Simple Summary
Invasive ant species are increasingly proving dangerous to native biodiversity and ecosystems, agriculture, other economic activities, and human health. Like many Mediterranean countries, Italy is witnessing a steady increase in non-native ant species of different origins and with different biological characteristics. Climate change is further posed to alter the region’s suitability for non-native ants; therefore, assessing their invasion potential is a crucial step in developing management strategies. We provide risk screenings for 15 non-native ant species already established in Italy and 12 that may be established in the future using a Terrestrial Species Invasiveness Screening Kit. The results indicate the Argentine ant, Linepithema humile, and the red imported fire ant, Solenopsis invicta, to be the most threatening species, followed by the electric ant, Wasmannia auropunctata; the Asian needle ant, Brachyponera chinensis; and the tropical fire ant, Solenopsis geminata. The harmfulness of other tropical species largely varies based on climatic predictions, while most species are far less dangerous. However, the impact of many ants is still undocumented, and the future role of climate change in their invasiveness is unclear. The detection of newly established species is often late and accidental, but public engagement could be crucial as most species first establish near cities.
Abstract
Over five hundred non-native ant species have spread worldwide, including many that have severe effects on biodiversity, are serious economic pests, or threaten human health and agriculture. The number of species in the Mediterranean is steadily increasing, with Italy being a prominent example. We provide risk screenings for non-native ant species in Italy using a Terrestrial Species Invasiveness Screening Kit using current climate conditions and future predictions. The screened species consist of 15 established and 12 horizon taxa. The results highlight the threat posed by Linepithema humile and Solenopsis invicta, followed by Wasmannia auropunctata, Brachyponera chinensis, and Solenopsis geminata. The threat posed by other tropical invaders such as Anoplolepis gracilipes and Pheidole megacephala depends on climate change scenarios. The Palearctic non-native Lasius neglectus and Tetramorium immigrans species are recognized as intermediate threats, while most screened species are far less threatening. The biology and ecology of most non-native ant species remain scarcely documented. Among the established species, B. chinensis, L. humile, and S. invicta deserve the most attention, while W. auropunctata is rapidly spreading in neighboring countries. Detection is still often accidental and late compared to establishment. Most species first establish around urban areas, making citizen science a promising tool for biosurveillance.
1. Introduction
More than five hundred ant species have been introduced outside their native range by human activities, almost always unintentionally. Some of these species are ranked among the world’s most damaging invasive species due to their environmental and economic impacts [1,2,3,4] and nineteen are listed in the Global Invasive Species Database [5]. Among invasive ants, some act as pests in agricultural and urban environments and threaten human health in a few cases [1,2,3]. A few successful species can cause severe disruption to the invaded ecosystems, displacing native ants and affecting other invertebrates, plants, and vertebrates through direct interactions and cascading effects [1,4]. At the same time, most non-native ants are restricted to heavily disturbed habitats, where they sometimes depend on special microclimatic conditions (e.g., heated buildings, greenhouses, irrigated gardens) and are considered to have little or no impact [1,4,6]. It is therefore crucial to distinguish between different non-native ant species and their invasion potential to plan management and control actions.
The Mediterranean region stands as a key terrestrial biodiversity hotspot, hosting native and rare ant species [7,8]. However, a steady and threatening increase in the number of non-native ant species has occurred over the last few decades [9,10,11,12]. Data from cargo hotspots indicate that an even larger number of non-native ant species may arrive [13]. Ants are among the most difficult invasive species to control because, given their small size, they are hard to detect and can easily spread without being noticed [14,15]. However, no invasion risk assessment has so far been conducted for non-native ants in the Mediterranean Region. Some introduced species have attracted significant attention from both scientists and media outlets. This is the case with the Argentine ant, Linepithema humile, first recorded in Portugal at the end of the 19th century [16], and the relatively recent arrivals of the red imported fire ant, Solenopsis invicta, in Italy [11,17] and the electric ant, Wasmannia auropunctata, in Cyprus, France, and Spain [12,18,19]. Conversely, other species known to cause serious impacts in other areas of the world have so far seemed to pose little threat to Mediterranean habitats, even following their occasional introduction (e.g., the African big-headed ant, Pheidole megacephala [20]). In Europe, while most established ant species are limited to urban or agricultural habitats, many of them can establish outdoors in Euro-Mediterranean countries [9,21,22]. Climate change represents a further source of uncertainty for the establishment potential of non-native ants in Europe, with non-anecdotal predictions currently available for only a handful of species [11,23,24,25].
