Impact on Ant Communities by Chemical Pesticides Applied in Controlling the Red Imported Fire Ant (Solenopsis invicta Buren) in the Field
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Environment and Test Pesticides
- Classification of Live Ant Nest Density per Unit Area
- Level I: Mild—an average of 0 to 0.1 live ant nests per 100 m2.
- Level II: Moderate—an average of 0.11 to 0.5 live ant nests per 100 m2.
- Level III: Moderately Heavy—an average of 0.51 to 1.0 live ant nests per 100 m2.
- Level IV: Heavy—an average of 1.1 to 10 live ant nests per 100 m2.
- Level V: Severe—an average of more than 10 live ant nests per 100 m2.
- 2.
- Classification of Foraging Worker Ant Density
- Level I: Light—an average of fewer than 20 workers per monitoring bait.
- Level II: Moderate—an average of 20.1 to 100 workers per monitoring bait.
- Level III: Moderately Heavy—an average of 100.1 to 150 workers per monitoring bait.
- Level IV: Heavy—an average of 150.1 to more than 300 workers per monitoring bait.
- Level V: Severe—an average of more than 301 workers per monitoring bait.
- 0.5% beta-cypermethrin dust (Guangzhou Ruifeng Biotechnology Co., Ltd., Guangzhou, China): the RIFA control area covered approximately 106 mu (1 mu ≈ 666.67 m2) and contained 103 active RIFA nests, with an average of 100.5 workers per monitoring bait, classified as level III in infestation severity.
