Dynamic Alterations of the Intestinal Microbiota of Fifth-Instar Silkworms (Bombyx mori) Fed an Artificial Diet or Mulberry Leaves
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. AD and MB Preparation
2.2. Silkworm Rearing
2.3. Assessment of Silkworm Feeding Efficiency and Silkworm Cocoon Quality
2.4. Intestinal Bacterial Community Analysis
2.5. Data Analysis
3. Results
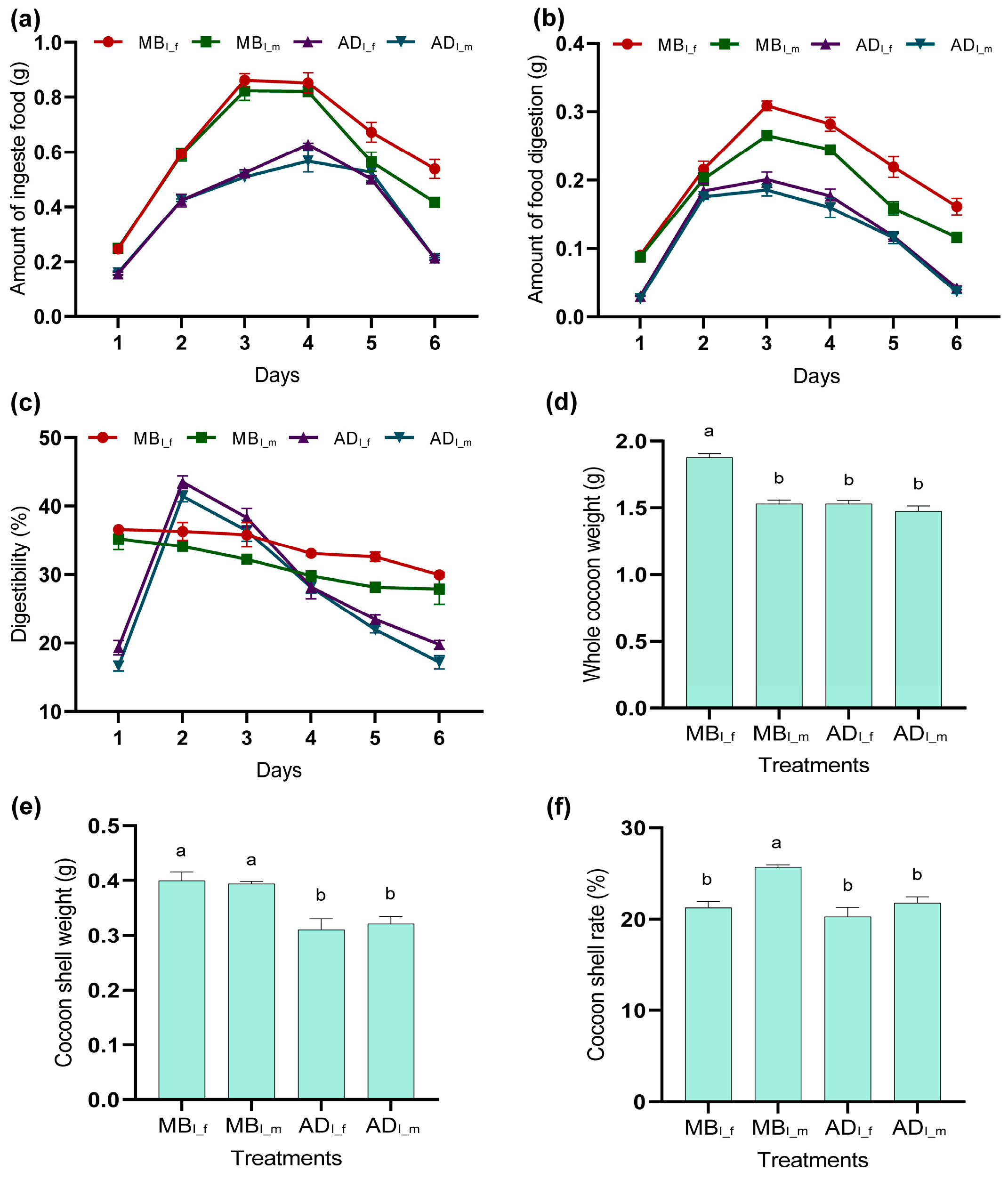
3.1. Effects of MB and AD on Silkworm Feeding Efficiency and Silkworm Cocoon Quality
3.2. Bacterial Compositionand α-Diversity
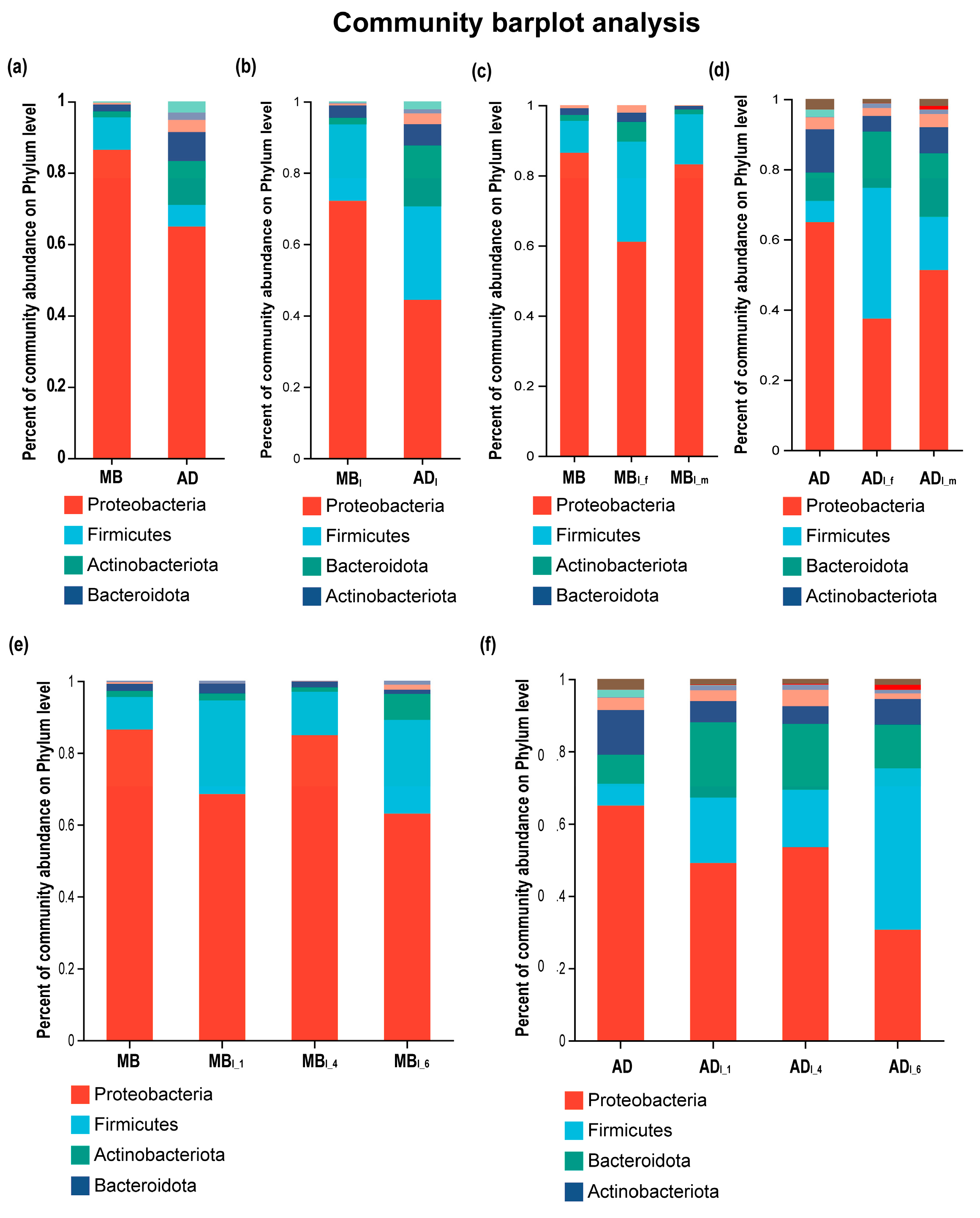
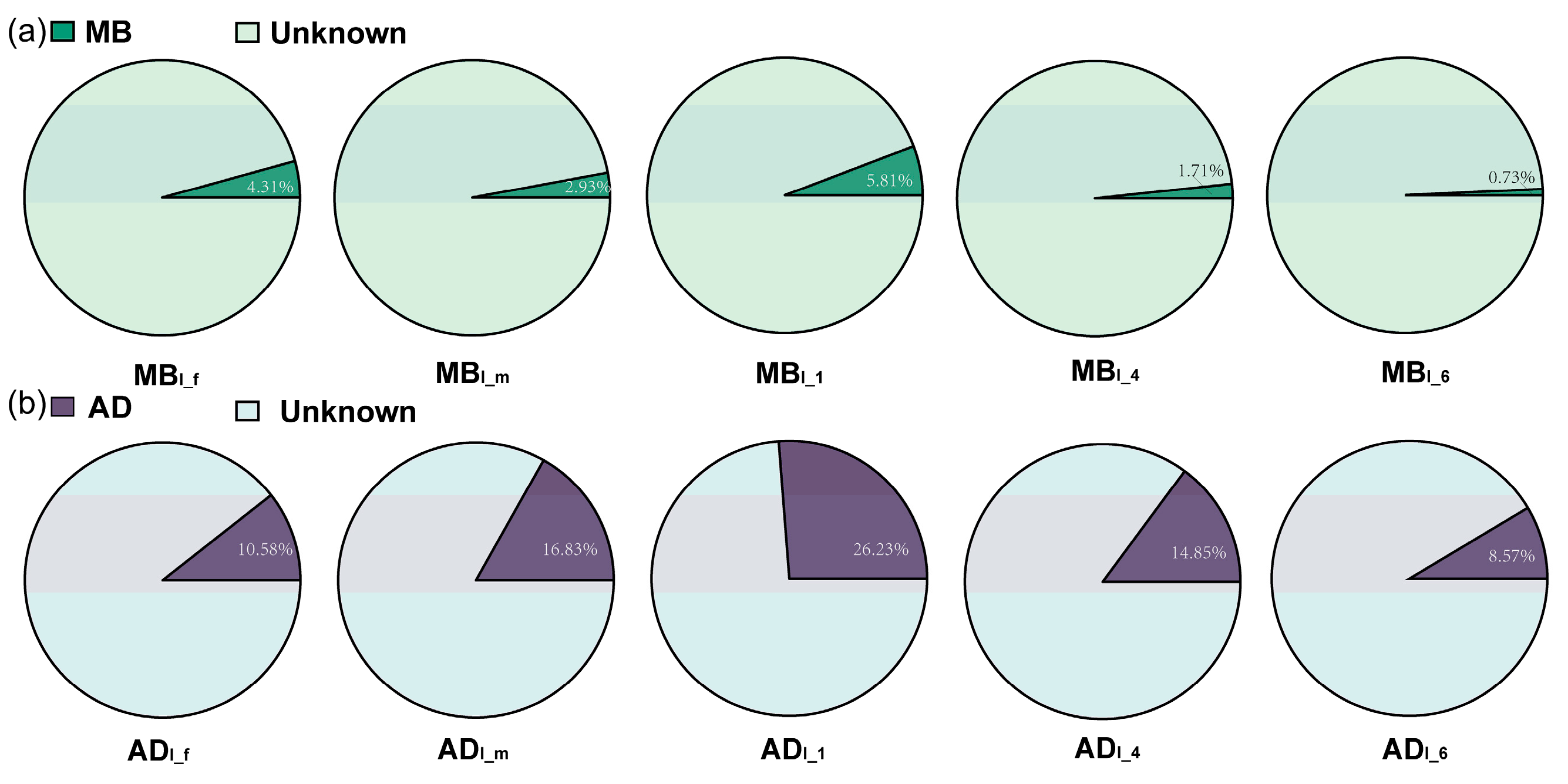
3.3. Taxonomic Analysis of Intestinal Microbiota
3.4. β-Diversity Analysis
3.5. Differential Bacterial Taxa
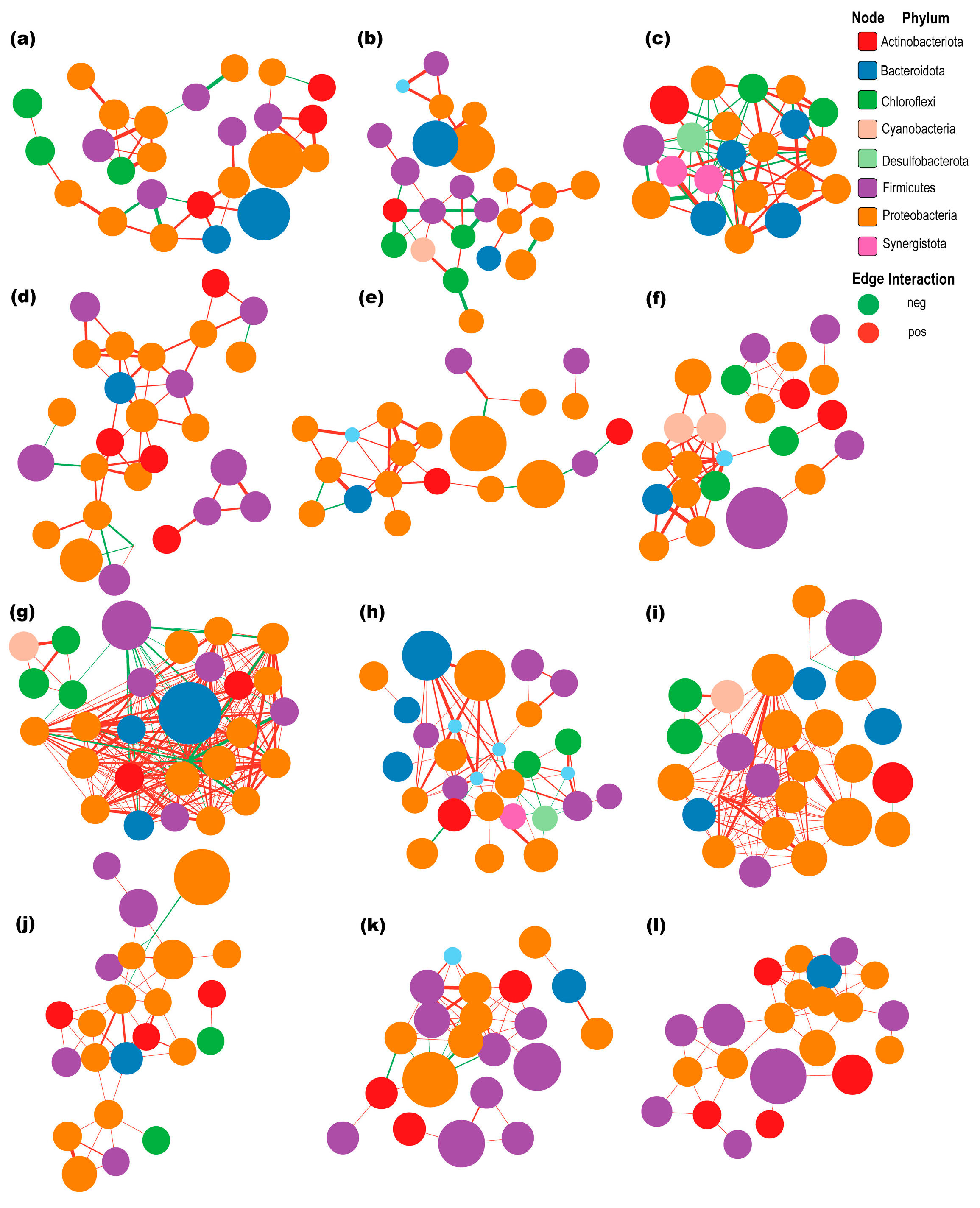
3.6. Correlation Network Analyses
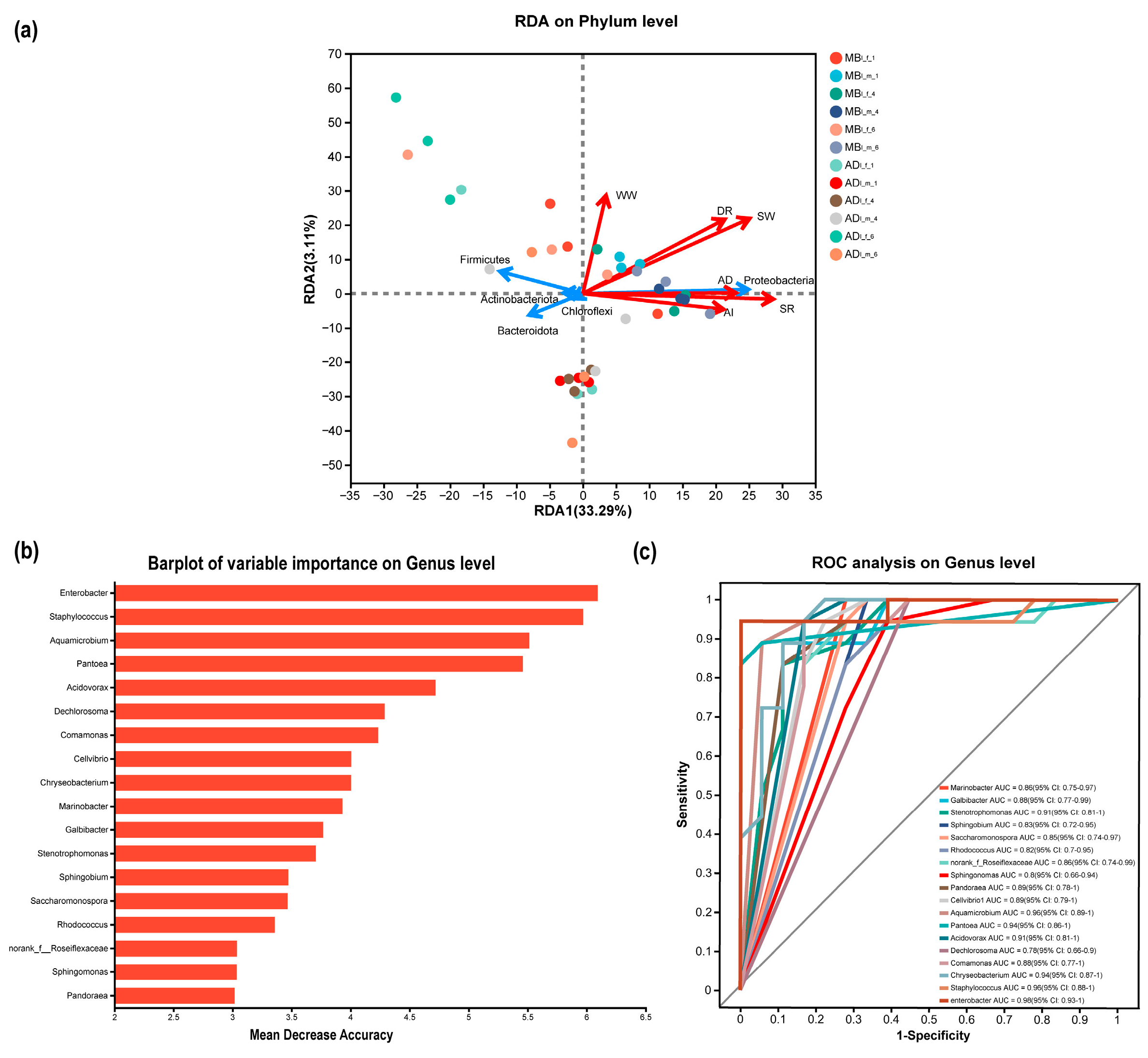