Italy hosts an increasing number of non-native ant species [9,26]. Recent discoveries include threatening species, such as the red imported fire ant and the Asian needle ant, Brachyponera chinensis, as well as Hypoponera ergatandria and Nylanderia vividula, bring the total number of established ants to at least 15 [10,11,27,28]. Although the ecological and economic threats posed by some of these non-native ant species have been well documented [1,11], there is a notable lack of information about the potential invasiveness of most of them. This gap makes effective management and control efforts challenging [2,3]. This study aims to address this deficiency by providing risk screenings for established and potentially invasive non-native ants in Italy. The objective is to provide a thorough evaluation of the risk potential for each species by applying a recently released decision support tool and considering both present and future climate predictions. This comprehensive method will be useful to promote the effective management and control of these species in Italy and will represent a valuable background for the evaluation of comparable risk screenings in other regions threatened by non-native ant species.
2. Materials and Methods
To identify potentially invasive non-native ants in Italy (the risk assessment area), a risk screening was conducted involving 27 species (Table 1). These included 15 species established in Italy (Brachyponera chinensis, Hypoponera ergatandria, Hypoopnera punctatissima, Lasius neglectus, Linepithema humile, Monomorium pharaonis, Nylanderia jaegerskioeldi, Nylanderia vividula, Paratrechina longicornis, Pheidole indica, Solenopsis invicta, Strumigenys membranifera, Tetramorium bicarinatum, Tetramorium immigrans, and Tetramorium lanuginosum) plus 12 horizon species chosen for their invasive potential and presence in the Mediterranean or in the whole of Europe (Anoplolepis gracilipes, Brachymyrmex patagonicus, Cardiocondyla obscurior, Monomorium carbonarium, Monomorium floricola, Pheidole megacephala, Plagiolepis alluaudi, Solenopsis geminata, Tapinoma melanocephalum, Technomyrmex pallipes, Trichomyrmex destructor, and Wasmannia auropunctata) [9,10,11,12,13,18,19,21,22,26,27,28,29,30]. The black imported fire ant, Solenopsis richteri, a species of concern according to the European Union, was not screened as it was deemed to be very similar to the red imported fire ant, Solenopsis invicta, while the remaining invasive species of Union concern according to the latest list (Commission Implementing Regulation (EU) 2022/1203 of 12 July 2022, amending Implementing Regulation (EU) 2016/1141 to update the list of invasive non-native species of Union concern) were all included: the aforementioned Solenopsis invicta and Wasmannia auropunctata and the tropical fire ant, Solenopsis geminata [5]. For the genera Nylanderia and Technomyrmex, both including several introduced species worldwide whose proper recognition has suffered from taxonomic difficulties, only the representatives that have a wider presence in the region were screened.

Table 1.
Non-native ant species screened with a Terrestrial Animal Species Invasiveness Screening Kit (TAS-ISK) for their invasion risk in Italy. The a priori categorization follows the four-step protocol of Vilizzi et al. [31]: (1) FishBase (www.fishbase.org (accessed on 13 September 2024)); (2) Global Invasive Species Database (GISD: www.iucngisd.org (accessed on 13 September 2024)); (3) European Alien Species Information Network (EASIN: https://easin.jrc.ec.europa.eu/easin (accessed on 13 September 2024)); (4) Google Scholar literature search. N = no impact/threat; Y = impact/threat; ‘–’ = absent; n.a. = not applicable. Species considered established in Italy are marked in bold.
Risk screening was undertaken using the Terrestrial Animal Species Invasiveness Screening Kit (TAS-ISK v2.4 [32]). This multilingual, taxon-generic decision support tool complies with the ‘minimum standards’ for screening non-native species under EC Regulation No. 1143/2014 on the prevention and management of the introduction and spread of invasive species [33]. The TAS-ISK consists of 55 questions of which 49 comprise the Basic Risk Assessment (BRA) and six the Climate Change Assessment (CCA). The latter component requires the assessor to predict how future predicted climatic conditions are likely to affect the BRA concerning risks of introduction, establishment, dispersal, and impact. All screenings were carried out by the first author.
The screening process followed the standard protocol by Vilizzi et al. [31], with the assessor providing a response, a confidence level, and a justification for each question [34]. Upon completion of a species’ screening, the BRA and BRA+CCA scores are computed. In both cases, a score < 1 indicates a ‘low risk’ of the species being or becoming invasive in the risk assessment area, whereas a score ≥ 1 indicates a ‘medium risk’ or a ‘high risk’. Distinction between medium-risk and high-risk species is made by computing a calibrated threshold through Receiver Operating Characteristic (ROC) curve analysis [31,35]. A measure of the accuracy of the calibration analysis is the area under the curve (AUC), whose values are interpreted as follows: 0.7 ≤ AUC < 0.8 = acceptable discriminatory power, 0.8 ≤ AUC < 0.9 = excellent, 0.9 ≤ AUC = outstanding [36]. An additional ad hoc threshold was also defined to distinguish within species classified as high risk those carrying a ‘very high risk’ of invasiveness (as per [37]). Following the identification of the threshold, an evaluation of the risk rankings to identify false/true negative/positive outcomes was not applied to the medium-risk species because their evaluation in a follow-up risk assessment depends on both management priorities and the availability of financial resources [31].