- 1.0% hydramethylnon bait (Wuhan Bile Health Technology Co., Ltd., Wuhan, China): the demonstration area covered approximately 90 mu, which contains 129 active RIFA nests, yielding an average of 120.33 workers per monitoring bait, also classified as level III in infestation severity.
- 0.1% indoxacarb bait (Kaiping Dahao Daily Chemical Technology Co., Ltd., Kaiping, China): the demonstration area covered approximately 90 mu and contained 135 active RIFA nests, with an average of 103.65 workers per monitoring bait, classified as level III in infestation severity.
- The control area covered approximately 50 mu and contained 94 active RIFA nests, with an average of 142.62 workers per monitoring bait, classified as level III in infestation severity.
2.2. Evaluation of the Control Effect of Three Pesticides on RIFAs
2.3. Evaluation Methods for Pesticide Control Efficacy
- WO: The average number of worker ants in the monitoring baits in the control area before treatment.
- WTi: The average number of worker ants in the monitoring baits in the treated area after treatment.
- WOi: The average number of worker ants in the monitoring baits in the control area after treatment.
- WTO: The average number of worker ants in the monitoring baits in the treated area before treatment.
- NO: The number of active ant nests in the control area before treatment.
- NTi: The number of active ant nests in the treated area after treatment.
- NOi: The number of active ant nests in the control area after treatment.
