of Adelges tsugae Annand (Hemiptera: Adelgidae) in Eastern Hemlock Stands: Implications for Sampling and Management. Insects 2024, 15, 751. https://doi.org/10.3390/insects15100751
Baek S, Park Y-L. Spatial Patterns of Adelges tsugae Annand (Hemiptera: Adelgidae) in Eastern Hemlock Stands: Implications for Sampling and Management. Insects. 2024; 15(10):751. https://doi.org/10.3390/insects15100751
Chicago/Turabian StyleBaek, Sunghoon, and Yong-Lak Park. 2024. "Spatial Patterns of Adelges tsugae Annand (Hemiptera: Adelgidae) in Eastern Hemlock Stands: Implications for Sampling and Management" Insects 15, no. 10: 751. https://doi.org/10.3390/insects15100751
APA StyleBaek, S., & Park, Y.-L. (2024). Spatial Patterns of Adelges tsugae Annand (Hemiptera: Adelgidae) in Eastern Hemlock Stands: Implications for Sampling and Management. Insects, 15(10), 751. https://doi.org/10.3390/insects15100751