3.7. Associations of Bacteria Community Composition with Silkworm Cocoon Quality and Silkworm Feeding Efficiency
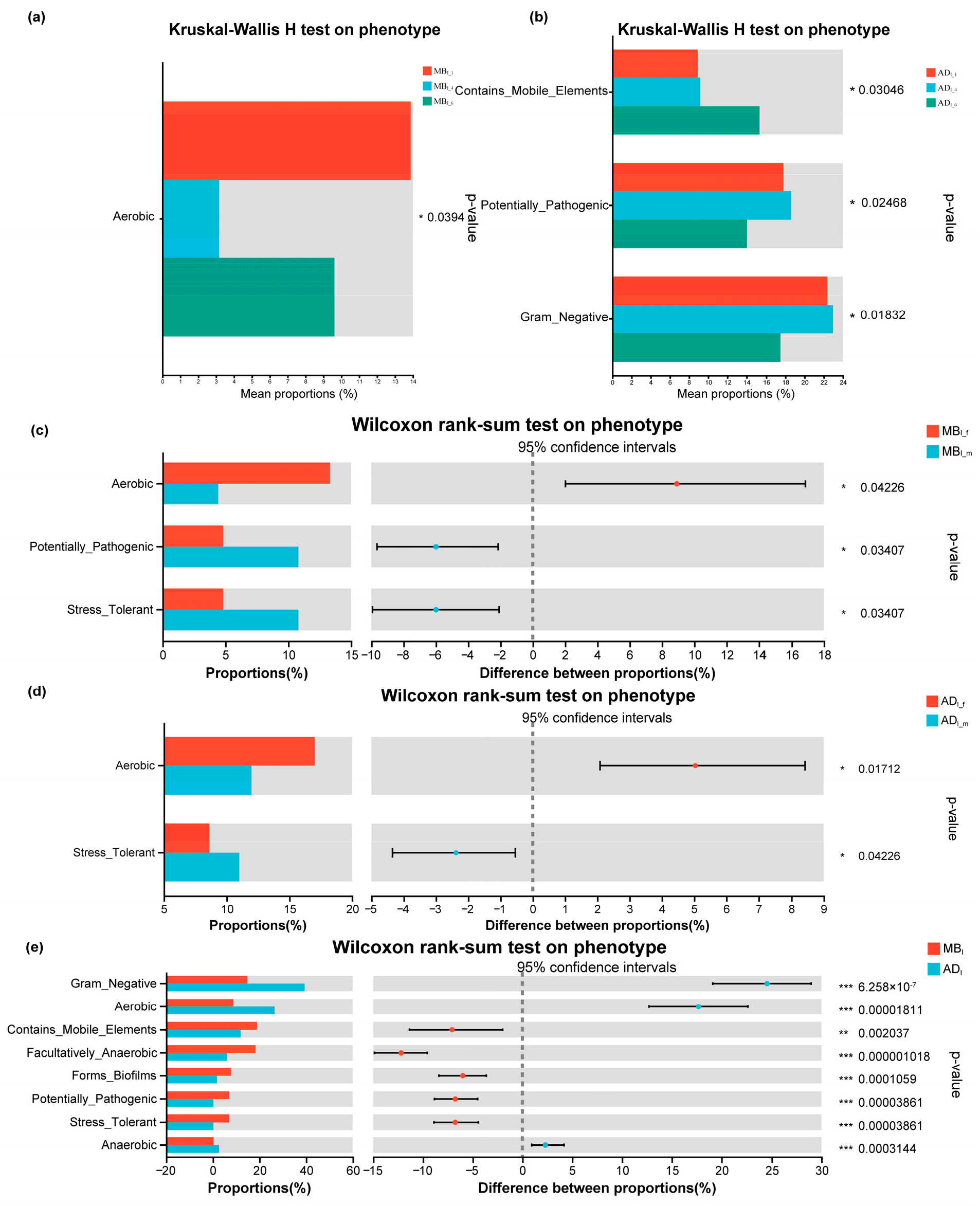
3.8. Predicted Functional Consequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, N.J.; Zhang, C.; Qi, X.W.; Zhao, S.C.; Tao, Y.; Yang, G.J.; Lee, T.H.; Wang, X.Y.; Cai, Q.L.; Li, D.; et al. Draft genome sequence of the mulberry tree Morus notabilis. Nat. Commun. 2013, 4, 2445. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Jha, S.; Mandal, P.; Ghosh, A. Artificial diet based silkworm rearing system-a review. Int. J. Pure App. Biosci. 2016, 4, 114–122. [Google Scholar] [CrossRef]
- Fukuda, T.; Suto, M.; Higuchi, Y. Silkworm raising on artificial food. Nature 1960, 187, 669–670. [Google Scholar] [CrossRef]
- Avramova, K.; Tzenov, P.; Grekov, D. Silkworm (Bombyx mori L.) rearing using artificial diet during the summer. Sci. Pap-Ser. D-Anim. Sci. 2020, 63, 19–24. [Google Scholar]
