Improving the Efficiency and Safety of Sentinel Stink Bug Eggs Using X-rays
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Colonies
2.2. Irradiation Process
2.3. Effect of X-ray Irradiation on Egg Eclosion
2.4. Effect of Egg Age on Radiosensitivity
2.5. Suitability of Irradiated Eggs for Parasitoid Development
2.6. Parasitoid Preference
2.7. Data Analysis
3. Results
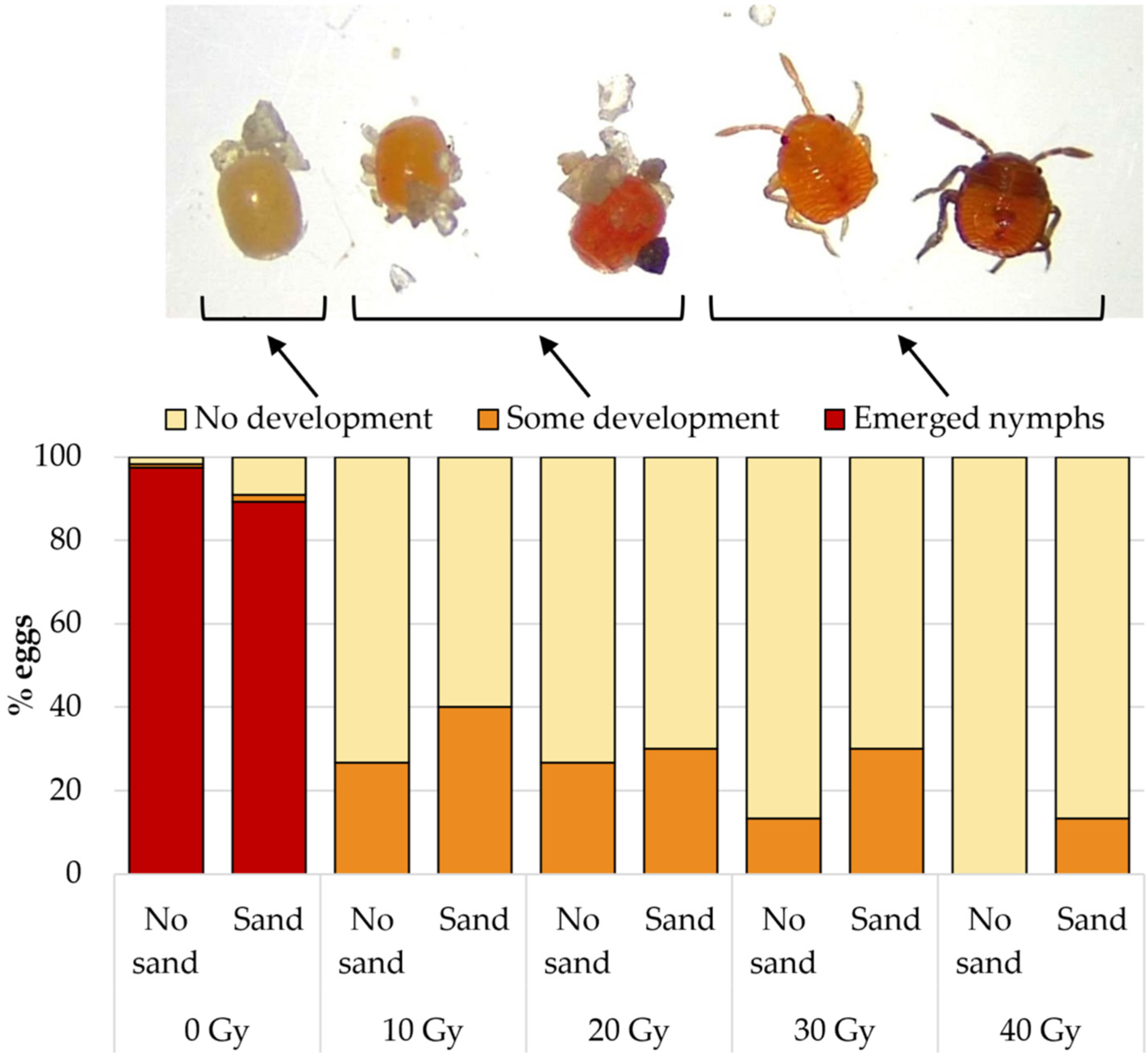
3.1. Effect of X-ray Irradiation on Egg Eclosion
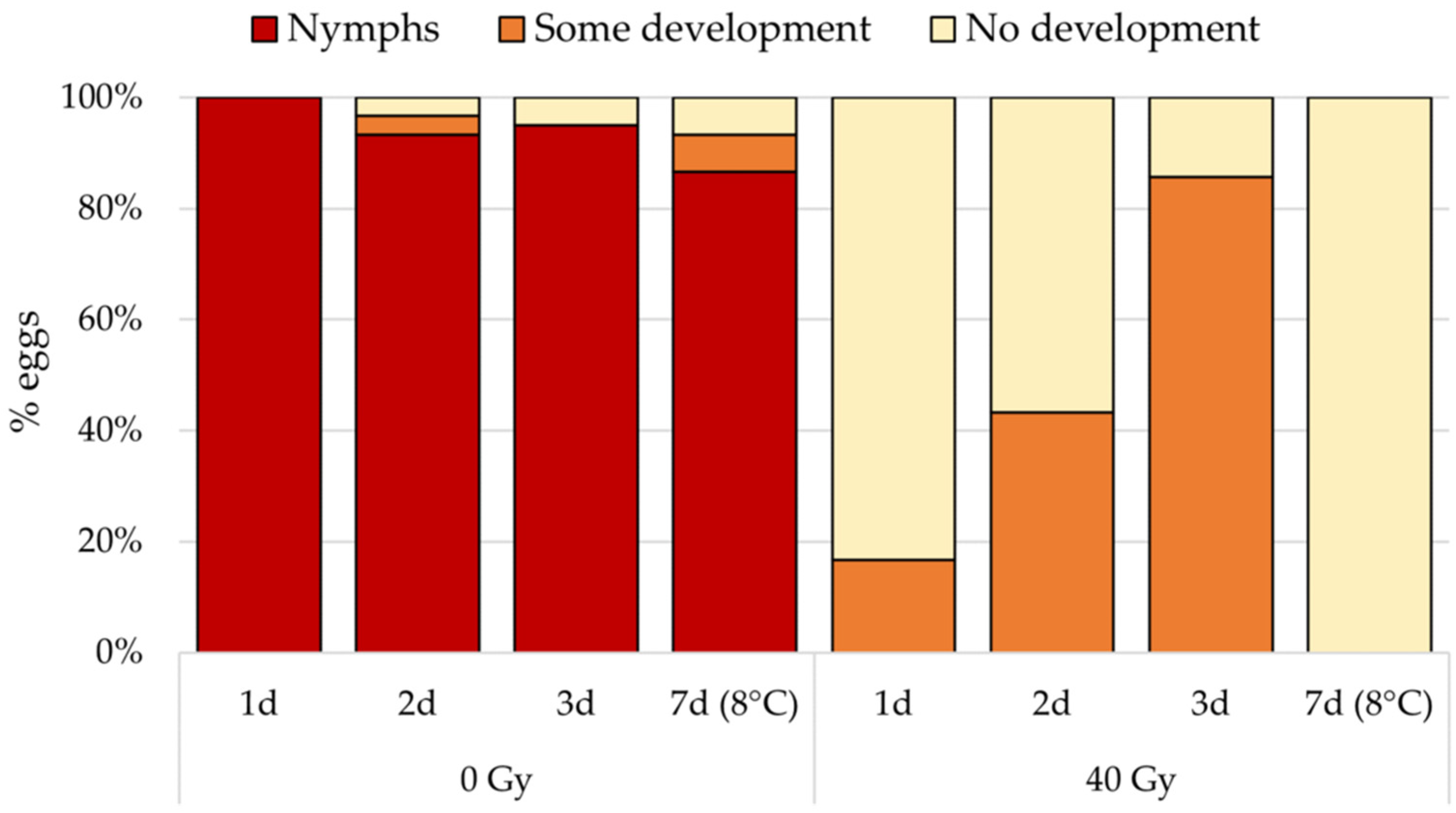
3.2. Effect of Egg Age on Radiosensitivity
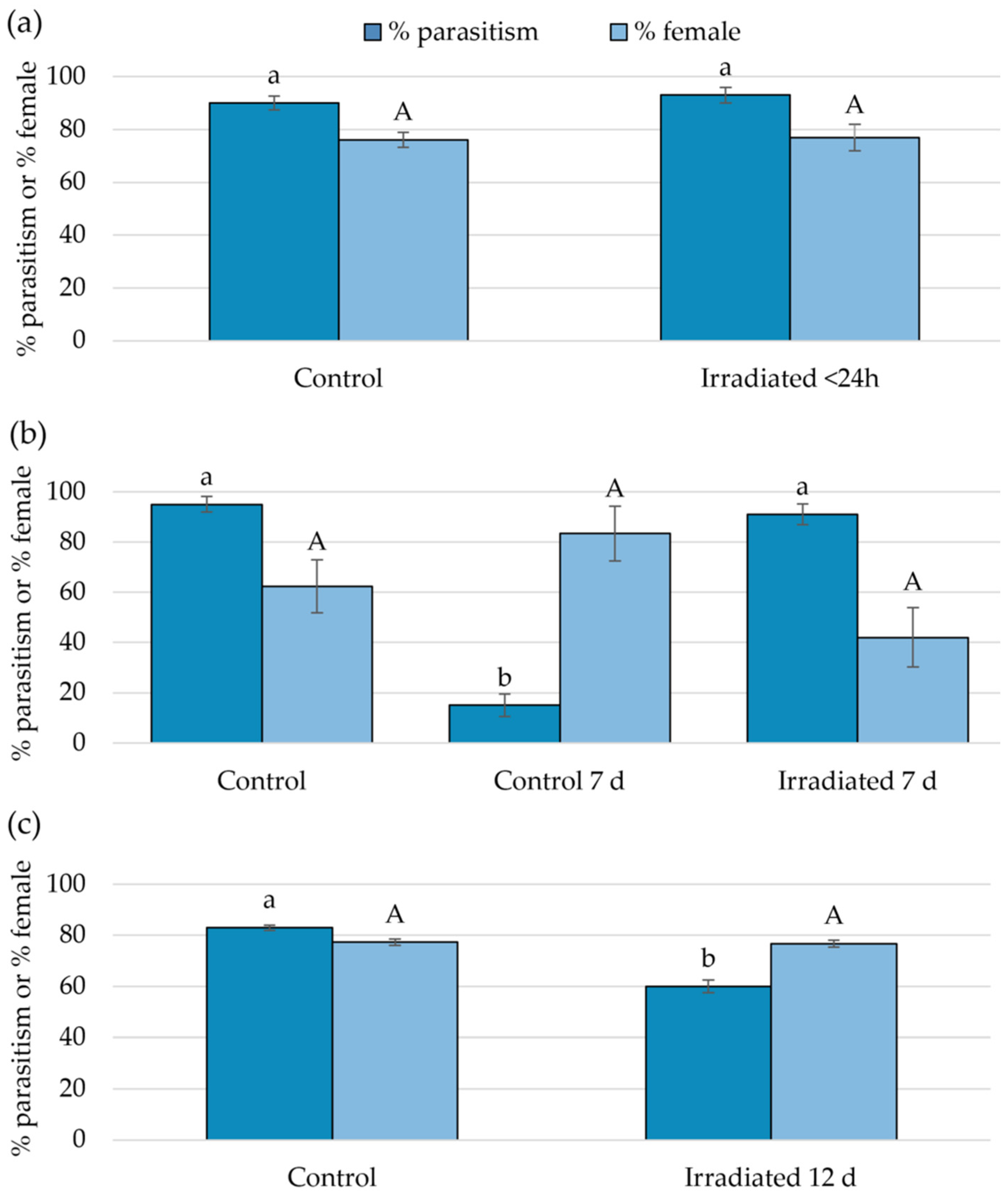
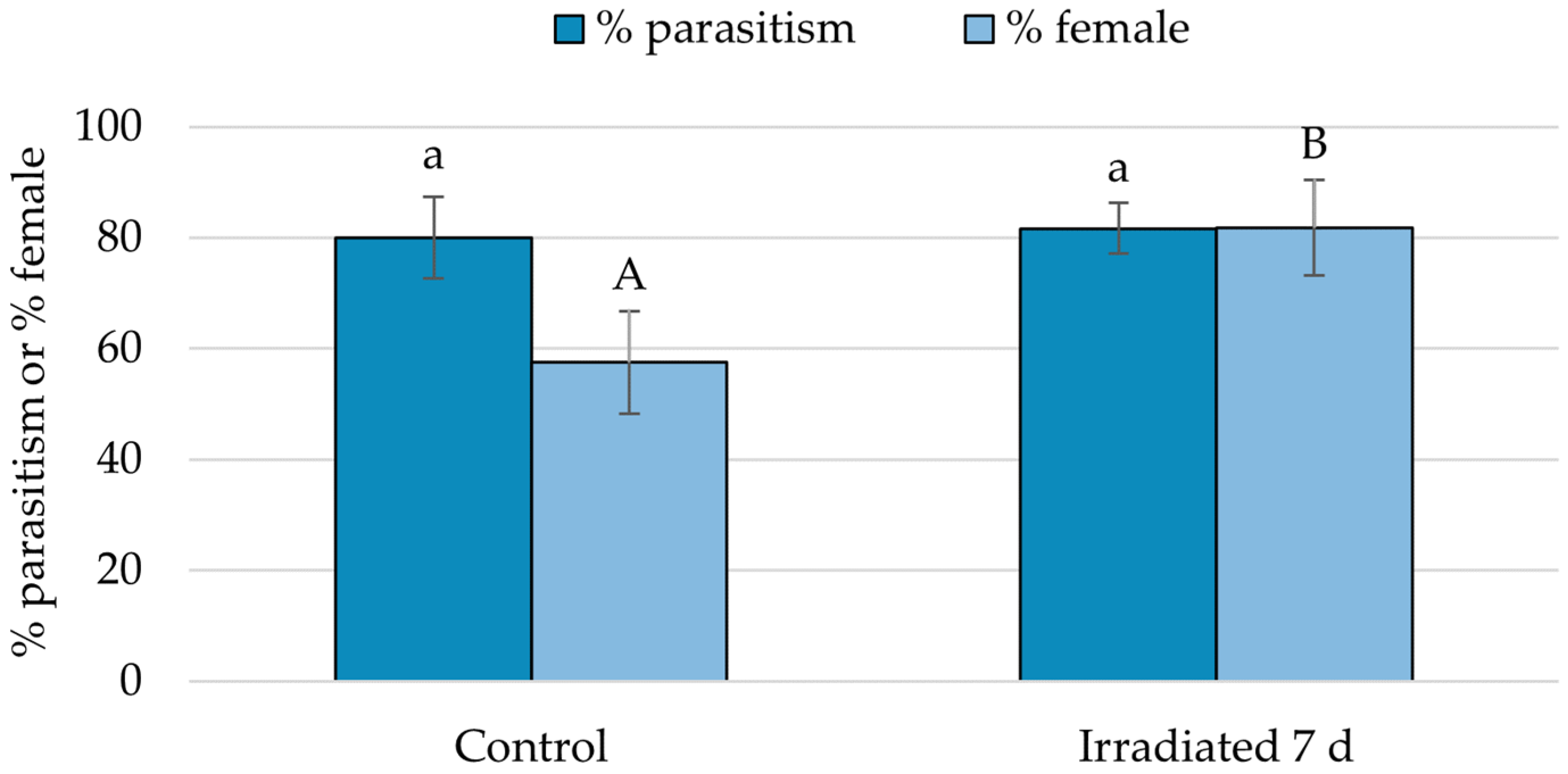
3.3. Suitability of Irradiated Eggs for Parasitoid Development
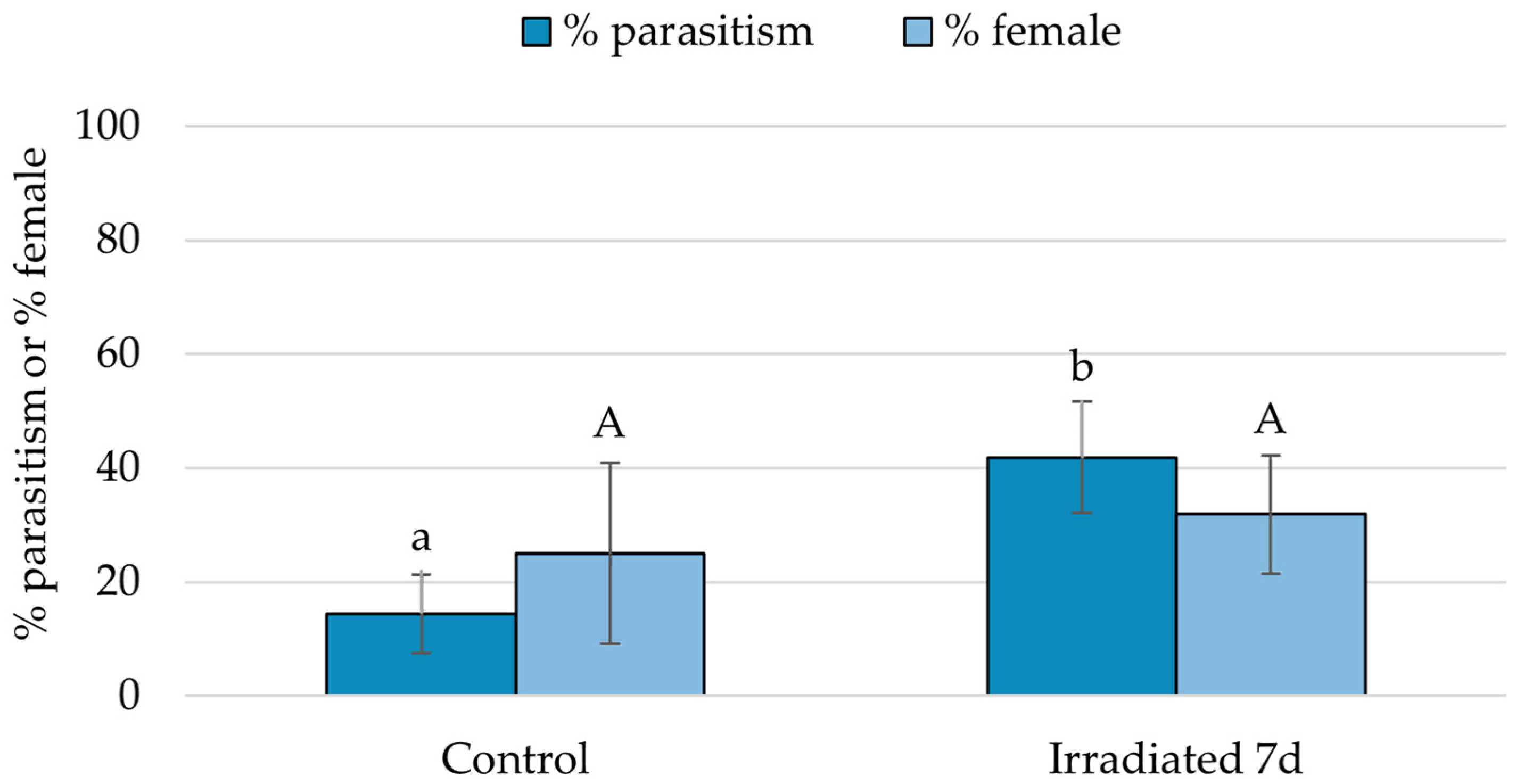
3.4. Parasitoid Preference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heimpel, G.E.; Mills, N.J. Biological Control; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Van Lenteren, J.C. Implementation of biological control. Am. J. Altern. Agric. 1988, 3, 102–109. [Google Scholar] [CrossRef]
- Lee, D.-H.; Short, B.D.; Joseph, S.V.; Bergh, J.C.; Leskey, T.C. Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 2013, 42, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.; Jones, W.A.; Bajwa, B.E.; Rashid, K. Egg parasitoids from Pakistan as possible classical biological control agents of the invasive pest Bagrada hilaris (Heteroptera: Pentatomidae). J. Entomol. Sci. 2015, 50, 147–149. [Google Scholar]
- Conti, E.; Avila, G.; Barratt, B.; Cingolani, F.; Colazza, S.; Guarino, S.; Hoelmer, K.; Laumann, R.A.; Maistrello, L.; Martel, G. Biological control of invasive stink bugs: Review of global state and future prospects. Entomol. Exp. Appl. 2021, 169, 28–51. [Google Scholar] [CrossRef]
