Wild Bee Diversity and Bee–Plant Interactions in Tropical and Temperate Forest Clearings in a Natural Protected Area in Central West Mexico
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
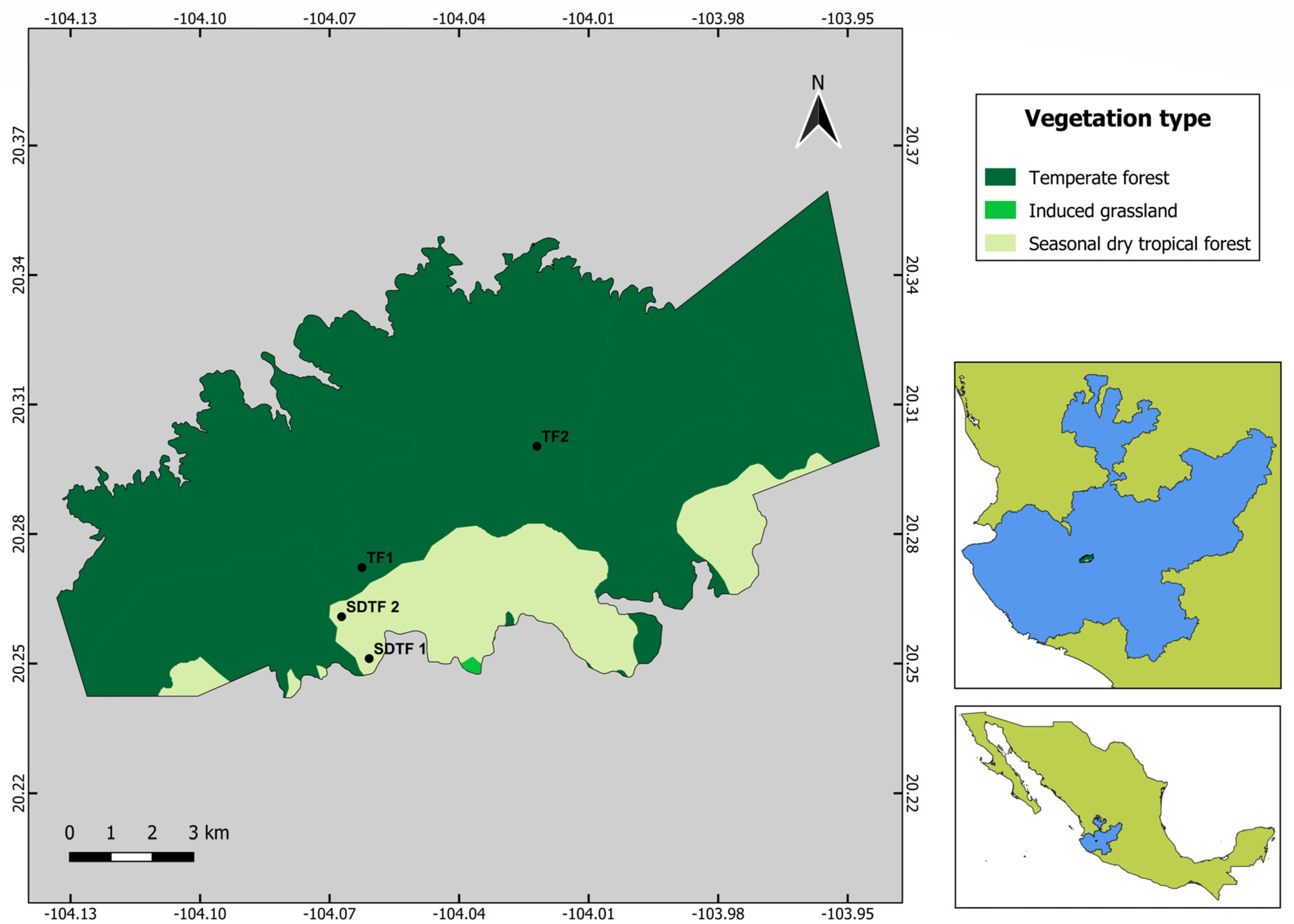
2.1. Description of the Study Area
2.2. Data Collection
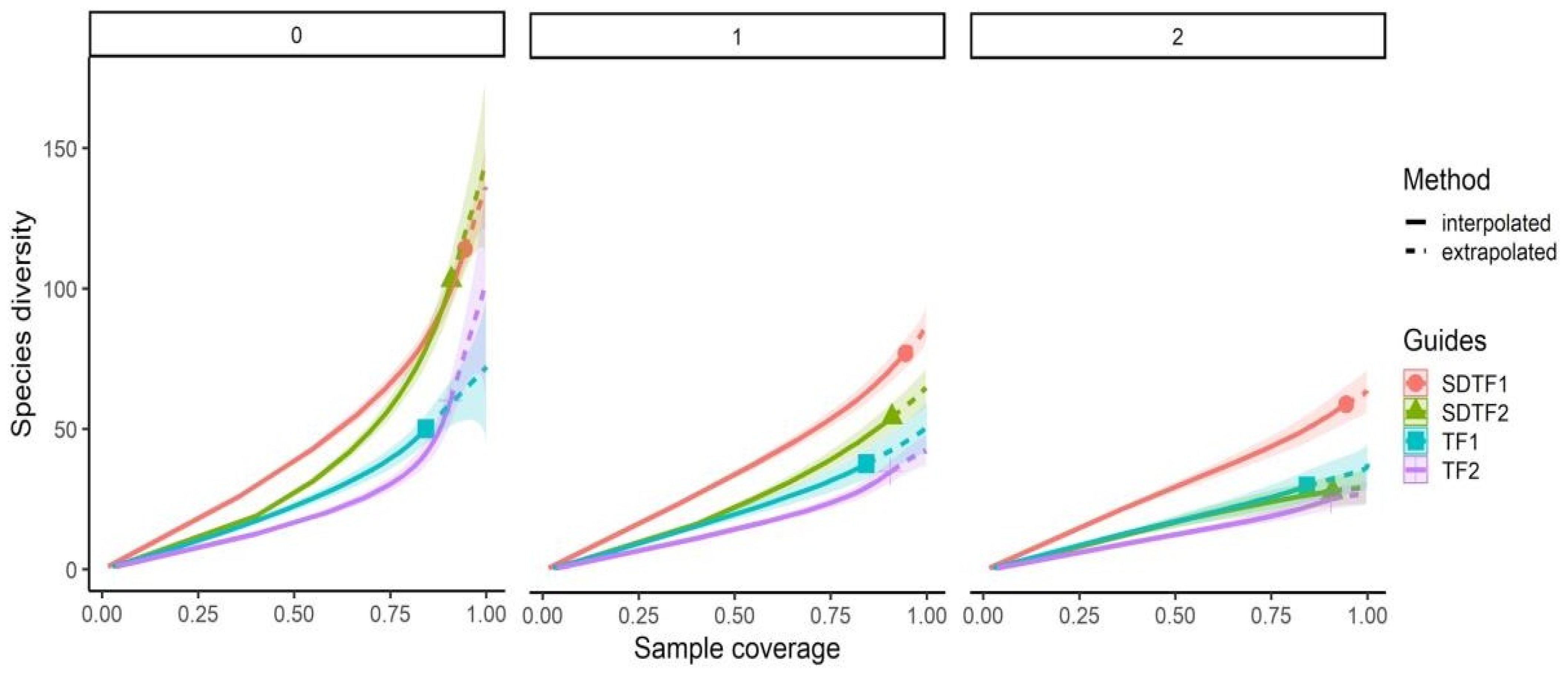
2.3. Data Analyses
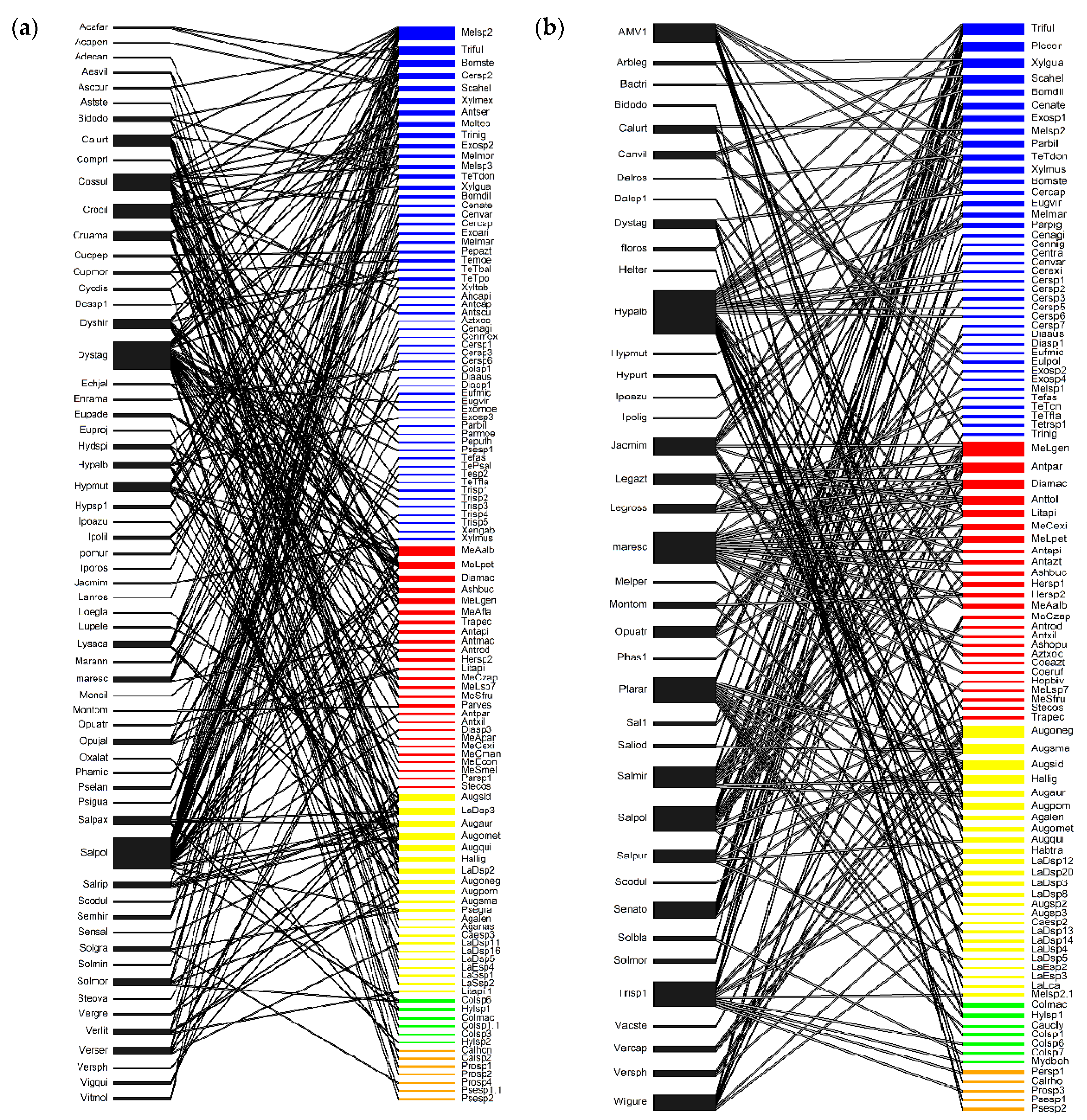
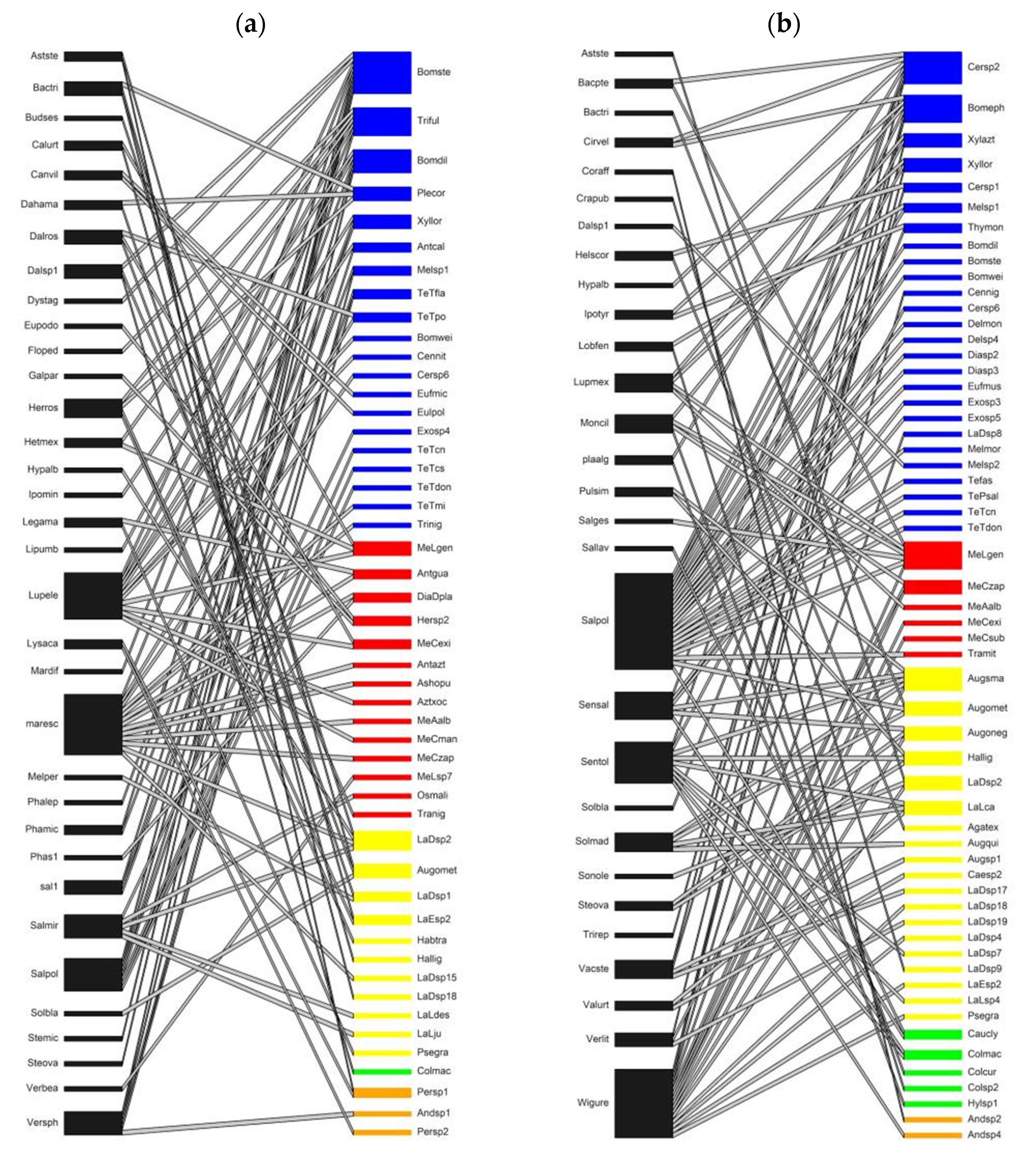
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyes-Novelo, E.; Méléndez, V.R.; Ayala, R.; Delfín, H.G. Bee faunas (Hymenoptera: Apoidea) of six natural protected areas in Yucatan, Mexico. Entomol. News 2009, 120, 530–544. [Google Scholar] [CrossRef]
- Nantes-Parra, G. Abejas silvestres y polinización. Manejo Integr. Plagas Agroecol. 2005, 75, 7–20. [Google Scholar]
- Jordano, P.; Vázquez, D.; Bascompte, J. Networks of Mutualistic Plant-Animal Interactions. In Ecology and Evolution of Plant-Animal Interactions: Concepts and Applications; Medel, R., Aizen, M.A., Zamora, R., Eds.; Editorial Universitaria: Santiago de Chile, Chile, 2009; pp. 17–42. [Google Scholar]
- Biesmeijer, J.C.; Roberts, S.P.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Winfree, R.; Aguilar, R.; Vázquez, D.P.; LeBuhn, G.; Aizen, M.A. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 2009, 90, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Martins, A.C.; Gonçalves, R.B.; Melo, G.A. Changes in wild bee fauna of a grassland in Brazil reveal negative effects associated with growing urbanization during the last 40 years. Zoologia 2013, 30, 157–176. [Google Scholar] [CrossRef]
