Limited Variation in Codon Usage across Mitochondrial Genomes of Non-Biting Midges (Diptera: Chironomidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Mitochondrial Genome Sequencing of Conchapelopia togamaculosa
2.3. Relative Synonymous Codon Usage Analysis of Chironomidae
2.4. Effective Number of Codons Analysis of Chironomidae
3. Results
3.1. Mitochondrial Genome of C. togamaculosa
3.2. Relative Synonymous Codon Usage across Chironomidae
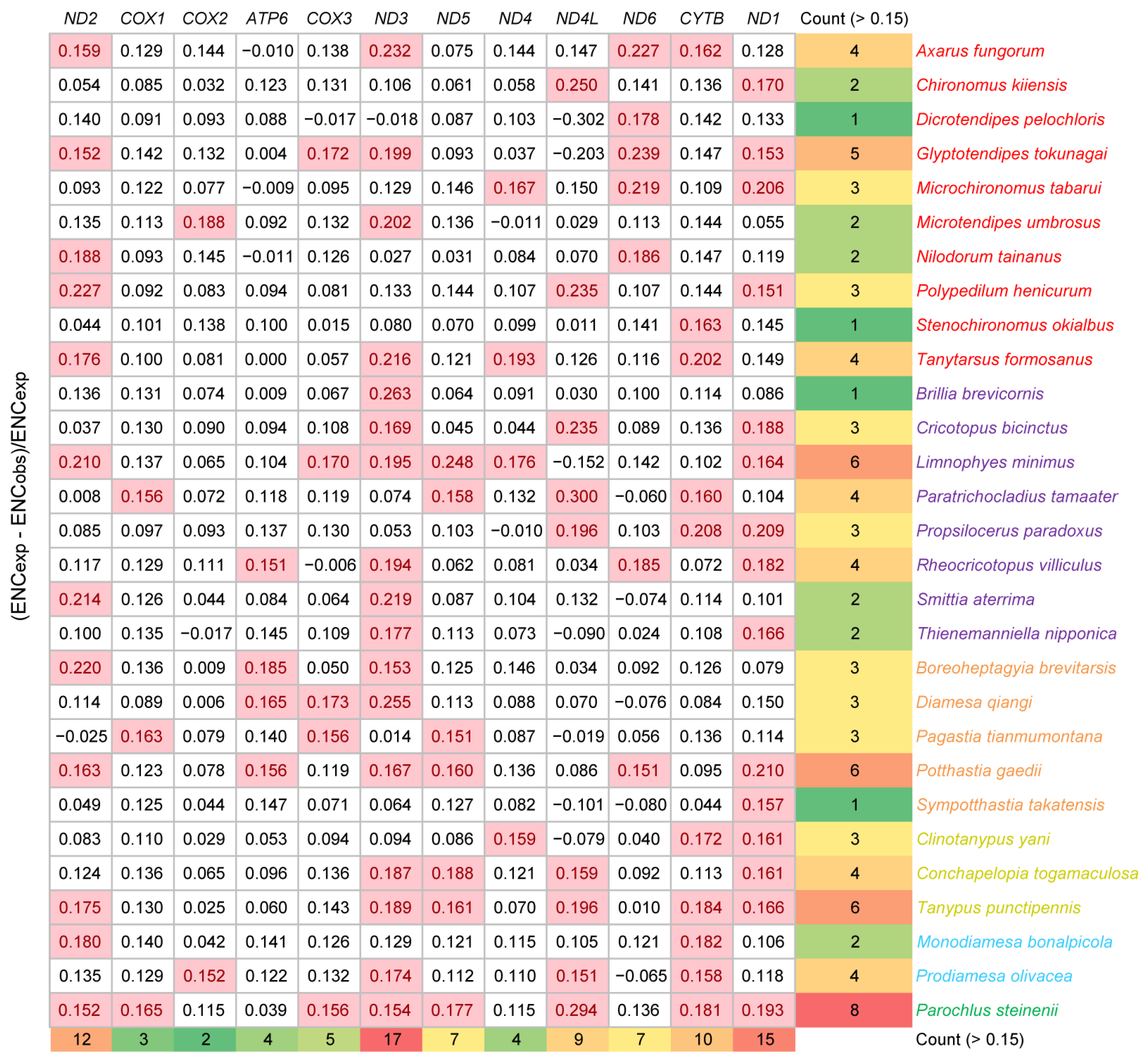
3.3. Effective Number of Codons across Chironomidae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferrington, L.C., Jr. Global diversity of non-biting midges (Chironomidae; Insecta-Diptera) in freshwater. Hydrobiologia 2008, 595, 447–455. [Google Scholar] [CrossRef]
- McLachlan, A.; Armitage, P.; Cranston, P.S.; Pinder, L.C.V. The Chironomidae. The biology and ecology of non-biting midges. J. Anim. Ecol. 1995, 64, 667. [Google Scholar] [CrossRef]
- Wei, L.; He, J.; Jia, X.; Qi, Q.; Liang, Z.; Zheng, H.; Ping, Y.; Liu, S.; Sun, J. Analysis of codon usage bias of mitochondrial genome in Bombyx mori and its relation to evolution. BMC Evol. Biol. 2014, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, J.; Cai, X.; Yang, D.; Li, X.; Liu, X. Comparative analysis of the mitochondrial genomes of Chloropidae and their implications for the phylogeny of the family. Int. J. Mol. Sci. 2024, 25, 2920. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, X.; Mao, B.; Xiao, Y.; Shen, M.; Fu, Y. Comparative mitogenome analyses of twelve non-biting flies and provide insights into the phylogeny of Chironomidae (Diptera: Culicomorpha). Sci. Rep. 2023, 13, 9200. [Google Scholar] [CrossRef]
- Lin, X.L.; Zhao, Y.M.; Yan, L.P.; Liu, W.B.; Bu, W.J.; Wang, X.H.; Zheng, C.G. Mitogenomes provide new insights into the evolutionary history of Prodiamesinae (Diptera: Chironomidae). Zool. Scr. 2021, 51, 119–132. [Google Scholar] [CrossRef]
- Lin, X.L.; Liu, Z.; Yan, L.P.; Duan, X.; Bu, W.J.; Wang, X.H.; Zheng, C.G. Mitogenomes provide new insights of evolutionary history of Boreheptagyiini and Diamesini (Diptera: Chironomidae: Diamesinae). Ecol. Evol. 2022, 12, e8957. [Google Scholar] [CrossRef]
- Zheng, C.G.; Zhu, X.X.; Yan, L.P.; Yao, Y.; Bu, W.J.; Wang, X.H.; Lin, X.L. First complete mitogenomes of Diamesinae, Orthocladiinae, Prodiamesinae, Tanypodinae (Diptera: Chironomidae) and their implication in phylogenetics. PeerJ 2021, 9, e11294. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Shin, S.C. Complete mitochondrial genome of the Antarctic midge Parochlus steinenii (Diptera: Chironomidae). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016, 27, 3475–3476. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.W.; Zhao, Y.M.; Lin, X.L. First report of the complete mitogenome of Tanypus punctipennis Meigen, 1818 (Diptera, Chironomidae) from Hebei Province, China. Mitochondrial DNA B Resour. 2022, 7, 215–216. [Google Scholar] [CrossRef]
- Parvathy, S.T.; Udayasuriyan, V.; Bhadana, V. Codon usage bias. Mol. Biol. Rep. 2021, 49, 539–565. [Google Scholar] [CrossRef] [PubMed]