- NTO: The number of active ant nests in the treated area before treatment.
- CO: The average level of the ant colonies in the control area before treatment.
- CTi: The average level of the ant colonies in the treatment area after treatment.
- COi: The average level of the ant colonies in the control area after treatment.
- CTO: The average level of the ant colonies in the treatment area before treatment.
- PN: The control effect on live ant nests.
- PW: The control effect on worker ants.
- PC: The control effect on the ant colony.
2.4. Impact of Pesticides on Ant Communities
- (1)
- Shannon–Wiener species diversity index formula:
- (2)
- Pielou’s uniformity index formula:
- (3)
- Simpson’s dominance index formula calculates the dominance index:
- (4)
- Margalef’s richness index formula:
2.5. Data Analysis
3. Results
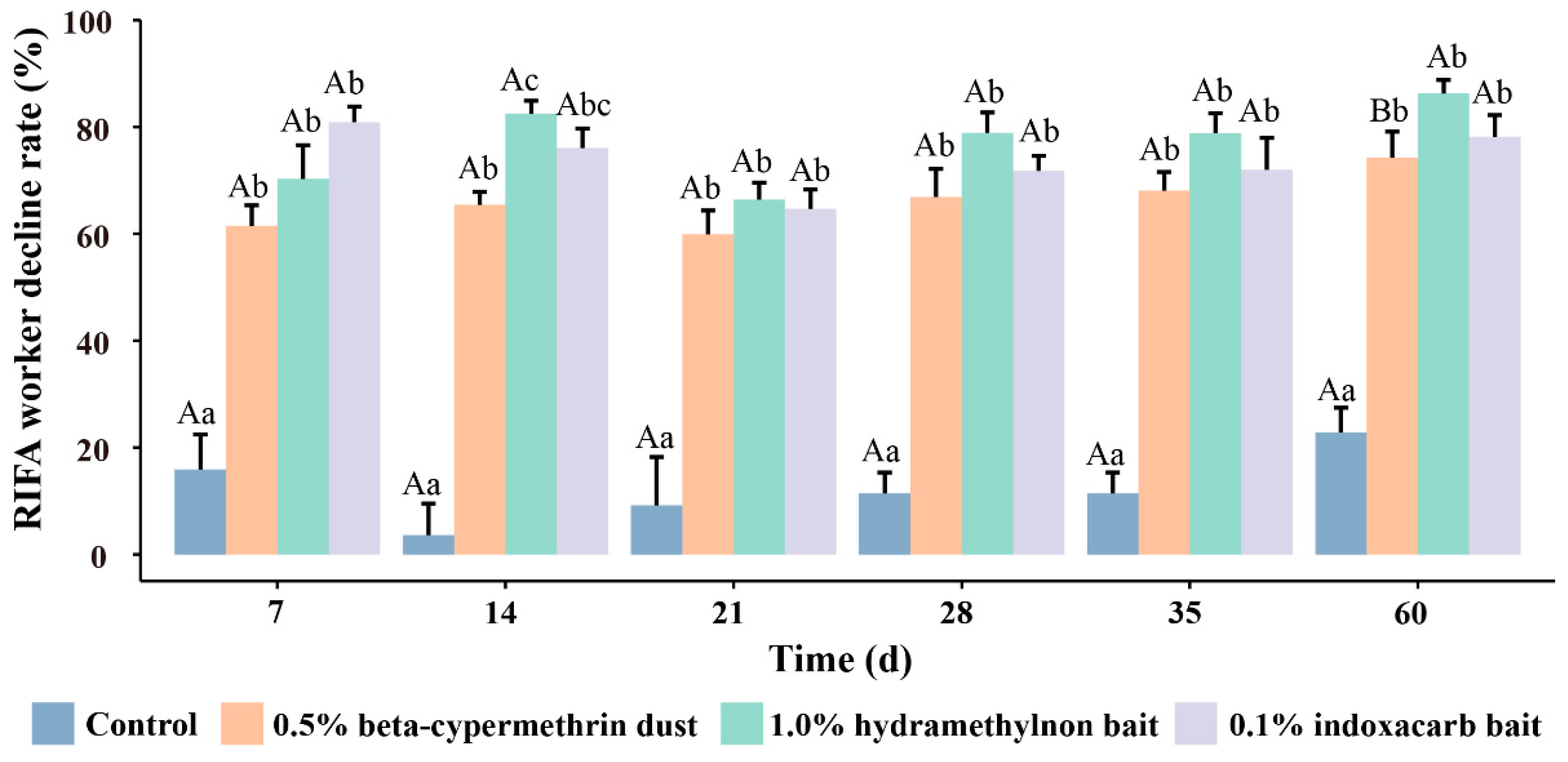
3.1. Control Effects of Three Pesticides on RIFA Workers
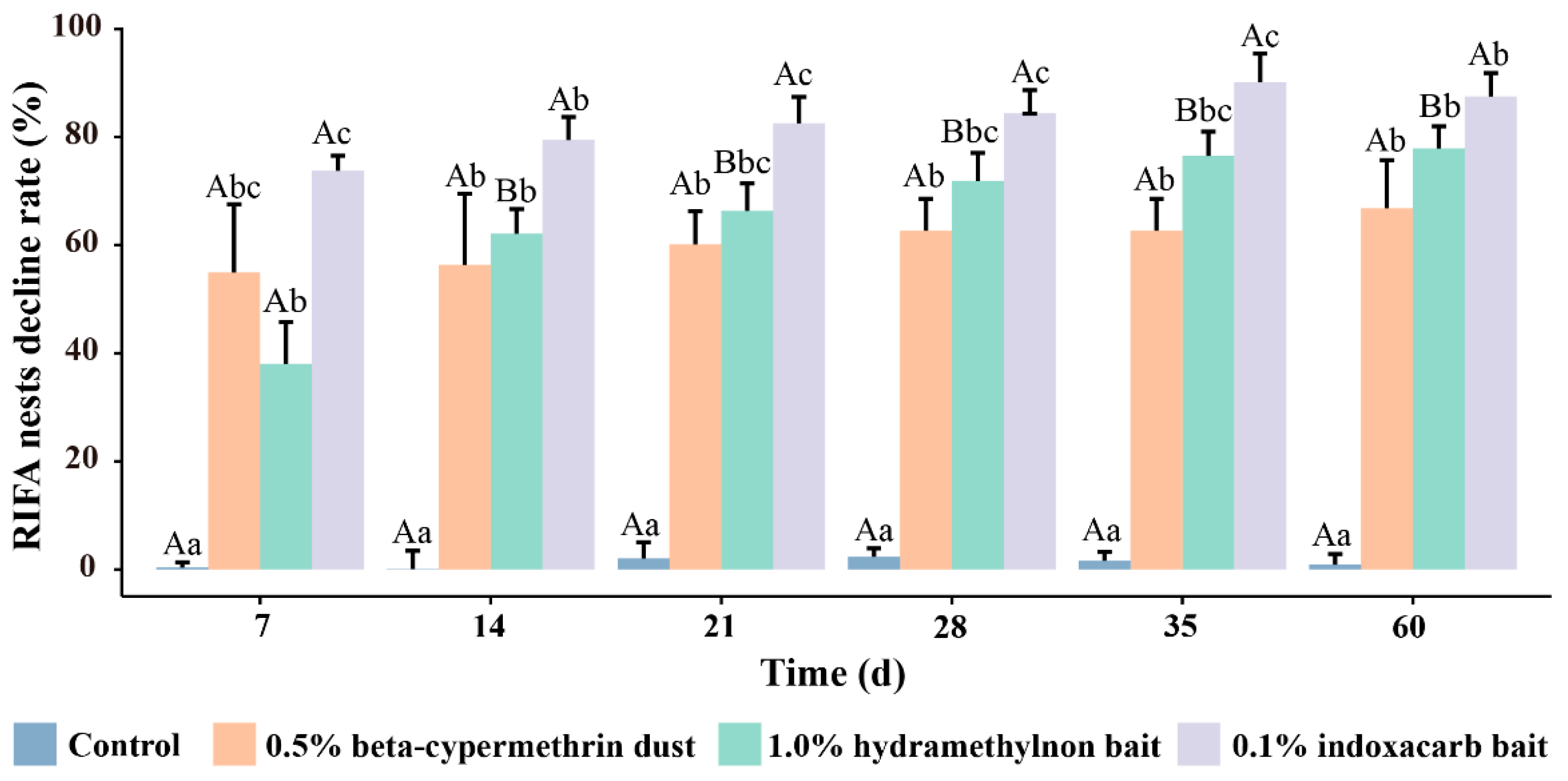
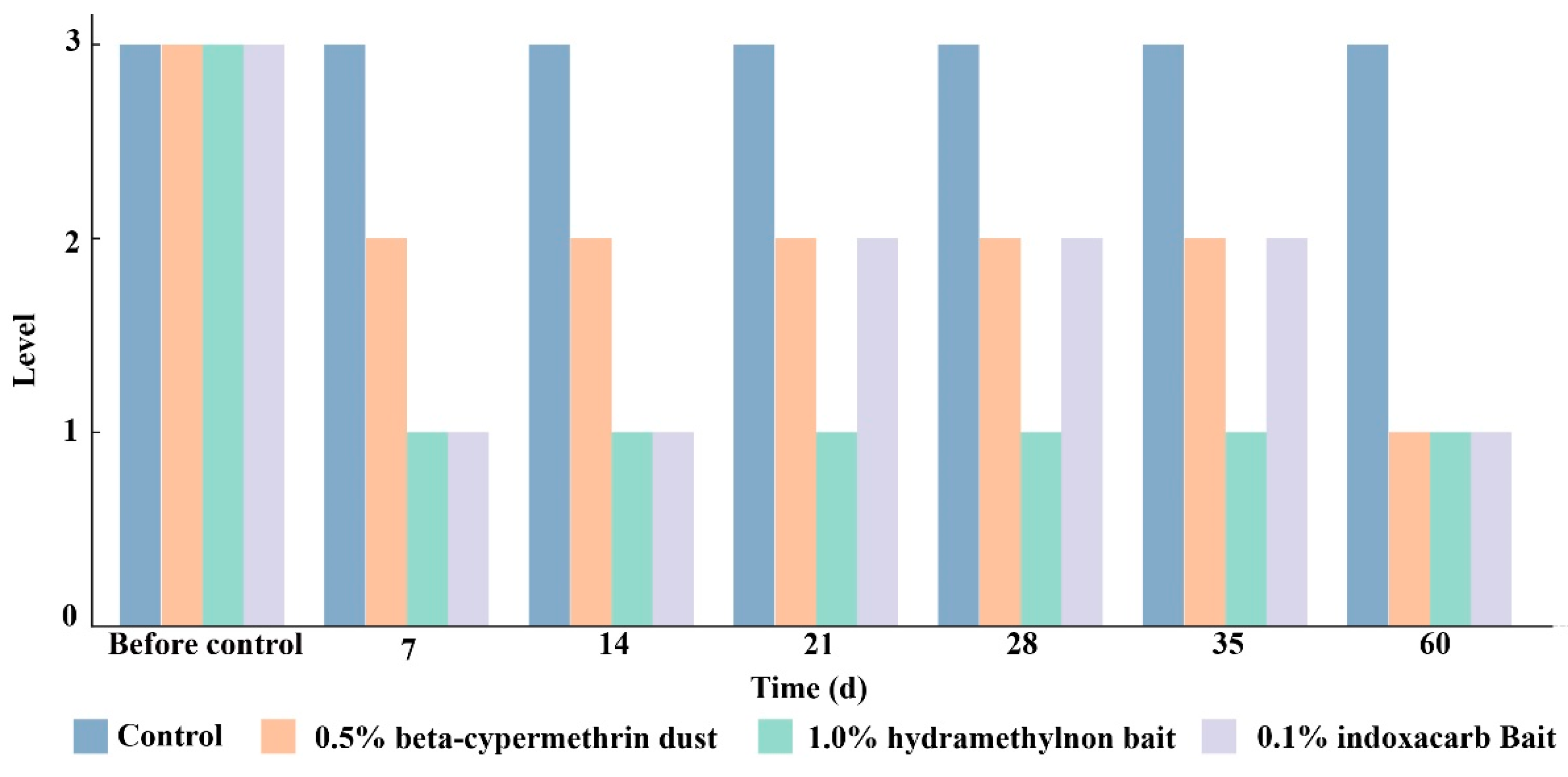
3.2. Control Effects of Three Pesticides on RIFA Nests
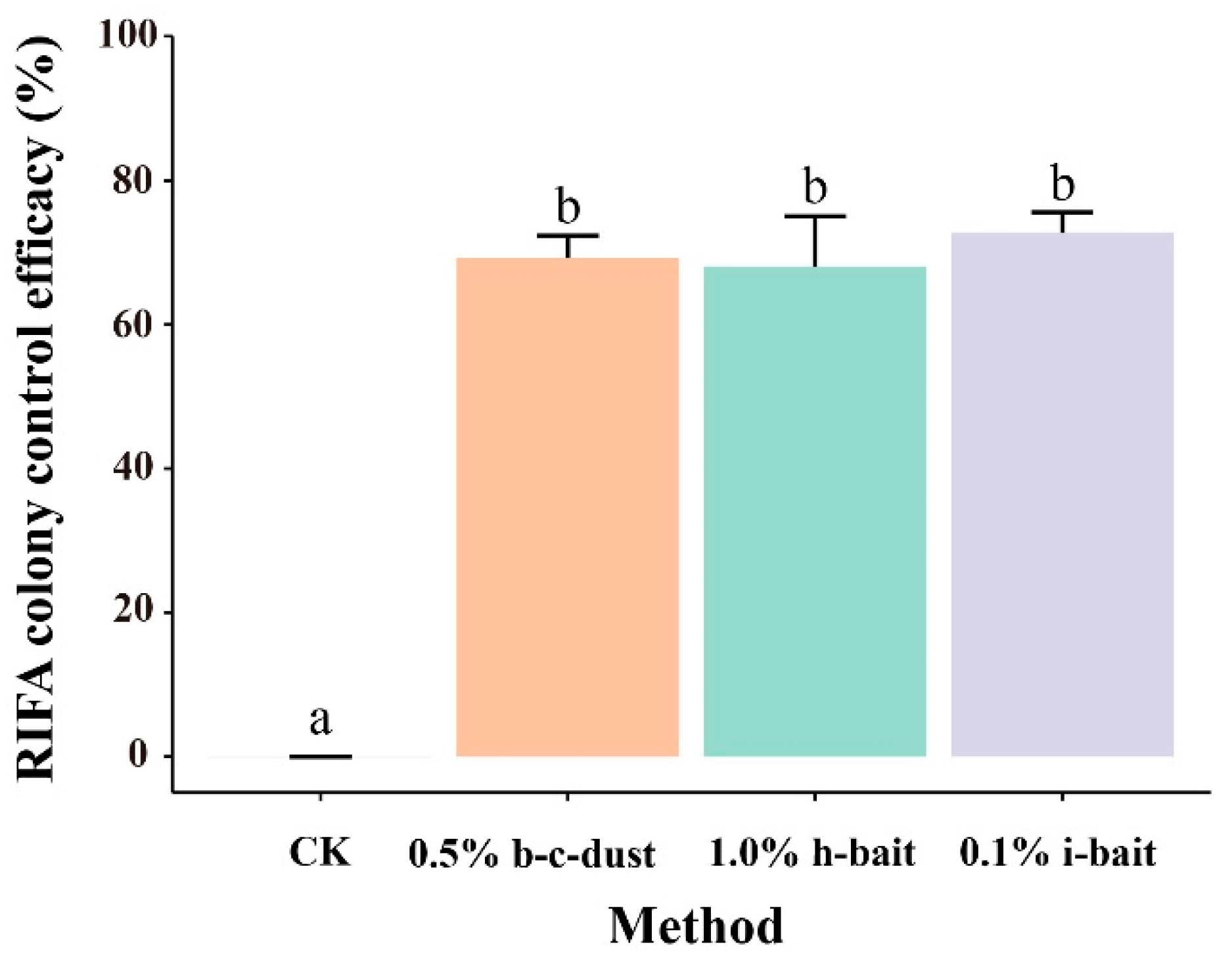
3.3. Effects of Three Pesticides on the Control of RIFA Colonies
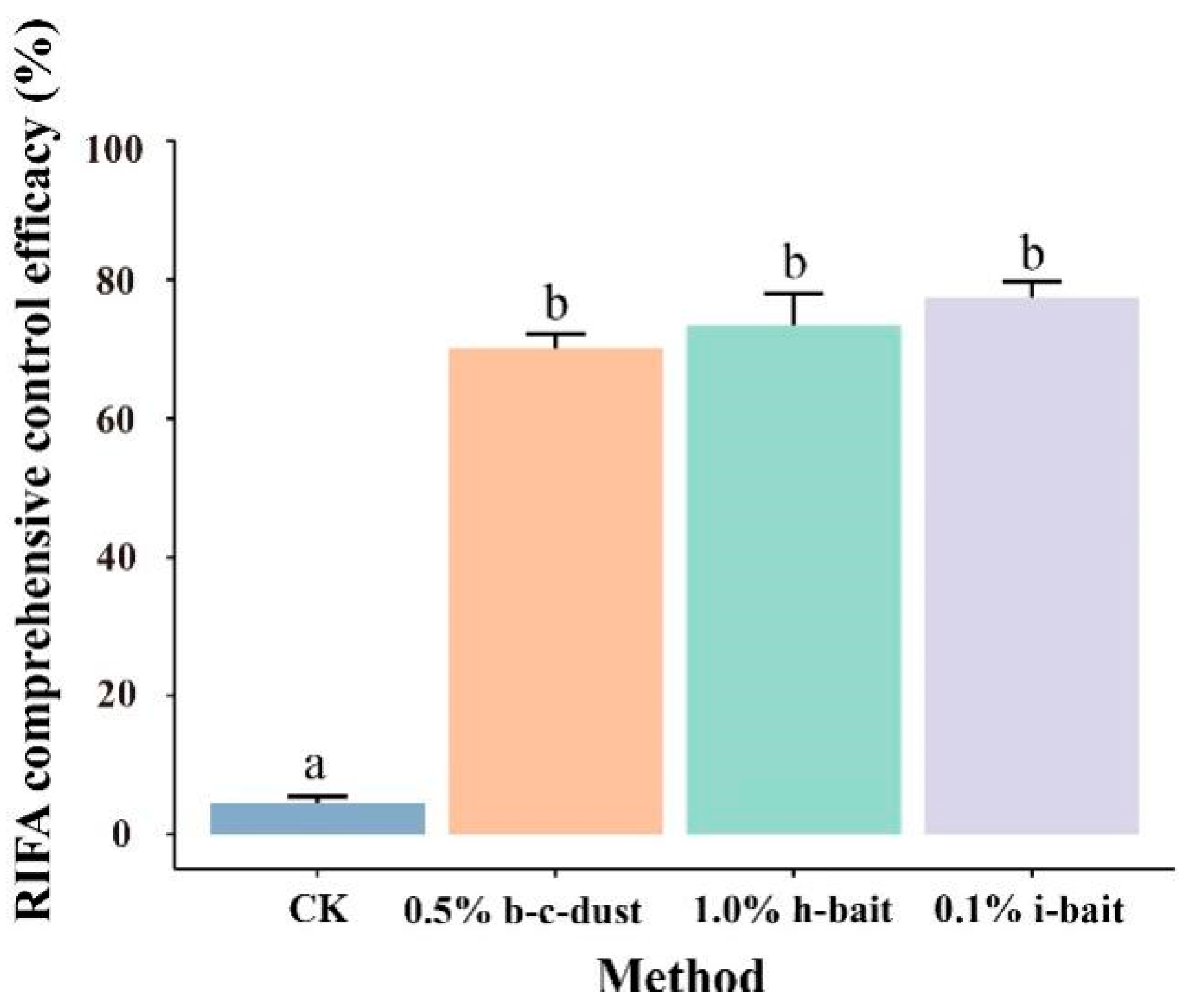
3.4. Comprehensive Control Effect of Three Pesticides
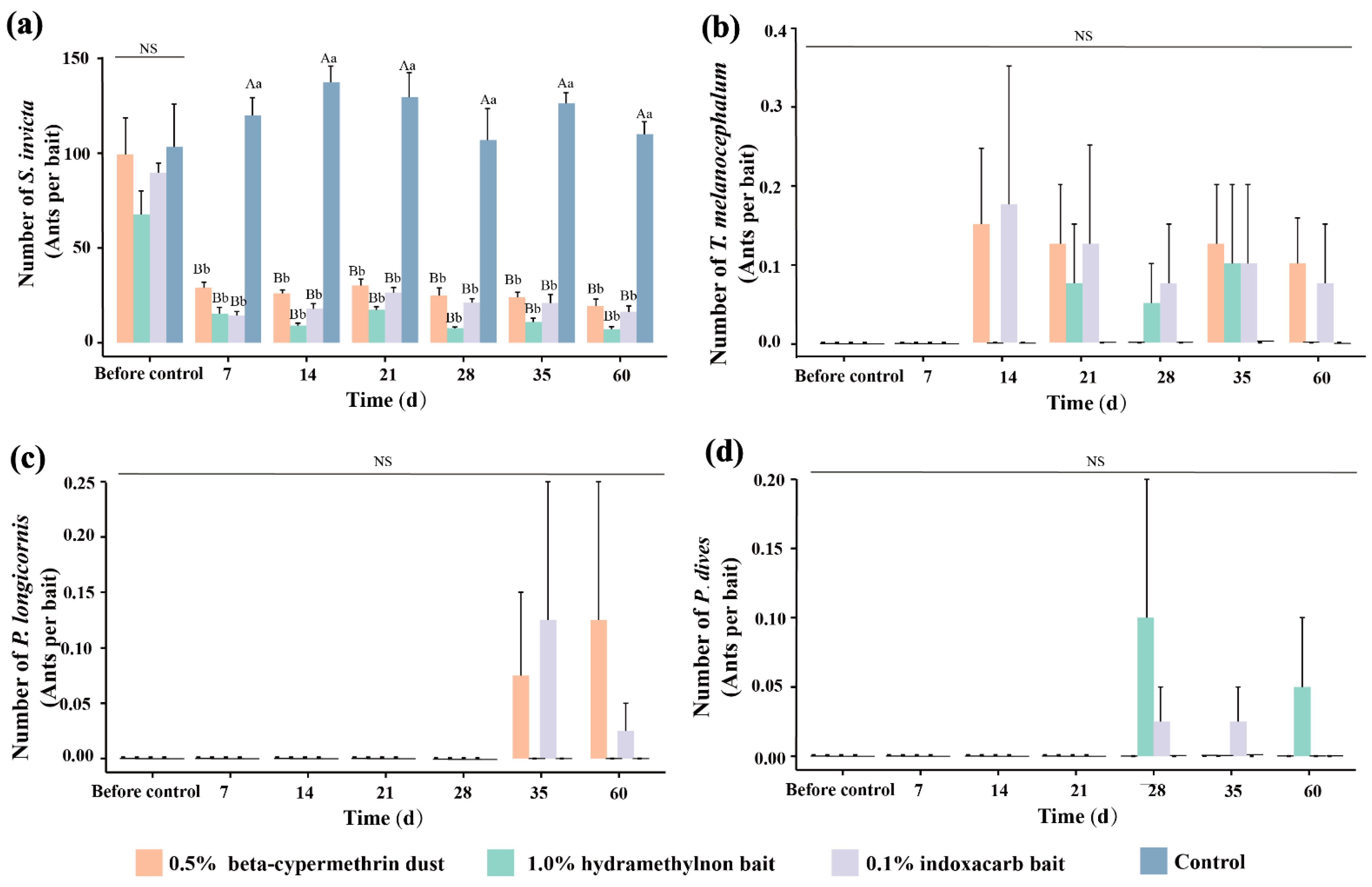
3.5. Effects of Three Pesticides on Ant Species