- Ademokoya, B.; Athey, K.; Ruberson, J. Natural enemies and biological control of stink bugs (Hemiptera: Heteroptera) in North America. Insects 2022, 13, 932. [Google Scholar] [CrossRef]
- Jones, A.L.; Jennings, D.E.; Hooks, C.R.; Shrewsbury, P.M. Sentinel eggs underestimate rates of parasitism of the exotic brown marmorated stink bug, Halyomorpha halys. Biol. Control 2014, 78, 61–66. [Google Scholar] [CrossRef]
- Cornelius, M.L.; Dieckhoff, C.; Vinyard, B.T.; Hoelmer, K.A. Parasitism and predation on sentinel egg masses of the brown marmorated stink bug (Hemiptera: Pentatomidae) in three vegetable crops: Importance of dissections for evaluating the impact of native parasitoids on an exotic pest. Environ. Entomol. 2016, 45, 1536–1542. [Google Scholar] [CrossRef]
- Costi, E.; Haye, T.; Maistrello, L. Surveying native egg parasitoids and predators of the invasive Halyomorpha halys in Northern Italy. J. Appl. Entomol. 2019, 143, 299–307. [Google Scholar] [CrossRef]
- Tillman, G.; Toews, M.; Blaauw, B.; Sial, A.; Cottrell, T.; Talamas, E.; Buntin, D.; Joseph, S.; Balusu, R.; Fadamiro, H. Parasitism and predation of sentinel eggs of the invasive brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), in the southeastern US. Biol. Control 2020, 145, 104247. [Google Scholar] [CrossRef]
- Ganjisaffar, F.; Talamas, E.J.; Bon, M.C.; Perring, T.M. First report and integrated analysis of two native Trissolcus species utilizing Bagrada hilaris eggs in California. J. Hymenopt. Res. 2020, 80, 49–70. [Google Scholar] [CrossRef]