- Bhavikatti, J.S.; Bodducharl, S.M.; Kamagond, R.S.; Desai, S.V.; Shet, A.R. Statistical optimisation of protease production using a freshwater bacterium chryseobacterium cucumeris sarjs-2 for multiple industrial applications. 3 Biotech 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Li, J.; Deng, J.; Deng, X.; Liu, L.; Zha, X. Metabonomic analysis of silkworm midgut reveals differences between the physiological effects of an artificial and mulberry leaf diet. Insects 2023, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Floch, M.H. Advances in intestinal microecology: The microbiome, prebiotics, and probiotics. Nutr. Clin. Pract. 2012, 27, 193–194. [Google Scholar] [CrossRef]
- Zhang, N.; He, J.; Shen, X.; Sun, C.; Muhammad, A.; Shao, Y. Contribution of sample processing to gut microbiome analysis in the model Lepidoptera, silkworm Bombyx mori. Comput. Struct. Biotechnol. J. 2021, 19, 4658–4668. [Google Scholar] [CrossRef]
- Li, Y.; Chang, L.; Xu, K.; Zhang, S.; Gao, F.; Fan, Y. Research progresses on the function and detection methods of insect gut microbes. Microorganisms 2023, 11, 1208. [Google Scholar] [CrossRef]
- Brune, A.; Dietrich, C. The gut microbiota of termites: Digesting the diversity in the light of ecology and evolution. Annu. Rev. Microbiol. 2015, 69, 145–166. [Google Scholar] [CrossRef]
- Grieco, M.B.; Lopes, F.A.C.; Oliveira, L.S.; Tschoeke, D.A.; Popov, C.C.; Thompson, C.C.; Gonçalves, L.C.; Constantino, R.; Martins, O.B.; Kruger, R.H. Metagenomic analysis of the whole gut microbiota in brazilian termitidae termites Cornitermes cumulans, Cyrilliotermes strictinasus, Syntermes dirus, Nasutitermes jaraguae, Nasutitermes aquilinus, Grigiotermes bequaerti, and Orthognathotermes mirim. Curr. Microbiol. 