3.6. Change Incommunity Characteristic Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, L.R., Jr.; Van der Meer, R.K.; Porter, S.D. Red imported fire ants expand their range across the west indies. Florida Entomologist. 2001, 84, 735–736. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Maywald, G. A climate model of the red imported fire ant, Solenopsis invicta buren (hymenoptera: Formicidae): Implications for invasion of new regions, particularly oceania. Environ. Entomol. 2005, 34, 317–335. [Google Scholar] [CrossRef]
- Zeng, L.; Lu, Y.Y.; He, X.F.; Zhang, W.Q.; Liang, G.W. Identification of red imported fire ant, Solenopsis invicta, to invade mainland china and infestation in wuchuan, guangdong. Chin. Bull. Entomol. 2005, 42, 144–148, 230–231. [Google Scholar]
- Zhang, R.Z.; Li, Y.C.; Liu, N.; Porter, S.D. An overview of the red imported fire ant (hymenoptera: Formicidae) in mainland china. Fla. Entomol. 2007, 90, 723–731. [Google Scholar] [CrossRef]
- Sung, S.; Kwon, Y.S.; Lee, D.K.; Cho, Y. Predicting the potential distribution of an invasive species, Solenopsis invicta buren (hymenoptera: Formicidae), under climate change using species distribution models. Entomol. Res. 2018, 48, 505–513. [Google Scholar] [CrossRef]
- Lin, C.H.; Wen, T.H.; Liu, Y.H.; Huang, R.N.; Liu, H.K.H. Elucidating how the red imported fire ant (Solenopsis invicta) diffused spatiotemporally among different landscapes in north taiwan, 2008–2015. Ecol. Evol. 2021, 11, 18604–18614. [Google Scholar] [CrossRef]