- Hogg, B.N.; Grettenberger, I.M.; Borkent, C.J.; Stokes, K.; Zalom, F.G.; Pickett, C.H. Natural biological control of Bagrada hilaris by egg predators and parasitoids in north-central California. Biol. Control 2022, 171, 104942. [Google Scholar] [CrossRef]
- Tillman, G.; Cottrell, T.; Balusu, R.; Fadamiro, H.; Buntin, D.; Sial, A.; Vinson, E.; Toews, M.; Patel, D.; Grabarczyk, E. Effect of duration of deployment on parasitism and predation of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) sentinel egg masses in various host plants. Fla. Entomol. 2022, 105, 44–52. [Google Scholar] [CrossRef]
- Herlihy, M.V.; Talamas, E.J.; Weber, D.C. Attack and success of native and exotic parasitoids on eggs of Halyomorpha halys in three Maryland habitats. PLoS ONE 2016, 11, e0150275. [Google Scholar] [CrossRef] [PubMed]
- Federico, R.P.; Francesco, B.; Leonardo, M.; Elena, C.; Lara, M.; Giuseppino, S.P. Searching for native egg-parasitoids of the invasive alien species Halyomorpha halys Stål (Heteroptera Pentatomidae) in Southern Europe. J. Zool. 2016, 99, 63–70. [Google Scholar]
- Power, N.; Ganjisaffar, F.; Xu, K.; Perring, T.M. Effects of parasitoid age, host egg age, and host egg freezing on reproductive success of Ooencyrtus mirus (Hymenoptera: Encyrtidae) on Bagrada hilaris (Hemiptera: Pentatomidae) eggs. Environ. Entomol. 2021, 50, 58–68. [Google Scholar] [CrossRef]
- Cantón-Ramos, J.M.; Callejón Ferre, Á.J. Raising Trissolcus basalis for the biological control of Nezara viridula in greenhouses of Almeria (Spain). Afr. J. Agric. Res. 2010, 5, 3207–3212. [Google Scholar]