The a priori categorization of species to implement ROC curve analysis followed Vilizzi et al. [31]. Fitting of the ROC curve was performed with pROC [38] for R x64 v4.3.2 [39]. Permutational ANOVA following normalization of the data was used to test for differences in the confidence factor (CF: see [31]) between the BRA and BRA + CCA using a Bray–Curtis dissimilarity measure, 9999 unrestricted permutations of the raw data, and with statistical effects evaluated at α = 0.05.
3. Results
The ROC curve analysis resulted in a threshold of 16.5 and an AUC of 0.8294 (0.6602–0.9986 95% CI); hence, it showed excellent discriminatory power. The threshold was therefore used for the calibration of the BRA and BRA+CCA scores to distinguish between medium- and high-risk species under current and predicted climate conditions, respectively (Table 2).

Table 2.
Risk outcomes for the non-native ant species screened with the TAS-ISK for Italy. For each species, the following information is provided: a priori categorization of invasiveness (N = non-invasive; Y = invasive: see Table 1); Basic Risk Assessment (BRA) and BRA + Climate Change Assessment (BRA + CCA) scores with corresponding risk ranks based on a calibrated threshold of 16.5 (M = medium; H = high; VH = very high, based on an ad hoc threshold ≥ 40. See text for details); classification (Class: FP = false positive; TP = true positive; ‘–’ = not implemented as medium risk; n.a. = not applicable. See text for details); CCA as the difference between BRA + CCA and BRA scores; and confidence factor (CF). Risk outcomes for the BRA scores (within the interval): M [1, 16.5[, H ]16.5, 40[, VH [40, 72]. Risk outcomes for the BRA + CCA scores: M [1, 16.5[, H ]16.5, 40[, VH [40, 82]. Note the reverse bracket notation indicating an open interval.
Based on the BRA scores (Table 2, Figure 1a), 3 (11.1%) species were ranked as very high risk, 13 (48.2%) as high risk, and 11 (40.7%) as medium risk. Of the 17 species categorized a priori as invasive, 3 were ranked as very high risk (Argentine ant, Linepithema humile; tropical fire ant, Solenopsis geminata; red imported fire ant, Solenopsis invicta) and 13 as high risk (true positives: yellow crazy ant, Anoplolepis gracilipes; dark rover ant, Brachymyrmex patagonicus; Asian needle ant, Brachyponera chinensis; invasive garden ant, Lasius neglectus; longhorn crazy ant, Paratrechina longicornis; Indian big-headed ant, Pheidole indica; African big-headed ant, Pheidole megacephala; ghost ant, Tapinoma melanocephalum; tramp ant, Tetramorium bicarinatum; destroyer ant, Trichomyrmex destructor; and electric ant, Wasmannia auropunctata). Of the ten species categorized a priori as non-invasive, two were ranked as high risk (false positive: little yellow ant, Plagiolepis alluaudi, and pavement ant, Tetramorium immigrans). Of the eleven medium-risk species, eight were a priori non-invasive (Hypoponera ergatandria; Roger’s ant, Hypoponera punctatissima; Monomorium carbonarium; Nylanderia jaegerskioeldi; Nylanderia vividula; membraniferous dacetine ant, Strumigenys membranifera; white-footed ant, Technomyrmex pallipes; wooly ant, Tetramorium lanuginosum) and three were invasive (Cardiocondyla obscurior, bicolored trailing ant, Monomorium floricola; pharaoh ant, Monomorium pharaonis).