- Selva Kumar, C.; Nair, R.R.; Sivaramakrishnan, K.G.; Ganesh, D.; Janarthanan, S.; Arunachalam, M.; Sivaruban, T. Influence of certain forces on evolution of synonymous codon usage bias in certain species of three basal orders of aquatic insects. Mitochondrial DNA 2012, 23, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.K.; Lei, T.; Gu, J.J.; Song, C.; Qi, X. Codon bias analysis of the mitochondrial genome reveals natural selection in the nonbiting midge Microtendipes umbrosus Freeman, 1955 (Diptera: Chironomidae). Pan-Pac. Entomol. 2023, 99, 217–225. [Google Scholar] [CrossRef]
- Niitsuma, H.; Tang, H. Notes on the genus Conchapelopia Fittkau (Diptera: Chironomidae: Tanypodinae) from southern China, with description of a new species. Zootaxa 2017, 4236, zootaxa.4236.2.7. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty:de novoassembly of organelle genomes from whole genome data. Nucleic Acids Res. 2016, 45, e18. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenetics Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Sharp, P.M.; Tuohy, T.M.F.; Mosurski, K.R. Codon usage in yeast: Cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res. 1986, 14, 5125–5143. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Duan, X.; Jiao, K.L.; Lin, X.L. First complete mitogenome of Axarus fungorum (Albu, 1980) from Guizhou Province, China (Diptera, Chironomidae). Mitochondrial DNA B Resour. 2022, 7, 1807–1809. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, G.; Lei, T.; Qi, X. The complete mitochondrial genome of a tropical midge Chironomus kiiensis Tokunaga, 1936 (Diptera: Chironomidae). Mitochondrial DNA B Resour. 2022, 7, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Q.; Zhao, Y.C.; Chen, J.L.; Lin, X.L. First report of the complete mitogenome of Microchironomus tabarui Sasa, 1987 (Diptera, Chironomidae) from Hebei Province, China. Mitochondrial DNA B Resour. 2021, 6, 2845–2846. [Google Scholar] [CrossRef]
- Di, X.; Lei, T.; Mou, K.; Song, C.; Qi, X. Complete mitochondrial genome of the non-biting midge Polypedilum henicurum Wang, 1995 (Diptera: Chironomidae). Mitochondrial DNA B Resour. 2023, 8, 908–911. [Google Scholar] [CrossRef]
- Zheng, C.G.; Liu, Z.; Zhao, Y.M.; Wang, Y.; Bu, W.J.; Wang, X.H.; Lin, X.L. First report on mitochondrial gene rearrangement in non-biting midges, revealing a synapomorphy in Stenochironomus Kieffer (Diptera: Chironomidae). Insects 2022, 13, 115. [Google Scholar] [CrossRef]
- Li, S.Y.; Chen, M.H.; Sun, L.; Wang, R.H.; Li, C.H.; Gresens, S.; Li, Z.; Lin, X.L. New mitogenomes from the genus Cricotopus (Diptera: Chironomidae, Orthocladiinae): Characterization and phylogenetic implications. Arch. Insect Biochem. Physiol. 2023, 115, e22067. [Google Scholar] [CrossRef]
- Fang, X.; Li, X.; Lu, T.; Fu, J.; Shen, M.; Xiao, Y.; Fu, Y. Complete mitochondrial genome of Limnophyes minimus (Diptera: Chironomidae). Mitochondrial DNA B Resour. 2022, 7, 280–282. [Google Scholar] [CrossRef]
- Wright, F. The ‘effective number of codons’ used in a gene. Gene 1990, 87, 23–29. [Google Scholar] [CrossRef]