- Haye, T.; Fischer, S.; Zhang, J.; Gariepy, T. Can native egg parasitoids adopt the invasive brown marmorated stink bug, Halyomorpha halys (Heteroptera: Pentatomidae), in Europe? J. Pest Sci. 2015, 88, 693–705. [Google Scholar] [CrossRef]
- Martel, G.; Auge, M.; Talamas, E.; Roche, M.; Smith, L.; Sforza, R.F.H. First laboratory evaluation of Gryon gonikopalense (Hymenoptera: Scelionidae), as potential biological control agent of Bagrada hilaris (Hemiptera: Pentatomidae). Biol. Control 2019, 135, 48–56. [Google Scholar] [CrossRef]
- Egwuatu, R.; Taylor, T.A. Development of Gryon gnidus (Nixon) (Hymenoptera: Scelionidae) in eggs of Acanthomia tomentosicollis (Stål) (Hemiptera: Coreidae) killed either by gamma irradiation or by freezing. Bull. Entomol. Res. 1977, 67, 31–33. [Google Scholar] [CrossRef]
- Morandin, L.A.; Long, R.F.; Kremen, C. Hedgerows enhance beneficial insects on adjacent tomato fields in an intensive agricultural landscape. Agric. Ecosyst. Environ. 2014, 189, 164–170. [Google Scholar] [CrossRef]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021; p. 1216. [Google Scholar]
- Roselli, G.; Anfora, G.; Sasso, R.; Zapponi, L.; Musmeci, S.; Cemmi, A.; Suckling, D.M.; Hoelmer, K.A.; Ioriatti, C.; Cristofaro, M. Combining Irradiation and Biological Control against Brown Marmorated Stink Bug: Are Sterile Eggs a Suitable Substrate for the Egg Parasitoid Trissolcus japonicus? Insects 2023, 14, 654. [Google Scholar] [CrossRef] [PubMed]
- Kohli, A.K. Gamma Irradiator Technology: Challenges and Future Prospects. Univers. J. Appl. Sci. 2018, 12, 47–51. [Google Scholar] [CrossRef][Green Version]