- van der Sluijs, J.P.; Vaage, N.S. Pollinators and global food security: The need for holistic global stewardship. Food Ethics 2016, 1, 75–91. [Google Scholar] [CrossRef]
- Nantes-Parra, G.; Palacios, E.; Parra, A. Efecto del cambio del paisaje en la estructura de la comunidad de abejas sin aguijón (Hymenoptera: Apidae) en Meta, Colombia. Rev. Biol. Trop. 2008, 56, 1295–1308. [Google Scholar]
- Widhiono, I.; Sudiana, E.; Sucianto, E.T. Insect pollinator diversity along a habitat quality gradient on Mount Slamet, Central Java, Indonesia. Biodiversitas 2016, 17, 746–752. [Google Scholar] [CrossRef]
- Alvarenga, A.S.; Silveira, F.A.; dos Santos Júnior, J.E.; de Novais, S.M.A.; Quesada, M.; Neves, F.D.S. Vegetation composition and structure determine wild bee communities in a tropical dry forest. J. Insect Conserv. 2020, 24, 487–498. [Google Scholar] [CrossRef]
- Winfree, R.; Kremen, C. Are ecosystem services stabilized by differences among species? A test using crop pollination. Proc. R. Soc. B 2009, 276, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.M.; Peters, V.E.; Rehan, S.M. Tropical bee species abundance differs within a narrow elevational gradient. Sci. Rep. 2021, 11, 23368. [Google Scholar] [CrossRef]
- Hoiss, B.; Krauss, J.; Potts, S.G.; Roberts, S.; Steffan-Dewenter, I. Altitude Acts as an Environmental Filter on Phylogenetic Composition, Traits and Diversity in Bee Communities. Proc. R. Soc. B 2012, 279, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Perillo, L.N.; Neves, F.D.S.; Antonini, Y.; Martins, R.P. Compositional changes in bee and wasp communities along Neotropical mountain altitudinal gradient. PLoS ONE 2017, 12, e0182054. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Canadas, S.; Flores-Hernández, N.; Sánchez-Ortiz, T.; Valiente-Banuet, A. Changes in the structure and composition of the ‘Mexical’ scrubland bee community along an elevational gradient. PLoS ONE 2021, 16, e0254072. [Google Scholar] [CrossRef] [PubMed]
- Perillo, L.N.; Castro, F.S.D.; Solar, R.; Neves, F.D.S. Disentangling the effects of latitudinal and elevational gradients on bee, wasp, and ant diversity in an ancient neotropical mountain range. J. Biogeogr. 2021, 48, 1564–1578. [Google Scholar] [CrossRef]
- Classen, A.; Steffan-Dewenter, I.; Kindeketa, W.J.; Peters, M.K. Integrating intraspecific variation in community ecology unifies theories on body size shifts along climatic gradients. Funct. Ecol. 2017, 31, 768–777. [Google Scholar] [CrossRef]
- Arnold, S.E.; Savolainen, V.; Chittka, L. Flower colours along an alpine altitude gradient, seen through the eyes of fly and bee pollinators. Arthropod-Plant Interact. 2009, 3, 27–43. [Google Scholar] [CrossRef]
- Sponsler, D.B.; Requier, F.; Kallnik, K.; Classen, A.; Maihoff, F.; Sieger, J.; Steffan-Dewenter, I. Contrasting patterns of richness, abundance, and turnover in mountain bumble bees and their floral hosts. Ecology 2022, 103, e3712. [Google Scholar] [CrossRef] [PubMed]