- Menchetti, M.; Schifani, E.; Alicata, A.; Cardador, L.; Sbrega, E.; Toro-Delgado, E.; Vila, R. The invasive ant Solenopsis invicta is established in europe. Curr. Biol. 2023, 33, R896–R897. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.T.; Banks, W.A.; Lofgren, C.S.; Smittle, B.J.; Harlan, D.P. Impact of the red imported fire ant, Solenopsis invicta (hymenoptera: Formicidae), on the growth and yield of soybeans. J. Econ. Entomol. 1983, 76, 1129–1132. [Google Scholar] [CrossRef]
- Giuliano, W.M.; Allen, C.R.; Lutz, R.S.; Demarais, S. Effects of red imported fire ants on northern bobwhite chicks. J. Wildl. Manag. 1996, 60, 309–313. [Google Scholar] [CrossRef]
- Gutrich, J.J.; VanGelder, E.; Loope, L. Potential economic impact of introduction and spread of the red imported fire ant, Solenopsis invicta, in hawaii. Environ. Sci. Policy 2007, 10, 685–696. [Google Scholar] [CrossRef]
- Vinson, S.B. Impact of the invasion of the imported fire ant. Insect Sci. 2013, 20, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Hung, S.H.; Lin, X.R.; Huang, R.N. Herbal plants as alternatives for the management of the red imported fire ant, Solenopsis invicta. J. Appl. Entomol. 2022, 146, 975–989. [Google Scholar] [CrossRef]
- Malone, M.; Ivanov, K.; Taylor, S.V.; Schürch, R. Fast range expansion of the red imported fire ant in virginia and prediction of future spread in the united states. Ecosphere 2023, 14, e4652. [Google Scholar] [CrossRef]