- Mastrangelo, T.; Parker, A.G.; Jessup, A.; Pereira, R.; Orozco-Dávila, D.; Islam, A.; Dammalage, T.; Walder, J.M.M. A New Generation of X Ray Irradiators for Insect Sterilization. J. Econ. Entomol. 2010, 103, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kaboré, B.A.; Nawaj, A.; Maiga, H.; Soukia, O.; Pagabeleguem, S.; Ouédraogo/Sanon, M.S.G.; Vreysen, M.J.; Mach, R.L.; De Beer, C.J. X-rays are as effective as gamma-rays for the sterilization of Glossina palpalis gambiensis Vanderplank, 1911 (Diptera: Glossinidae) for use in the sterile insect technique. Sci. Rep. 2023, 13, 17633. [Google Scholar] [CrossRef]
- Congress.Gov. Text—H.R.5515—115th Congress (2017–2018): An Act to Authorize Appropriations for Fiscal Year 2019 for Military Activities of the Department of Defense, for Military Construction, and for Defense Activities of the Department of Energy, to Prescribe Military Personnel Strengths for Such Fiscal Year, and for Other Purposes. Available online: https://www.congress.gov/bill/115th-congress/house-bill/5515/text (accessed on 28 May 2024).
- Bundy, C.S.; Grasswitz, T.R.; Sutherland, C. First report of the invasive stink bug Bagrada hilaris (Burmeister) (Heteroptera: Pentatomidae) from New Mexico, with notes on its biology. Southwest. Entomol. 2012, 37, 411–414. [Google Scholar] [CrossRef]
- Reed, D.A.; Palumbo, J.C.; Perring, T.M.; May, C. Bagrada hilaris (Hemiptera: Pentatomidae), an invasive stink bug attacking cole crops in the southwestern United States. J. Integr. Pest Manag. 2013, 4, C1–C7. [Google Scholar] [CrossRef]
- Torres-Acosta, R.I.; Sánchez-Peña, S.R. Geographical distribution of Bagrada hilaris (Hemiptera: Pentatomidae) in Mexico. J. Entomol. Sci. 2016, 51, 165–167. [Google Scholar]