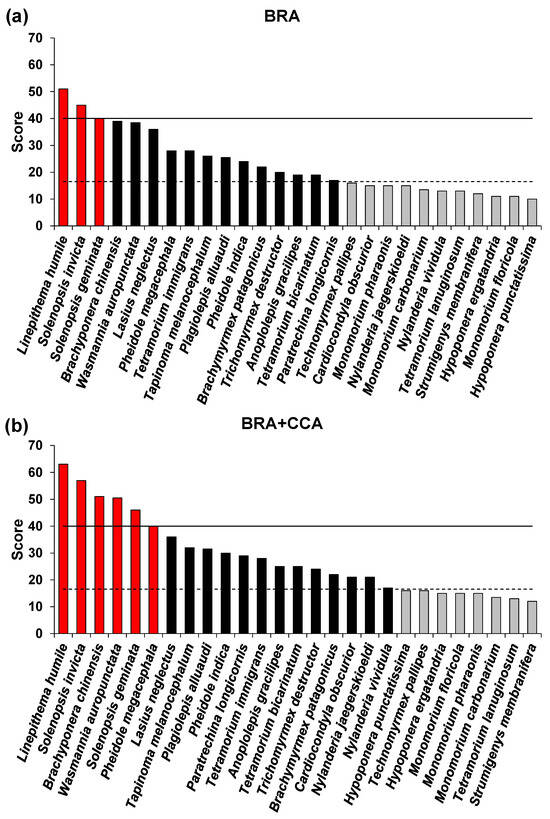
Figure 1.
Risk outcome scores for the non-native ants screened with the Terrestrial Animal Species Invasiveness Screening Kit (TAS-ISK) in Italy. (a) Basic Risk Assessment (BRA) scores; (b) BRA + Climate Change Assessment (BRA+CCA) scores. Red bars = very-high-risk species. Black bars = high-risk species. Gray bars = medium-risk species. Solid line = very-high-risk (VH) threshold. Hatched line = high-risk (H) threshold. Thresholds as per Table 2.
Based on the BRA+CCA scores (Table 2, Figure 1b), 19 (70.4%) species were ranked as very high or high risk and 8 (29.6%) we ranked as medium risk. Of the a priori invasive species, 15 were ranked as high or very high risk (same species as for the BRA plus Cardiocondyla obscurior), and of the a priori non-invasive species 4 were ranked as high risk (same species as per BRA plus Nylanderia jaegerskioeldi and Nylanderia vividula). Of the eight medium-risk species, six were a priori non-invasive (Hypoponera ergatandria, Hypoponera punctatissima, Monomorium carbonarium, Strumigenys membranifera, Technomyrmex pallipes, Tetramorium lanuginosum) and two were invasive (Monomorium floricola and Monomorium pharaonis).
Based on an ad hoc threshold ≥40, Linepithema humile, Solenopsis invicta, and Solenopsis geminata were ranked as very high risk for both the BRA and BRA+CCA, and an additional three species were ranked for the BRA+CCA only (i.e., Brachyponera chinensis, Pheidole megacephala, and Wasmannia auropunctata) (Table 2, Figure 1). The number of species ranked as high (and very high) risk increased from 16 (59.3%) under the BRA to 19 (70.4%) under the BRA+CCA. The CCA resulted in an increase in the BRA score (cf. BRA+CCA score) for 16 (70.4%) species and in no change for 8 (29.6%) (Table 2).
The mean CFTotal was 0.741 ± 0.012 SE, the mean CFBRA was 0.779 ± 0.011 SE, and the mean CFCCA was 0.427 ± 0.044 SE, hence indicating lower confidence for the CCA (Table 2). The mean CFBRA was higher than mean CFCCA (F#1,52 = 60.35, p# < 0.001; # = permutational value).
4. Discussion
This study represents the first risk screening focusing on non-native ant species in a Mediterranean country and the second in a European country after the one by Báthori et al. [40]. The results fill a knowledge gap by providing a comprehensive evaluation of the risk posed by non-native ant species in a representative country of the Mediterranean region and provide a valuable framework for future risk assessment. This study also contributes to increasing the knowledge of the potential hazards posed by the screened species, underlining the need for focused monitoring and management measures considering the increasingly complex ecological interactions triggered by climate change.
Linepithema humile and Solenopsis invicta were the species associated with the highest risk scores under both current and predicted climate conditions. There is an overwhelming body of literature describing the environmental impact of both species, which are regarded among the worst invasives globally [4,24,41,42]. Linepithema humile is the only invasive species that has already proved to be highly capable of invading and deteriorating Mediterranean ecosystems, with an extraordinary supercolonial organization ranging through southwestern Europe [42]. It is mainly associated with coastal and highly disturbed habitats in Italy, where it was first detected in the early 20th century [16]. On the other hand, Solenopsis invicta has only recently been discovered in Italy, the first European or Mediterranean country to witness its establishment, which may have occurred since at least 2015 [10,11]. While both species are considered serious ecological threats and can also harm agricultural activities, Solenopsis invicta is also a threat to electric infrastructures and human health [41]. Both species are expected to significantly expand their suitable range in Europe due to climate change [10,23].