- Ayala, R.; Griswold, T.L.; Yanega, D. Apoidea (Hymenoptera). In Biodiversidad, Taxonomía, y Biogeografía de Artrópodos de México: Hacia una Síntesis de su Conocimiento; Llorente, J., García, A., González, E., Eds.; UNAM-CONABIO: Ciudad de México, Mexico, 1996; pp. 423–464. [Google Scholar]
- Escobedo Escobedo-Kenefic, N.; Landaverde-González, P.; Theodorou, P.; Cardona, E.; Dardón, M.J.; Martínez, O.; Domínguez, C.A. Disentangling the effects of local resources, landscape heterogeneity and climatic seasonality on bee diversity and plant-pollinator networks in tropical highlands. Oecologia 2020, 194, 333–344. [Google Scholar] [CrossRef]
- Vizentin Vizentin-Bugoni, J.; Maruyama, P.K.; de Souza, C.S.; Ollerton, J.; Rech, A.R.; Sazima, M. Plant-pollinator networks in the tropics: A review. In Ecological Networks in the Tropics: An Integrative Overview of Species Interactions from Some of the Most Species-Rich Habitats on Earth; Dáttilo, W., Rico-Gray, V., Eds.; Springer: Cham, Switzerland, 2018; pp. 73–91. [Google Scholar] [CrossRef]
- Dalmazzo, M. Diversidad y aspectos biológicos de abejas silvestres de un ambiente urbano y otro natural de la región central de Santa Fe, Argentina. Rev. Soc. Entomol. Argent. 2010, 69, 33–44. [Google Scholar]
- Ayala, R. Fauna de abejas silvestres (Hymenoptera: Apoidea). In Artrópodos de Chamela; García, A.N., Ayala, R., Eds.; UNAM: Ciudad de México, Mexico, 2004; pp. 193–219. [Google Scholar]
- Trejo, I.; Dirzo, R. Deforestation of seasonally dry tropical forest: A national and local analysis in Mexico. Biol. Conserv. 2000, 94, 133–142. [Google Scholar] [CrossRef]