- Faúndez, E.I. From agricultural to household pest: The case of the painted bug Bagrada hilaris (Burmeister) (Heteroptera: Pentatomidae) in Chile. J. Med. Entomol. 2018, 55, 1365–1368. [Google Scholar] [CrossRef]
- Colazza, S.; Guarino, S.; Peri, E. Bagrada hilaris (Burmeister) (Heteroptera: Pentatomidae) a pest of capper in the island of Pantelleria [Capparis spinosa L.; Sicily]. Inf. Fitopatol. 2004, 54, 30–34. [Google Scholar]
- Palumbo, J.C.; Perring, T.M.; Millar, J.G.; Reed, D.A. Biology, ecology, and management of an invasive stink bug, Bagrada hilaris, in North America. Annu. Rev. Entomol. 2016, 61, 453–473. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Bundy, C.S.; McPherson, J.E. Unusual ovipositional behavior of the stink bug Bagrada hilaris (Hemiptera: Heteroptera: Pentatomidae). Ann. Entomol. Soc. Am. 2014, 107, 872–877. [Google Scholar] [CrossRef]
- Tofangsazi, N.; Hogg, B.N.; Hougardy, E.; Stokes, K.; Pratt, P.D. Host searching behavior of Gryon gonikopalense and Trissolcus hyalinipennis (Hymenoptera: Scelionidae), two candidate biological control agents for Bagrada hilaris (Hemiptera: Pentatomidae). Biol. Control 2020, 151, 104397. [Google Scholar] [CrossRef]
- Hogg, B.N.; Hougardy, E.; Talamas, E. Adventive Gryon aetherium Talamas (Hymenoptera, Scelionidae) associated with eggs of Bagrada hilaris (Burmeister) (Hemiptera, Pentatomidae) in the USA. J. Hymenopt. Res. 2021, 87, 481. [Google Scholar] [CrossRef]
- Martel, G.; Sforza, R.F. Evaluation of three cold storage methods of Bagrada hilaris (Hemiptera: Pentatomidae) and the effects of host deprivation for an optimized rearing of the biocontrol candidate Gryon gonikopalense (Hymenoptera: Scelionidae). Biol. Control 2021, 163, 104759. [Google Scholar] [CrossRef]