- Beckenbach, A.T. Mitochondrial genome sequences of Nematocera (Lower Diptera): Evidence of rearrangement following a complete genome duplication in a winter crane fly. Genome Biol. Evol. 2012, 4, 89–101. [Google Scholar] [CrossRef]
- Wolstenholme, D.R. Animal mitochondrial DNA: Structure and evolution. Int. Rev. Cytol. 1992, 141, 173–216. [Google Scholar] [CrossRef]
- Moreno-Carmona, M.; Cameron, S.L.; Prada Quiroga, C.F. How are the mitochondrial genomes reorganized in Hexapoda? Differential evolution and the first report of convergences within Hexapoda. Gene 2021, 791, 145719. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Yanase, T.; Tsuda, T.; Noda, H. Species-specific mitochondrial gene rearrangements in biting midges and vector species identification. Med. Vet. Entomol. 2009, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wei, D.W.; Ma, N.B.; Xu, C.; Li, Y.F.; Wang, H.; Bu, W.J.; Jiao, K.L. The rearrangement features and phylogenetic signals of mitochondrial genomes of gall midge subfamily Cecidomyiinae (Diptera: Cecidomyiidae) with first complete mitogenome of an economically important mango gall midge pest, Procontarina fructiculi from Guangxi, China. Orient. Insects 2024, 1–13. [Google Scholar] [CrossRef]
- Pei, W.; Xu, W.; Li, H.; Yan, L.; Gai, Y.; Yang, N.; Yang, J.; Chen, J.; Peng, H.; Pape, T.; et al. Unusual rearrangements of mitogenomes in Diptera revealed by comparative analysis of 135 tachinid species (Insecta, Diptera, Tachinidae). Int. J. Biol. Macromol. 2024, 258, 128997. [Google Scholar] [CrossRef] [PubMed]
- Kabiraj, D.; Chetia, H.; Nath, A.; Sharma, P.; Mosahari, P.V.; Singh, D.; Dutta, P.; Neog, K.; Bora, U. Mitogenome-wise codon usage pattern from comparative analysis of the first mitogenome of Blepharipa sp. (Muga uzifly) with other Oestroid flies. Sci. Rep. 2022, 12, 7028. [Google Scholar] [CrossRef] [PubMed]
- Rico, E.; Quesada, A. Distribution and ecology of chironomids (Diptera, Chironomidae) on Byers Peninsula, Maritime Antarctica. Antarct. Sci. 2013, 25, 288–291. [Google Scholar] [CrossRef]
- Montooth, K.L.; Abt, D.N.; Hofmann, J.W.; Rand, D.M. Comparative genomics of Drosophila mtDNA: Novel features of conservation and change across functional domains and lineages. J. Mol. Evol. 2009, 69, 94–114. [Google Scholar] [CrossRef]
- Song, C.; Wang, L.; Lei, T.; Qi, X. New color-patterned species of Microtendipes Kieffer, 1913 (Diptera: Chironomidae) and a deep intraspecific divergence of species by DNA barcodes. Insects 2023, 14, 227. [Google Scholar] [CrossRef]
- Ibrahim, K.; Chatanga, E.; Mohamed, N.S.; Ahmed, A.; Alasmari, S.; Almathen, F.; Nakao, R.; Salim, B. Intra- and interspecies variation and population dynamics of Fasciola gigantica among ruminants in Sudan. Parasitol. Res. 2024, 123, 210. [Google Scholar] [CrossRef]
- Tissaoui, G.; Suchentrunk, F.; Awadi, A.; Smith, S.; Weber, A.; Ben Slimen, H. Evolutionary characteristics of the mitochondrial NADH dehydrogenase subunit 6 gene in some populations of four sympatric Mustela species (Mustelidae, Mammalia) from central Europe. Mol. Biol. Rep. 2024, 51, 575. [Google Scholar] [CrossRef] [PubMed]