A high risk was also associated with Wasmannia auropunctata, Solenopsis geminate, and Brachyponera chinensis, which are all ecologically damaging and regarded as health threats due to their stinging abilities [3,43,44]. Brachyponera chinensis has been found in two far-apart Italian localities very recently, and is otherwise unknown elsewhere in Europe, while its invasive abilities were mostly studied in North America [10,28]. On the other hand, Wasmannia auropunctata, traditionally considered a tropical species, has been detected in three Euro-Mediterranean countries very recently, establishing viable and growing populations [12,18,19,45]. Finally, while past introductions of Solenopsis geminata in the region did not result in established outdoor populations or were based on misidentifications, a potential invasion of the species is still considered high risk due to its invasive abilities, environmental impact, and powerful sting, and it has been included in the list of species of Union concern by the EU alongside Solenopsis invicta, Solenopsis richteri, and Wasmannia auropunctata [22,46].
When climate change scenarios are considered, there is an increase in the risk level associated with several species, most evident in Pheidole megacephala, Paratrechina longicornis, Solenopsis invicta, and Wasmannia auropunctata. Among the species with increased risk levels, Solenopsis geminata, Pheidole megacephala, and Anoplolepis gracilipes have a serious invasive record in the tropics but never had significant success in the Mediterranean in past introductions. None of them seems to be currently established in Italy (Pheidole megacephala only temporarily established at Malpensa airport, Milan (see [13])). While their suitability models suggest a future increase in Europe, this is not necessarily decisive enough or the evidence is not conclusive [47,48,49]. In general, most of the species assessed are native to warm tropical climates and are predicted to increase their invasion risk with climate change, but predictions are still often anecdotal (low confidence) and these results must be treated accordingly.
Two Palearctic species supposedly native to Anatolia and Caucasus or Central Asia, namely Lasius neglectus and Tetramorium immigrans, have somewhat intermediate invasion risk scores: the two have been successfully colonizing urban and disturbed habitats across Europe and have locally been considered pests, with Tetramorium immigrans being a cryptic invader of recent recognition and unknown introduction time that is particularly widespread [50,51,52,53]. Most of the species have lower scores, as none of them are expected to pose a significant threat to either the environment or human activities, and most are likely to be confined within buildings or near urban areas [9]. However, information on their ecological role is extremely scarce in most cases—including for those species that have already established in Italy or the Mediterranean region.
The number of non-native ants in Italy rapidly passed from six at the beginning of the 21st century [9] to the current number of at least fifteen [10,11,27,28]. Most species discovered during the last few years have been spotted near urban areas, and most were first detected by non-professional researchers, pest controllers, or curious citizens before being identified by specialists [9,10,11,13,17,27,28,29]. This pattern is overall similar across different European countries, with stinging species attracting significant attention [9,10,11,12,17,19,28]. While dangerous lag is often observed between first establishment and detection, given non-native ants’ preference for anthropogenic habitats, citizen science and public engagement could play a key role in aiding biosurveillance efforts and achieving earlier detection of threats [17,54]. However, the increasing number of either established in Italy and in Mediterranean Europe, in general, requires us to establish priorities and distinguish between species posing different threats. Hence, the use of TAS-ISK and similar decision tools to evaluate the risks posed by non-native species could be useful to monitor the risk posed by incoming species and implement control and management strategies.
5. Conclusions
The present results clearly suggest that some species, like the already established Linepithema humile and Solenopsis invicta, are those posing the highest risk under both current and predicted climate conditions. The concurrence of their well-known capacities to negatively impact biodiversity with all associated costs, together with their high potential to rapidly expand their range of invasion because of climate change, suggest that the urgent implementation of monitoring and management measures should become a priority. The results of this study also highlight the importance of integrating decision support tools like TAS-ISK to evaluate the levels of threat posed by different species in order to promote a more strategic allocation of management efforts. Also, the risks associated with some species that are traditionally not successful in the Mediterranean appear to be worsened under predicted climate change scenarios.
Public outreach and citizen science programs are promising considering the increasing number of non-native ant species that are being reported from Italy and which are frequently detected for the first time by non-specialists, often with a significant delay from their establishment. While their correct identification normally requires highly trained taxonomists, increasing public participation and awareness can improve early detection and monitoring initiatives. In addition to official surveillance efforts, programs that would promote the reporting of new non-native ant species occurrences and assist and increase awareness about the potential consequences of these species can be useful to enhance biosurveillance measures in general. Effective monitoring and management measures are important considering the growing number of non-native species and the potential risks they pose. More in-depth research about the ecological effects of less-studied species, especially those with lower risk scores, is also encouraged.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15110875/s1, Table S1: Screenings of established and horizon non-native ant species following the terrestrial species invasiveness screening kit (TAS-ISK).