- Liu, Y.S.; Huang, S.A.; Lin, I.L.; Lin, C.C.; Lai, H.K.; Yang, C.H.; Huang, R.N. Establishment and social impacts of the red imported fire ant, Solenopsis invicta, (hymenoptera: Formicidae) in taiwan. Int. J. Environ. Res. Public Health 2021, 18, 5055. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zeng, L.; Xu, Y.J.; Liang, G.W.; Wang, L. Research progress of invasion biology and management of red imported fire ant. J. South China Agric. Univ. 2019, 40, 149–160. [Google Scholar] [CrossRef]
- Wu, P.; Dong, Y.J.; Chen, J.H.; Yang, W.G.; Yao, J.P. Analysis of the reasons for the difficulty in controlling red imported fire ants. Agric. Disaster Res. 2023, 13, 1–3. [Google Scholar]
- Jin, Y.H.; Ji, D.C.; Xiong, Z.P. Current status of research on red imported fire ant control. Plant Quar. 2023, 37, 22–26. [Google Scholar] [CrossRef]
- Huang, Y.T. The Impact of Flavor Enhancers on the Survival and Behavior of Red Imported Fire Ants. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2017. [Google Scholar]
- Stringer, C.E., Jr.; Lofgren, C.S.; Bartlett, F.J. Imported fire ant toxic bait studies: Evaluation of toxicants. J. Econ. Entomol. 1964, 57, 941–945. [Google Scholar] [CrossRef]
- Drees, B.M.; Frisbie, R. Overview of the texas imported fire ant research and management project. Southwest. Entomol. 2001, 25, 1–5. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20033011280 (accessed on 5 October 2024).