- Galicia, L.; Zarco-Arista, A.E. Multiple ecosystem services, possible trade-offs and synergies in a temperate forest ecosystem in Mexico: A review. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2014, 5, 275–288. [Google Scholar] [CrossRef]
- Hill, R.; Nates-Parra, G.; Quezada-Euán, J.J.G.; Buchori, D.; LeBuhn, G.; Maués, M.M.; Roué, M. Biocultural approaches to pollinator conservation. Nat. Sustain. 2019, 2, 214–222. [Google Scholar] [CrossRef]
- Villavicencio, R.; Ávila, C.R. Mapa de uso de Suelo y Vegetación y Zona de Influencia del Área Natural Protegida Sierra de Quila; Final Technical Report; Universidad de Guadalajara: Guadalajara, Mexico, 2015. [Google Scholar]
- Potts, S.G.; Vulliamy, B.; Dafni, A.; Ne’eman, G.; Willmer, P. Linking bees and flowers: How do floral communities structure pollinator communities? Ecology 2003, 84, 2628–2642. [Google Scholar] [CrossRef]
- Michener, C.D.; McGinley, R.J.; Danforth, B.N. The Bee Genera of North and Central America (Hymenoptera: Apoidea); Smithsonian Institution Press: Washington, DC, USA, 1994; pp. 1–208. [Google Scholar]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: Interpolation and Extrapolation for Species Diversity. R package, Version 2.0.5. Available online: https://github.com/JohnsonHsieh (accessed on 14 July 2023).
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 14 July 2023).
- Dormann, C.F.; Gruber, B.; Freund, J. Introducing the bipartite Package: Analyzing Ecological Networks. Interaction 2008, 8, 8–11. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 14 July 2024).