- Mansour, M. Effects of gamma radiation on the Mediterranean flour moth, Ephestia kuehniella, eggs and acceptability of irradiated eggs by Trichogramma cacoeciae females. J. Pest Sci. 2010, 83, 243–249. [Google Scholar] [CrossRef]
- Haff, R.; Jackson, E.; Gomez, J.; Light, D.; Follett, P.; Simmons, G.; Higbee, B. Building lab-scale X-ray tube based irradiators. Radiat. Phys. Chem. 2016, 121, 43–49. [Google Scholar] [CrossRef]
- Liang, P.-S.; Haff, R.P.; Ovchinnikova, I.; Light, D.M.; Mahoney, N.E.; Kim, J.H. Curcumin and quercetin as potential radioprotectors and/or radiosensitizers for X-ray-based sterilization of male navel orangeworm larvae. Sci. Rep. 2019, 9, 2016. [Google Scholar] [CrossRef]
- Hougardy, E.; Hogg, B.N. Host patch use and potential competitive interactions between two egg parasitoids from the family Scelionidae, candidate biological control agents of Bagrada hilaris (Hemiptera: Pentatomidae). J. Econ. Entomol. 2021, 114, 611–619. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Jalali, M.A.; Michaud, J.; Ziaaddini, M.; Hashemirad, H. Multiparasitism of stink bug eggs: Competitive interactions between Ooencyrtus pityocampae and Trissolcus agriope. Biocontrol 2014, 59, 279–286. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Ozyardimci, B.; Cetinkaya, N.; Denli, E.; Ic, E.; Alabay, M. Inhibition of egg and larval development of the Indian meal moth Plodia interpunctella (Hübner) and almond moth Ephestia cautella (Walker) by gamma radiation in decorticated hazelnuts. J. Stored Prod. Res. 2006, 42, 183–196. [Google Scholar] [CrossRef]