- Vogt, J.T.; Shelton, T.G.; Merchant, M.E.; Russell, S.A.; Tanley, M.J.; Appel, A.G. Efficacy of three citrus oil formulations against Solenopsis invicta buren (hymenoptera: Formicidae), the red imported fire ant. J. Agric. Urban Entomol. 2002, 19, 159–171. [Google Scholar]
- Guo, W.J. The Contact Toxicity of Indoxacarb on Red Imported Fire Ants and the Foraging Study of its Bait. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2016. [Google Scholar]
- Verza, S.S.; Diniz, E.A.; Chiarelli, M.F.; Mussury, R.M.; Bueno, O.C. Waste of leaf-cutting ants: Disposal, nest structure, and abiotic soil factors around internal waste chambers. Acta Ethologica 2017, 20, 119–126. [Google Scholar] [CrossRef]
- Sun, Q.; Haynes, K.F.; Zhou, X.G. Dynamic changes in death cues modulate risks and rewards of corpse management in a social insect. Funct. Ecol. 2017, 31, 697–706. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20173184599 (accessed on 5 October 2024). [CrossRef]
- Pan, F.X. Toxicity Determination of Multiple Pesticides on Red Imported Fire Ants and Evaluation of Bait Efficacy. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2017. [Google Scholar]
- Morrill, W.L. Red imported fire ant [Solenopsis invicta] control with mound drenches [insect pests]. J. Econ. Entomol. 1976, 69, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zeng, L.; Liang, G.W.; Lu, Y.Y.; Xu, Y.J.; Gao, Y.B.; Zhang, Q.T.; Zhang, S.Q.; Yang, H.Z.; Chen, Z.N.; et al. Demonstration of eradication technology for red imported fire ant. Chin. J. Plant Prot. 2007, 8, 41–43. [Google Scholar]
- Tan, D.L.; Li, X.; Zeng, L.; Xu, Y.J. Evaluation of the field control effect of beta-cyfluthrin microcapsule suspension and the two-stage method on red imported fire ants. J. Environ. Entomol. 2015, 37, 1227–1231. [Google Scholar]
- Wen, C.; Shen, L.M.; Chen, J.; Zhang, J.L.; Feng, Y.; Wang, Z.; Chen, X.; Cai, J.C.; Wang, L.; He, Y.H. Red imported fire ants cover the insecticide-treated surfaces with particles to reduce contact toxicity. J. Pest Sci. 2022, 95, 1135–1150. [Google Scholar] [CrossRef]
- Greenberg, L.; Martinez, M.; Tilzer, A.; Nelson, K.; Koenig, S.; Cummings, R. Comparison of different protocols for control of the red imported fire ant, Solenopsis invicta buren (hymenoptera: Formicidae), in orange county, california, including a list of co-occurring ants. Southwest. Entomol. 2015, 40, 297–305. [Google Scholar] [CrossRef]
- Plentovich, S.; Swenson, C.; Reimer, N.; Richardson, M.; Garon, N. The effects of hydramethylnon on the tropical fire ant, solenopsis geminata (hymenoptera: Formicidae), and non-target arthropods on spit island, midway atoll, hawaii. J. Insect Conserv. 2010, 14, 459–465. [Google Scholar] [CrossRef]
- Zakharov, A.A.; Thompson, L.C. Effects of repeated use of fenoxycarb and hydramethylnon baits on nontarget ants. J. Entomol. Sci. 1998, 33, 212–220. [Google Scholar] [CrossRef]
- Batista, C.H.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Indoxacarb effects on non-target predator, podisus distinctus (hemiptera: Pentatomidae). Environ. Sci. Pollut. Res. 2022, 29, 29967–29975. [Google Scholar] [CrossRef]
- GB/T 23626-2009; Guidelines for Quarantine Surveillance of Solenopsis invicta Buren. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China; China National Standardization Management Committee: Beijing, China, 2009.
- Song, Z.D.; Xu, Y.J.; Lu, Y.Y.; Huang, J.; Zeng, L. Impact of chemical control on red imported fire ants and native ants in green belts. Acta Ecol. Sin. 2009, 29, 6148–6155. [Google Scholar]
- GB/T 17980.149-2009; Pesticide—Guidelines for the Field Efficacy Trials(2)—Part 149: Insecticides Against Solenopsis invicta Buren. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China; China National Standardization Management Committee: Beijing, China, 2009.
- Ward, D.F.; New, T.R.; Yen, A.L. Effects of pitfall trap spacing on the abundance, richness and composition of invertebrate catches. J. Insect Conserv. 2001, 5, 47–53. [Google Scholar] [CrossRef]
- Hohbein, R.R.; Conway, C.J. Pitfall traps: A review of methods for estimating arthropod abundance. Wildl. Soc. Bull. 2018, 42, 597–606. [Google Scholar] [CrossRef]
- Chen, Z.L.; YU, Z.M.; Zhou, S.Y. Guangxi Huaping Ant Atlas; China Scientific Book Services Co. Ltd.: Beijing, China, 2021. [Google Scholar]
- Zhou, S.Y.; Chen, Z.L. A Photographic Ecological Guide to Common ants in China; Henan Science and Technology Publishing House: Zhengzhou, China, 2020. [Google Scholar]
- Chan, K.H.; Guénard, B. Ecological and socio-economic impacts of the red import fire ant, Solenopsis invicta (hymenoptera: Formicidae), on urban agricultural ecosystems. Urban Ecosyst. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Cook, J.L. Conservation of biodiversity in an area impacted by the red imported fire ant, Solenopsis invicta (hymenoptera: Formicidae). Biodivers. Conserv. 2003, 12, 187–195. [Google Scholar] [CrossRef]
- Drees, B.M.; Gold, R.E. Development of integrated pest management programs for the red imported fire ant (hymenoptera: Formicidae). J. Entomol. Sci. 2003, 38, 170–180. [Google Scholar] [CrossRef]