- Jordano, P. Patterns of mutualistic interactions in pollination and seed dispersal: Connectance, dependence asymmetries, and coevolution. Am. Nat. 1987, 129, 657–677. [Google Scholar] [CrossRef]
- Rodríguez-Gironés, M.A.; Santamaría, L. A new algorithm to calculate the nestedness temperature of presence-absence matrices. J. Biogeogr. 2006, 33, 924–935. [Google Scholar] [CrossRef]
- Blüthgen, N.; Menzel, F.; Hovestadt, T.; Fiala, B.; Blüthgen, N. Specialization, constraints, and conflicting interests in mutualistic networks. Curr. Biol. 2007, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Novotny, V. Beta diversity of plant-insect food webs in tropical forests: A conceptual framework. Insect Conserv. Divers. 2009, 2, 5–9. [Google Scholar] [CrossRef]
- Poisot, T.; Canard, E.; Mouillot, D.; Mouquet, N.; Gravel, D.; Jordan, F. The dissimilarity of species interaction networks. Ecol. Lett. 2012, 15, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Michener, C.D. The Bees of the World, 1st ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2000; pp. 1–830. [Google Scholar]
- Potts, S.G.; Vulliamy, B.; Dafni, A.; Ne’eman, G.; O’Toole, C.; Roberts, S.; Willmer, P. Response of plant-pollinator communities to fire: Changes in diversity, abundance and floral reward structure. Oikos 2003, 101, 103–112. [Google Scholar] [CrossRef]
- Ayala, R. Abejas silvestres (Hymenoptera: Apoidea) de Chamela, Jalisco, México. Folia Entomol. Mex. 1988, 73, 395–493. [Google Scholar]
- Hinojosa, I.A.D. Distribución Altitudinal de las Abejas Silvestres (Hymenoptera: Apoidea) en el Declive sur de la Sierra de Chichinautzin. Morelos. Master’s Thesis, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, 2021. [Google Scholar]
- Novelo-Rincón, L.F.; Delfín-González, H.; Ayala, B.R.; Contreras-Acosta, H. Estructura de las comunidades de abejas nativas de cuatro tipos de vegetación en el estado de Yucatán, México. Folia Entomol. Mex. 2003, 42, 177–190. [Google Scholar]
- Ramírez, F.L. Abejas Nativas (Hymenoptera: Apoidea: Anthophila) Asociadas a la Vegetación de Nuevo León, México. Bachelor’s Thesis, Universidad Autónoma de Nuevo León, Monterrey, Mexico, 2012. [Google Scholar]
- Vandame, R. Diversidad de Abejas (Hymenoptera: Apoidea) de la Reserva de la Biosfera El Triunfo, Chiapas; Final Technical Report. SNIB-CONABIO; El Colegio de la Frontera del Sur, Unidad Tapachula: Tapachula, Mexico, 2012; pp. 1–80. [Google Scholar]
- Vergara, H.C.; Ayala, R. Diversity, phenology and biogeography of the bees (Hymenoptera: Apoidea) of Zapotitlán de Salinas, Puebla, México. J. Kansas Entomol. Soc. 2002, 75, 16–30. [Google Scholar]
- Smith-Pardo, A.; González, V.H. Diversidad de abejas (Hymenoptera: Apoidea) en estados sucesionales del bosque húmedo tropical. Acta Biol. Col. 2007, 12, 43–56. [Google Scholar]
- Fierros-López, H.E. Abejas silvestres (Hymenoptera: Apoidea) del Volcán de Tequila, Jalisco, México. Folia Entomol. Mex. 1998, 4, 21–70. [Google Scholar]
- Chang-Martínez, L.; Domínguez-Vázquez, G. Distribución altitudinal del polen en un gradiente altitudinal en Michoacán, México. Rev. Mex. Biodivers. 2013, 84, 876–883. [Google Scholar] [CrossRef]
- Baumann, K.; Keune, J.; Wolters, V.; Jauker, F. Distribution and pollination services of wild bees and hoverflies along an altitudinal gradient in mountain hay meadows. Ecol. Evol. 2021, 11, 11345–11351. [Google Scholar] [CrossRef]
- Roubik, D.W. Ecology and Natural History of Tropical Bees; Cambridge University Press: Cambridge, UK, 1989; pp. 1–514. [Google Scholar]
- Domínguez-Álvarez, A.; Cano-Santana, Z.; Ayala-Barajas, R. Estructura y fenología de la comunidad de abejas nativas (Hymenoptera: Apoidea). Div. Hábitats Ecol. Comunidades 2009, 2, 421–432. [Google Scholar]
- Lara-Romero, C.; Seguí, J.; Pérez-Delgado, A.; Nogales, M.; Traveset, A. Beta diversity and specialization in plant–pollinator networks along an elevational gradient. J. Biogeogr. 2019, 46, 1598–1610. [Google Scholar] [CrossRef]