Author Contributions
Conceptualization, E.S., D.G. and L.V.; methodology, D.G. and L.V.; validation, E.S., D.G. and L.V.; formal analysis, L.V.; investigation, E.S.; resources, D.G. and L.V.; data curation, E.S., D.G. and L.V.; writing—original draft preparation, E.S.; writing—review and editing, D.G. and L.V.; visualization, L.V.; supervision, D.G. and L.V.; project administration, D.G. and L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data supporting the conclusions of this article are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The causes and consequences of ant invasions. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Angulo, E.; Hoffmann, B.D.; Ballesteros-Mejia, L.; Taheri, A.; Balzani, P.; Bang, A.; Renault, D.; Cordonnier, M.; Bellard, C.; Diagne, C.; et al. Economic costs of invasive alien ants worldwide. Biol. Invasions 2022, 24, 2041–2060. [Google Scholar] [CrossRef]
- Wong, M.K.; Economo, E.P.; Guénard, B. The global spread and invasion capacities of alien ants. Curr. Biol. 2023, 33, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Global Invasive Species Database. Available online: http://www.iucngisd.org/gisd/100_worst.php (accessed on 13 September 2024).
- Arnan, X.; Angulo, E.; Boulay, R.; Molowny-Horas, R.; Cerdá, X.; Retana, J. Introduced ant species occupy empty climatic niches in Europe. Sci. Rep. 2021, 11, 3280. [Google Scholar] [CrossRef] [PubMed]
- Kass, J.M.; Guénard, B.; Dudley, K.L.; Jenkins, C.N.; Azuma, F.; Fisher, B.L.; Parr, C.L.; Gibb, H.; Longino, J.T.; Ward, P.S.; et al. The global distribution of known and undiscovered ant biodiversity. Sci. Adv. 2022, 8, eabp9908. [Google Scholar] [CrossRef]
- Wang, R.; Kass, J.M.; Galkowski, C.; Garcia, F.; Hamer, M.T.; Radchenko, A.; Salata, S.; Schifani, E.; Yusupov, Z.M.; Economo, E.P.; et al. Geographic and climatic constraints on bioregionalization of European ants. J. Biogeogr. 2023, 50, 503–514. [Google Scholar] [CrossRef]
- Schifani, E. Exotic ants (Hymenoptera, Formicidae) invading Mediterranean Europe: A brief summary over about 200 years of documented introductions. Sociobiology 2019, 66, 198–208. [Google Scholar] [CrossRef]
- Menchetti, M.; Schifani, E.; Gentile, V.; Vila, R. The worrying arrival of the invasive Asian needle ant Brachyponera chinensis in Europe (Hymenoptera: Formicidae). Zootaxa 2022, 5115, 146–150. [Google Scholar] [CrossRef]
- Menchetti, M.; Schifani, E.; Alicata, A.; Cardador, L.; Sbrega, E.; Toro-Delgado, E.; Vila, R. The invasive ant Solenopsis invicta is established in Europe. Curr. Biol. 2023, 33, R896–R897. [Google Scholar] [CrossRef]
- Blight, O.; Thomas, T.; Jourdan, H.; Bichaton, J.Y.; Colindre, L.; Galkowski, C. Detection and early impacts of France’s first established population of the little fire ant, Wasmannia auropunctata. Biol. Invasions 2023, 26, 627–631. [Google Scholar] [CrossRef]
- Jucker, C.; Rigato, F.; Regalin, R. Exotic ant records from Italy (Hymenoptera, Formicidae). Boll. Zool. Agr. Bachicolt. 2008, 40, 99–107. [Google Scholar]
- Stohlgren, T.J.; Schnase, J.L. Risk analysis for biological hazards: What we need to know about invasive species. Risk Anal. 2006, 26, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.V.; Haight, R.G.; Homans, F.R.; Polasky, S.; Venette, R.C. Optimal detection and control strategies for invasive species management. Ecol. Econ. 2007, 61, 237–245. [Google Scholar] [CrossRef]
- Wetterer, J.K.; Wild, A.L.; Suarez, A.V.; Roura-Pascual, N.; Espadaler, X. Worldwide spread of the Argentine ant, Linepithema humile (Hymenoptera: Formicidae). Myrmecol. News 2009, 12, 187–194. [Google Scholar]
- Menchetti, M.; Schifani, E.; Alicata, A.; Cardador, L.; Sbrega, E.; Toro-Delgado, E.; Vila, R. Response to Genovesi et al.: Ant biosurveillance should come before invasion. Curr. Biol. 2024, 34, R37–R52. [Google Scholar] [CrossRef]