2019, 76, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Rangberg, A.; Diep, D.B.; Rudi, K.; Amdam, G.V. Paratransgenesis: An approach to improve colony health and molecular insight in honey bees (Apis mellifera)? Integr. Comp. Biol. 2012, 52, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.Y.; Wang, X.M.; Hu, H.L.; Qin, L. Comparison of bacterial communities between midgut and midgut contents in two silkworms, Antheraea pernyi and Bombyx mori. Sci. Rep. 2020, 10, 12966. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I.; Xu, Y.J. Role of insect gut microbiota in pesticide degradation: A review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhang, S.K.; Xu, L.T. The pivotal roles of gut microbiota in insect plant interactions for sustainable pest management. NPJ Biofilms Microbiomes 2023, 9, 66. [Google Scholar] [CrossRef]
- Habineza, P.; Muhammad, A.; Ji, T.L.; Xiao, R.; Yin, X.Y.; Hou, Y.M.; Shi, Z.H. The promoting effect of gut microbiota on growth and development of red palm weevil, Rhynchophorus ferrugineus (Olivier) (coleoptera: Dryophthoridae) by modulating its nutritional metabolism. Front. Microbiol. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Schmidt, K.; Engel, P. Mechanisms underlying gut microbiota-host interactions in insects. J. Exp. Biol. 2021, 224 Pt 2, jeb207696. [Google Scholar] [CrossRef]
- Savio, C.; Mugo-Kamiri, L.; Upfold, J.K. Bugs in bugs: The role of probiotics and prebiotics in maintenance of health in mass-reared insects. Insects 2022, 13, 376. [Google Scholar] [CrossRef]