- Minachilis, K.; Kantsa, A.; Devalez, J.; Vujic, A.; Pauly, A.; Petanidou, T. High species turnover and unique plant–pollinator interactions make a hyperdiverse mountain. J. Anim. Ecol. 2023, 92, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Tonial, M.L.S.; Silva, H.L.R.; Tonial, I.J.; Costa, M.C.D.; Silva Júnior, N.J.D.; Diniz-Filho, J.A.F. Geographical patterns and partition of turnover and richness components of beta-diversity in faunas from Tocantins river valley. Braz. J. Biol. 2012, 72, 497–504. [Google Scholar] [CrossRef]
- Cristóbal-Perez, E.J.; Barrantes, G.; Cascante-Marín, A.; Hanson, P.; Picado, B.; Gamboa-Barrantes, N.; Rojas-Malavasi, G.; Zumbado, M.A.; Madrigal-Brenes, R.; Martén-Rodríguez, S.; et al. Elevational and seasonal patterns of plant-pollinator networks in two highland tropical ecosystems in Costa Rica. PLoS ONE 2024, 19, e0295258. [Google Scholar] [CrossRef]
- Cortés-Flores, J.; Lopezaraiza-Mikel, M.; de Santiago-Hernández, M.H.; Martén-Rodríguez, S.; Cristóbal-Pérez, E.J.; Aguilar-Aguilar, M.J.; Quesada, M. Successional and phenological effects on plant-floral visitor interaction networks of a tropical dry forest. J. Ecol. 2023, 111, 927–942. [Google Scholar] [CrossRef]
- Williams, N.; Crone, E.; Roulston, T.; Minckley, R.; Packer, L.; Potts, S. Ecological and life-history traits predict bee species responses to environmental disturbances. Biol. Conserv. 2010, 143, 2280–2291. [Google Scholar] [CrossRef]
- Adedoja, O.; Kehinde, T. Changes in interaction network topology and species composition of flower-visiting insects across three land use types. Afr. J. Ecol. 2018, 56, 964–971. [Google Scholar] [CrossRef]
- Beltrán, R.; Traveset, A. Redes de interacción entre flores e himenópteros en dos comunidades costeras: Efectos de la pérdida de hábitat. Rev. Ecosistemas 2017, 27, 102–114. [Google Scholar] [CrossRef]
- Jauker, F.; Jauker, B.; Grass, I.; Steffan-Dewenter, I.; Wolters, V. Partitioning wild bee and hoverfly contributions to plant–pollinator network structure in fragmented habitats. Ecology 2018, 100, e02569. [Google Scholar] [CrossRef]
- Walker, B.H. Biodiversity and ecological redundancy. Conserv. Biol. 1992, 6, 18–23. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin III, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of Biodiversity on Ecosystem Functioning: A Consensus of Current Knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Baldock, K.C.; Memmott, J.; Ruiz-Guajardo, J.C.; Roze, D.; Stone, G.N. Daily temporal structure in African savanna flower visitation networks and consequences for network sampling. Ecology 2011, 92, 687–698. [Google Scholar] [CrossRef]
- Valido, A.; Rodríguez-Rodríguez, M.C.; Jordano, P. Impacto de la introducción de la abeja doméstica (Apis mellifera, Apidae) en el Parque Nacional del Teide (Tenerife, Islas Canarias). Ecosistemas 2014, 23, 58–66. [Google Scholar] [CrossRef]
- Quesada, M.; Rosas, F.; Lópezaraiza, M.; Aguilar, R.; Ashworth, L.; Rosas, G.V.; Sánchez, M.G.; Martén, R.S. Ecología y Conservación Biológica de Sistemas de Polinización de Plantas Tropicales. In Ecología y Evolución de las Interacciones Bióticas; del Val, E., Bouge, K., Eds.; Fondo de Cultura Económica; CIECO y UNAM: Ciudad de México, Mexico, 2012; pp. 75–100. [Google Scholar]
- Tylianakis, J.M.; Laliberté, E.; Nielsen, A.; Bascompte, J. Conservation of species interaction networks. Biol. Conserv. 2010, 143, 2270–2279. [Google Scholar] [CrossRef]
- Calizza, E.; Costantini, M.L.; Rossi, L. Effect of multiple disturbances on food web vulnerability to biodiversity loss in detritus-based systems. Ecosphere 2015, 6, 1–20. [Google Scholar] [CrossRef]
- Dune, J.A.; Williams, R.J.; Martínez, N.D. Network Topology and Species Loss in Food Webs: Robustness Increases with Connectance. Ecol. Lett. 2002, 5, 558–567. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Redes mutualistas de especies. Investig. Cienc. 2008, 384, 50–59. [Google Scholar]