- Nuryadi, H.; Mandagi, I.F.; Masengi, K.W.A.; Kusumi, J.; Inomata, N.; Yamahira, K. Evidence for hybridization-driven heteroplasmy maintained across generations in a ricefish endemic to a Wallacean ancient lake. Biol. Lett. 2024, 20, 20230385. [Google Scholar] [CrossRef] [PubMed]
- Haukisalmi, V.; Ribas, A.; Hugot, J.P.; Morand, S.; Chaisiri, K.; Junker, K.; Matthee, S.; Spickett, A.; Lehtonen, J.T.; Feliu, C.; et al. Phylogenetic relationships and systematics of tapeworms of the family Davaineidae (Cestoda, Cyclophyllidea), with emphasis on species in rodents. Folia Parasitol. 2024, 71, 011. [Google Scholar] [CrossRef] [PubMed]




| Subfamily | Species | GenBank Accession Number | Reference |
|---|---|---|---|
| Chironominae | Axarus fungorum | ON099430 | [22] |
| Chironominae | Chironomus kiiensis | MZ150770 | [23] |
| Chironominae | Dicrotendipes pelochloris | ON838257 | [5] |
| Chironominae | Glyptotendipes tokunagai | MZ747091 | [5] |
| Chironominae | Microchironomus tabarui | MZ261913 | [24] |
| Chironominae | Microtendipes umbrosus | MZ981734 | [13] |
| Chironominae | Nilodorum tainanus | ON838256 | [5] |
| Chironominae | Polypedilum henicurum | MZ981735 | [25] |
| Chironominae | Stenochironomus okialbus | OL753645 | [26] |
| Chironominae | Tanytarsus formosanus | ON838255 | [5] |
| Diamesinae | Boreoheptagyia brevitarsis | MZ043575 | [7] |
| Diamesinae | Diamesa qiangi | MZ127839 | [7] |
| Diamesinae | Pagastia tianmumontana | MZ231025 | [7] |
| Diamesinae | Potthastia gaedii | OM302504 | [7] |
| Diamesinae | Sympotthastia takatensis | MZ231026 | [7] |
| Orthocladiinae | Brillia brevicornis | MZ424311 | [6] |
| Orthocladiinae | Cricotopus bicinctus | OP006251 | [27] |
| Orthocladiinae | Limnophyes minimus | MZ041033 | [28] |
| Orthocladiinae | Paratrichocladius tamaater | MW837768 | [6] |
| Orthocladiinae | Propsilocerus paradoxus | MW846254 | [6] |
| Orthocladiinae | Rheocricotopus villiculus | MW373526 | [8] |
| Orthocladiinae | Smittia aterrima | ON838254 | [5] |
| Orthocladiinae | Thienemanniella nipponica | MZ424312 | [6] |
| Podonominae | Parochlus steinenii | KT003702 | [9] |
| Prodiamesinae | Monodiamesa bonalpicola | MW837770 | [6] |
| Prodiamesinae | Prodiamesa olivacea | MW373525 | [8] |
| Tanypodinae | Clinotanypus yani | MW373524 | [8] |
| Tanypodinae | Conchapelopia togamaculosa | PP831866 | This study |
| Tanypodinae | Tanypus punctipennis | MZ475054 | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, T.; Zheng, X.; Song, C.; Jin, H.; Chen, L.; Qi, X. Limited Variation in Codon Usage across Mitochondrial Genomes of Non-Biting Midges (Diptera: Chironomidae). Insects 2024, 15, 752. https://doi.org/10.3390/insects15100752
Lei T, Zheng X, Song C, Jin H, Chen L, Qi X. Limited Variation in Codon Usage across Mitochondrial Genomes of Non-Biting Midges (Diptera: Chironomidae). Insects. 2024; 15(10):752. https://doi.org/10.3390/insects15100752
Chicago/Turabian StyleLei, Teng, Xiaojun Zheng, Chao Song, Haobo Jin, Lingjun Chen, and Xin Qi. 2024. "Limited Variation in Codon Usage across Mitochondrial Genomes of Non-Biting Midges (Diptera: Chironomidae)" Insects 15, no. 10: 752. https://doi.org/10.3390/insects15100752