- Eubanks, M.D. Estimates of the direct and indirect effects of red imported fire ants on biological control in field crops. Biol. Control 2001, 21, 35–43. [Google Scholar] [CrossRef]
- Gruber, M.A.M.; Janssen-May, S.; Santoro, D.; Cooling, M.; Wylie, R. Predicting socio-economic and biodiversity impacts of invasive species: Red imported fire ant in the developing western pacific. Ecol. Manag. Restor. 2021, 22, 89–99. [Google Scholar] [CrossRef]
- Zhou, M. Trial and application demonstration of integrated innovation model for red imported fire ant control technology. Guangxi Plant Prot. 2023, 36, 19–22. [Google Scholar]
- Chen, J.; Zhang, W.J.; Yin, Q.X.; Ning, Q.L. Demonstration of the effectiveness of pesticide control of red imported fire ants. Guangxi Plant Prot. 2020, 33, 26–28. [Google Scholar]
- Morrison, L.W. Mechanisms of interspecific competition among an invasive and two native fire ants. Oikos 2000, 90, 238–252. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Arnett, A.E. Biogeographic effects of red fire ant invasion. Ecol. Lett. 2000, 3, 257–261. [Google Scholar] [CrossRef]
- Zhang, B.; Lv, L.H.; Chen, J.; Gao, Y.; Zhong, F.; Qi, G.J.; He, Y.R. Study on the food composition of red imported fire ants in mulberry fields and wastelands in south china. Guangdong Agric. Sci. 2012, 39, 83–86. [Google Scholar] [CrossRef]
- Liu, J.; Qi, G.J.; Lv, L.H.; HE, Y.R. Impact of indoxacarb on the diversity of ant communities in green spaces for controlling red imported fire ants. J. Appl. Entomol. 2015, 52, 1385–1391. [Google Scholar]
- Roura-Pascual, N.; Bas, J.M.; Hui, C. The spread of the argentine ant: Environmental determinants and impacts on native ant communities. Biol. Invasions 2010, 12, 2399–2412. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Wu, B.Q.; Xu, Y.J.; Zeng, L. Effects of red imported fire ants (Solenopsis invicta) on the species structure of ant communities in south china. Sociobiology 2012, 59, 275–285. [Google Scholar] [CrossRef]
- Chen, J. Study on the Recovery of Arthropod Diversity in Different Habitats after the Decline of Red Imported Fire Ants. Master’s Thesis, Fujian Agricultural and Forestry University, Fuzhou, China, 2008. [Google Scholar]
- Eubanks, M.D.; Blackwell, S.A.; Parrish, C.J.; Delamar, Z.D.; Hull-Sanders, H. Intraguild predation of beneficial arthropods by red imported fire ants in cotton. Environ. Entomol. 2002, 31, 1168–1174. [Google Scholar] [CrossRef]
- Liang, M.R.; Liu, Z.; Chen, T.; Yue, X.L.; Song, S.C.; Jiao, Y.T.; Lu, Y.Y.; Xu, Y.J.; Wang, L. Evaluation of the control effect of three plant-derived pesticides on red imported fire ants and their impact on local ant communities. J. Environ. Entomol. 2022, 44, 1569–1581. [Google Scholar]
- Porter, S.D.; Savignano, D.A. Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 1990, 71, 2095–2106. [Google Scholar] [CrossRef]
- Gibbons, L.; Simberloff, D. Interaction of hybrid imported fire ants (Solenopsis invicta× s. Richteri) with native ants at baits in southeastern tennessee. Southeast. Nat. 2005, 4, 303–320. [Google Scholar] [CrossRef]
- Stuble, K.L.; Kirkman, L.K.; Carroll, C.R. Patterns of abundance of fire ants and native ants in a native ecosystem. Ecol. Entomol. 2009, 34, 520–526. [Google Scholar] [CrossRef]
- King, J.R.; Tschinkel, W.R. Experimental evidence that human impacts drive fire ant invasions and ecological change. Proc. Natl. Acad. Sci. USA 2008, 105, 20339–20343. [Google Scholar] [CrossRef] [PubMed]



| Species | Indigenous Species/Alien Species | Control Area | Treatment Zones | ||
|---|---|---|---|---|---|
| 0.5% Beta-Cypermethrin Dust | 1.0% Hydramethylnon Bait | 0.1% Indoxacarb Bait | |||
| Tapinoma melanocephalum(Fabricius) | Alien species | - | + | + | + |
| Polyrhachis dives (Smith) | Indigenous species | - | - | + | + |
| Paratrechina longicornis (Latreille) | Alien species | - | + | + | + |
| Solenopsis invicta (Buren) | Alien species | + | + | + | + |
| Total | 1 | 3 | 4 | 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Chen, M.; Wu, J.; Hong, J.; Ouyang, T.; Liang, Y.; Liang, M.; Lu, Y. Impact on Ant Communities by Chemical Pesticides Applied in Controlling the Red Imported Fire Ant (Solenopsis invicta Buren) in the Field. Insects 2024, 15, 876. https://doi.org/10.3390/insects15110876
Song Y, Chen M, Wu J, Hong J, Ouyang T, Liang Y, Liang M, Lu Y. Impact on Ant Communities by Chemical Pesticides Applied in Controlling the Red Imported Fire Ant (Solenopsis invicta Buren) in the Field. Insects. 2024; 15(11):876. https://doi.org/10.3390/insects15110876
Chicago/Turabian StyleSong, Yunbo, Meng Chen, Jiarui Wu, Jingxin Hong, Ting Ouyang, Yuling Liang, Mingrong Liang, and Yongyue Lu. 2024. "Impact on Ant Communities by Chemical Pesticides Applied in Controlling the Red Imported Fire Ant (Solenopsis invicta Buren) in the Field" Insects 15, no. 11: 876. https://doi.org/10.3390/insects15110876
APA StyleSong, Y., Chen, M., Wu, J., Hong, J., Ouyang, T., Liang, Y., Liang, M., & Lu, Y. (2024). Impact on Ant Communities by Chemical Pesticides Applied in Controlling the Red Imported Fire Ant (Solenopsis invicta Buren) in the Field. Insects, 15(11), 876. https://doi.org/10.3390/insects15110876