- Ayvaz, A.; Albayrak, S.; Karaborklu, S. Gamma radiation sensitivity of the eggs, larvae and pupae of Indian meal moth Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). Pest Manag. Sci. 2008, 64, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Donoughe, S. Insect egg morphology: Evolution, development, and ecology. Curr. Opin. Insect Sci. 2022, 50, 100868. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princenton, NJ, USA, 1994. [Google Scholar]
- Lara, J.R.; Pickett, C.H.; Kamiyama, M.T.; Figueroa, S.; Romo, M.; Cabanas, C.; Bazurto, V.; Strode, V.; Briseno, K.; Lewis, M. Physiological host range of Trissolcus japonicus in relation to Halyomorpha halys and other pentatomids from California. BioControl 2019, 64, 513–528. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hougardy, E.; Haff, R.P.; Hogg, B.N. Improving the Efficiency and Safety of Sentinel Stink Bug Eggs Using X-rays. Insects 2024, 15, 767. https://doi.org/10.3390/insects15100767
Hougardy E, Haff RP, Hogg BN. Improving the Efficiency and Safety of Sentinel Stink Bug Eggs Using X-rays. Insects. 2024; 15(10):767. https://doi.org/10.3390/insects15100767
Chicago/Turabian StyleHougardy, Evelyne, Ronald P. Haff, and Brian N. Hogg. 2024. "Improving the Efficiency and Safety of Sentinel Stink Bug Eggs Using X-rays" Insects 15, no. 10: 767. https://doi.org/10.3390/insects15100767
APA StyleHougardy, E., Haff, R. P., & Hogg, B. N. (2024). Improving the Efficiency and Safety of Sentinel Stink Bug Eggs Using X-rays. Insects, 15(10), 767. https://doi.org/10.3390/insects15100767