- Espadaler, X.; Pradera, C.; Santana, J.A. The first outdoor-nesting population of Wasmannia auropunctata in continental Europe (Hymenoptera, Formicidae). Iberomyrmex 2018, 10, 1–8. [Google Scholar]
- Demetriou, J.; Georgiadis, C.; Roy, H.; Martinou, A.; Borowiec, L.; Salata, S. One of the world’s worst invasive alien species Wasmannia auropunctata (Hymenoptera: Formicidae) detected in Cyprus. Sociobiology 2022, 69, e8536. [Google Scholar] [CrossRef]
- Sarnat, E.M.; Fischer, G.; Guénard, B.; Economo, E.P. Introduced Pheidole of the world: Taxonomy, biology and distribution. ZooKeys 2015, 543, 1. [Google Scholar] [CrossRef]
- Blatrix, R.; Colin, T.; Wegnez, P.; Galkowski, C.; Geniez, P. Introduced ants (Hymenoptera: Formicidae) of mainland France and Belgium, with a focus on greenhouses. Ann. Soc. Entomol. Fr. 2018, 54, 293–308. [Google Scholar] [CrossRef]
- Demetriou, J.; Georgiadis, C.; Koutsoukos, E.; Borowiec, L.; Salata, S. Alien ants (Hymenoptera, Formicidae) on a quest to conquer Greece: A review including an updated species checklist and guidance for future research. NeoBiota 2023, 86, 81–122. [Google Scholar] [CrossRef]
- Roura-Pascual, N.; Suarez, A.V.; Gómez, C.; Pons, P.; Touyama, Y.; Wild, A.L.; Peterson, A.T. Geographical potential of Argentine ants (Linepithema humile Mayr) in the face of global climate change. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Bertelsmeier, C.; Luque, G.M.; Hoffmann, B.D.; Courchamp, F. Worldwide ant invasions under climate change. Biodivers. Conserv. 2015, 24, 117–128. [Google Scholar] [CrossRef]
- Bertelsmeier, C.; Blight, O.; Courchamp, F. Invasions of ants (Hymenoptera: Formicidae) in light of global climate change. Myrmecol. News 2016, 22, 25–42. [Google Scholar]
- Schifani, E. The new Checklist of the Italian Fauna: Formicidae. Biogeographia 2022, 37, ucl006. [Google Scholar] [CrossRef]
- Schifani, E.; Pezzin, A.; Castracani, C.; Grasso, D.A. First record of the exotic ant Hypoponera ergatandria in Italy: Indoor alate swarms and stinging queens. Sociobiology 2024, 71, e10091. [Google Scholar] [CrossRef]
- Schifani, E.; Grunicke, D.; Montechiarini, A.; Pradera, C.; Vila, R.; Menchetti, M. Alien ants spreading through Europe: Brachyponera chinensis and Nylanderia vividula in Italy. Biodivers. Data J. 2024, 12, e123502. [Google Scholar] [CrossRef]
- Schifani, E.; Alicata, A. Exploring the myrmecofauna of Sicily: Thirty-two new ant species recorded, including six new to Italy and many new aliens (Hymenoptera, Formicidae). Pol. J. Entomol. 2018, 87, 323–348. [Google Scholar] [CrossRef]
- Schifani, E.; Georgiadis, C.; Menchetti, M. Cardiocondyla obscurior, a new alien ant in Crete (Hymenoptera, Formicidae). Biogeographia 2024, 39, a033. [Google Scholar] [CrossRef]
- Vilizzi, L.; Hill, J.E.; Piria, M.; Copp, G.H. A protocol for screening potentially invasive non-native species using weed risk assessment-type decision-support tools. Sci. Total Environ. 2022, 832, 154966. [Google Scholar] [CrossRef]
- Vilizzi, L.; Piria, M.; Pietraszewski, D.; Kopecký, O.; Špelić, I.; Radočaj, T.; Šprem, N.; Ta, K.A.T.; Tarkan, A.S.; Weiperth, A.; et al. Development and application of a multilingual electronic decision-support tool for risk screening non-native terrestrial animals under current and future climate conditions. NeoBiota 2022, 76, 211–236. [Google Scholar] [CrossRef]
- Roy, H.E.; Rabitsch, W.; Scalera, R.; Stewart, A.; Gallardo, B.; Genovesi, P.; Essl, F.; Adriaens, T.; Bacher, S.; Booy, O.; et al. Developing a framework of minimum standards for the risk assessment of alien species. J. Appl. Ecol. 2018, 55, 526–538. [Google Scholar] [CrossRef]
- Vilizzi, L.; Piria, M. Providing scientifically defensible evidence and correct calibrated thresholds for risk screening non-native species with second-generation Weed Risk Assessment-type decision-support tools. J. Vertebr. Biol. 2022, 71, 526–538. [Google Scholar] [CrossRef]