- Kratochwil, A.; Beil, M.; Schwabe, A. Complex structure of pollinator–plant interaction webs: Random, nested, with gradients or modules? Apidologie 2009, 40, 634–650. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. The structure of plant-animal mutualistic networks. In Ecological Networks; Pascual, M., Dunne, J.A., Eds.; Oxford University Press: New York, NY, USA, 2006; pp. 143–162. [Google Scholar]
- Lara-Rodríguez, N.Z.; Díaz-Valenzuela, R.; Martínez-García, V.; Mauricio-Lopéz, E.; Anaid-Díaz, S.; Valle, O.I.; Fisher-de León, A.; Lara, C.; Ortiz-Pulido, R. Redes de interacción colibrí-planta del centro-este de México. Rev. Mex. Biodivers. 2012, 83, 569–577. [Google Scholar] [CrossRef]
- Ashton, I.W.; Miller, A.E.; Bowman, W.D.; Suding, K.N. Niche complementarity due to plasticity in resource use: Plant partitioning of chemical N forms. Ecology 2010, 91, 3252–3260. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.M.; Presley, S.J.; Santos, G.M.M. Niche overlap and network specialization of flower-visiting bees in an agricultural system. Neotrop. Entomol. 2014, 43, 489–499. [Google Scholar] [CrossRef] [PubMed]
| Bees | βSor | βSim | βSne | ||||||
| SDTF1 | SDTF2 | TF1 | SDTF1 | SDTF2 | TF1 | SDTF1 | SDTF2 | TF1 | |
| SDTF2 | 0.4285 | 0.398 | 0.030 | ||||||
| TF1 | 0.731 | 0.634 | 0.56 | 0.44 | 0.172 | 0.194 | |||
| TF2 | 0.724 | 0.656 | 0.672 | 0.60 | 0.533 | 0.64 | 0.124 | 0.123 | 0.032 |
| Plants | |||||||||
| SDTF2 | 0.72 | 0.641 | 0.079 | ||||||
| TF1 | 0.766 | 0.583 | 0.551 | 0.428 | 0.215 | 0.155 | |||
| TF2 | 0.844 | 0.794 | 0.774 | 0.569 | 0.517 | 0.612 | 0.275 | 0.277 | 0.162 |
| βWN | βST | βOS | |||||||
| SDTF1 | SDTF2 | TF1 | SDTF1 | SDTF2 | TF1 | SDTF1 | SDTF2 | TF1 | |
| SDTF2 | 0.914 | 0.705 | 0.209 | ||||||
| TF1 | 0.964 | 0.943 | 0.845 | 0.778 | 0.119 | 0.165 | |||
| TF2 | 0.948 | 0.939 | 0.957 | 0.881 | 0.848 | 0.871 | 0.066 | 0.091 | 0.08 |
| Network Descriptor | SDTF1 | SDTF2 | TF1 | TF2 |
|---|---|---|---|---|
| Observed plant richness Estimated plant richness (CI 0.95) | 61 62 (51–73) | 39 39 (31–49) | 34 39 (30–45) | 29 29 (21–39) |
| Observed bee richness Estimated bee richness (CI 0.95) | 113 108 (98–120) | 101 101 (88–109) | 49 53 (45–61) | 58 50 (51–64) |
| Observed interactions richness Estimated interactions richness (CI 0.95) | 248 233 (211–255) | 201 201 (172–224) | 88 101 (83–120) | 108 108 (91–122) |
| Connectance | 0.035 | 0.05 | 0.05 | 0.06 |
| Links per species | 1.4 | 1.4 | 1.06 | 1.13 |
| Nestedness | 3.36 ** | 5.41 ** | 4.6 * | 4.58 ** |
| Web asymmetry | 0.31 | 0.44 | 0.18 | 0.33 |
| Niche overlap bees | 0.074 | 0.083 | 0.091 | 0.145 |
| Niche overlap plants | 0.03 | 0.04 | 0.061 | 0.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razo-León, A.E.; Muñoz-Urias, A.; Uribe-Mú, C.A.; Huerta-Martínez, F.M.; Fierros-López, H.E.; Vásquez-Bolaños, M.; Moya-Raygoza, G.; Carrillo-Reyes, P. Wild Bee Diversity and Bee–Plant Interactions in Tropical and Temperate Forest Clearings in a Natural Protected Area in Central West Mexico. Insects 2024, 15, 1009. https://doi.org/10.3390/insects15121009
Razo-León AE, Muñoz-Urias A, Uribe-Mú CA, Huerta-Martínez FM, Fierros-López HE, Vásquez-Bolaños M, Moya-Raygoza G, Carrillo-Reyes P. Wild Bee Diversity and Bee–Plant Interactions in Tropical and Temperate Forest Clearings in a Natural Protected Area in Central West Mexico. Insects. 2024; 15(12):1009. https://doi.org/10.3390/insects15121009
Chicago/Turabian StyleRazo-León, Alvaro Edwin, Alejandro Muñoz-Urias, Claudia Aurora Uribe-Mú, Francisco Martín Huerta-Martínez, Hugo Eduardo Fierros-López, Miguel Vásquez-Bolaños, Gustavo Moya-Raygoza, and Pablo Carrillo-Reyes. 2024. "Wild Bee Diversity and Bee–Plant Interactions in Tropical and Temperate Forest Clearings in a Natural Protected Area in Central West Mexico" Insects 15, no. 12: 1009. https://doi.org/10.3390/insects15121009
APA StyleRazo-León, A. E., Muñoz-Urias, A., Uribe-Mú, C. A., Huerta-Martínez, F. M., Fierros-López, H. E., Vásquez-Bolaños, M., Moya-Raygoza, G., & Carrillo-Reyes, P. (2024). Wild Bee Diversity and Bee–Plant Interactions in Tropical and Temperate Forest Clearings in a Natural Protected Area in Central West Mexico. Insects, 15(12), 1009. https://doi.org/10.3390/insects15121009