- Vilizzi, L.; Piria, M.; Copp, G.H. Which calibrated threshold is appropriate for ranking non-native species using scores generated by WRA-type screening toolkits that assess risks under both current and future climate conditions. Manag. Biol. Invasions 2022, 13, 593–608. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; p. 510. [Google Scholar]
- Britton, J.R.; Copp, G.H.; Brazier, M.; Davies, G.D. A modular assessment tool for managing introduced fishes according to risks of species and their populations, and impacts of management actions. Biol. Invasions 2011, 13, 2847–2860. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinformatics 2011, 12, 1–8. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Báthori, F.; Herczeg, G.; Vilizzi, L.; Jégh, T.; Kakas, C.; Petrovics, M.; Csősz, S. A survey and risk screening of non-native ant species colonising greenhouses in Hungary. Biol. Invasions 2024, 26, 1033–1044. [Google Scholar] [CrossRef]
- Tschinkel, W.R. The Fire Ants; Harvard University Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Angulo, E.; Guénard, B.; Balzani, P.; Bang, A.; Frizzi, F.; Masoni, A.; Abril, S.; Suarez, A.V.; Hoffmann, B.; Benelli, G.; et al. The Argentine ant, Linepithema humile: Natural history, ecology and impact of a successful invader. Entomol. Gen. 2024, 44, 41–61. [Google Scholar] [CrossRef]
- Guénard, B.; Wetterer, J.K.; MacGown, J.A. Global and temporal spread of a taxonomically challenging invasive ant, Brachyponera chinensis (Hymenoptera: Formicidae). Florida Entomol. 2018, 101, 649–656. [Google Scholar] [CrossRef]
- Wetterer, J.K. Worldwide spread of the little fire ant, Wasmannia auropunctata (Hymenoptera: Formicidae). Terrestrial Arthropod Rev. 2013, 6, 173–184. [Google Scholar] [CrossRef]
- Espadaler, X.; Pradera, C.; Santana, J.A.; Reyes, A.R. Dos nuevas poblaciones europeas de la pequeña hormiga de fuego, Wasmannia auropunctata (Roger, 1863) (Hymenoptera: Formicidae) en Andalucía (España). Bol. Soc. Andaluza Entomol. 2020, 30, 189–192. [Google Scholar]
- Wetterer, J.K. Worldwide spread of the tropical fire ant, Solenopsis geminata (Hymenoptera: Formicidae). Myrmecol. News 2011, 14, 21–35. [Google Scholar]
- Chen, Y. Global potential distribution of an invasive species, the yellow crazy ant (Anoplolepis gracilipes) under climate change. Integr. Zool. 2008, 3, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Lee, D.S.; Kwon, T.S.; Athar, M.; Park, Y.S. Predicting the global distribution of Solenopsis geminata (Hymenoptera: Formicidae) under climate change using the MaxEnt model. Insects 2021, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Mao, M.; Bamisile, S.; Li, Z.; Xu, Y. Predicting the potential distribution of Pheidole megacephala in light of present and future climate variations. J. Econ. Entomol. 2024, 117, 457–469. [Google Scholar] [CrossRef]
- Seifert, B. Rapid range expansion in Lasius neglectus (Hymenoptera, Formicidae)—An Asian invader swamps Europe. Dtsch. Entomol. Z. 2000, 47, 173–179. [Google Scholar]
- Tartally, A.; Antonova, V.; Espadaler, X.; Csősz, S.; Czechowski, W. Collapse of the invasive garden ant, Lasius neglectus, populations in four European countries. Biol. Invasions 2016, 18, 3127–3131. [Google Scholar] [CrossRef]
- Wagner, H.C.; Arthofer, W.; Seifert, B.; Muster, C.; Steiner, F.M.; Schlick-Steiner, B.C. Light at the end of the tunnel: Integrative taxonomy delimits cryptic species in the Tetramorium caespitum complex (Hymenoptera: Formicidae). Myrmecol. News 2017, 25, 95–129. [Google Scholar] [CrossRef]
- Moss, A.D.; Swallow, J.G.; Greene, M.J. Always under foot: Tetramorium immigrans (Hymenoptera: Formicidae), a review. Myrmecol. News 2022, 32, 75–92. [Google Scholar] [CrossRef]
- Castracani, C.; Spotti, F.A.; Schifani, E.; Giannetti, D.; Ghizzoni, M.; Grasso, D.A.; Mori, A. Public engagement provides first insights on Po Plain ant communities and reveals the ubiquity of the cryptic species Tetramorium immigrans (Hymenoptera, Formicidae). Insects 2020, 11, 678. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).