- Péchy-Tarr, M.; Bruck, D.J.; Maurhofer, M.; Fischer, E.; Vogne, C.; Henkels, M.D.; Donahue, K.M.; Grunder, J.; Loper, J.E.; Keel, C. Molecular analysis of a novel gene cluster encoding an insect toxin in plant-associated strains of Pseudomonas fluorescens. Environ. Microbiol. 2008, 10, 2368–2386. [Google Scholar] [CrossRef]
- Caccia, S.; Di Lelio, I.; La Storia, A.; Marinelli, A.; Varricchio, P.; Franzetti, E.; Banyuls, N.; Tettamanti, G.; Casartelli, M.; Giordana, B. Midgut microbiota and host immunocompetence underlie Bacillus thuringiensis killing mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, 9486–9491. [Google Scholar] [CrossRef]
- Shu, Q.L.; Wang, Y.F.; Gu, H.Y.; Zhu, Q.Y.; Liu, W.; Dai, Y.; Li, F.; Li, B. Effects of artificial diet breeding on intestinal microbial populations at the young stage of silkworm (Bombyx mori). Arch. Insect Biochem. Physiol. 2023, 113, e22019. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yu, T.; Xie, S.; Du, K.; Liang, X.; Lan, Y.; Sun, C.; Lu, X.; Shao, Y. Comparative shotgun metagenomic data of the silkworm Bombyx mori gut microbiome. Sci. Data 2018, 5, 180285. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, T.; Vankadara, S.; Ramasamy, S.; Lingaiah, K. Identification of potential probiotics in the midgut of mulberry silkworm, Bombyx mori through metagenomic approach. Probiotics Antimicrob. Proteins 2019, 12, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Fu, Y.M.; Tong, L.; Liu, H. Microbial shifts of the silkworm larval gut in response to lettuce leaf feeding. Appl. Microbiol. Biotechnol. 2014, 98, 3769–3776. [Google Scholar] [CrossRef]
- Chen, B.; Du, K.; Sun, C.; Vimalanathan, A.; Liang, X.; Li, Y.; Wang, B.; Lu, X.; Li, L.; Shao, Y. Gut bacterial and fungal communities of the domesticated silkworm (Bombyx mori) and wild mulberry-feeding relatives. ISME J. 2018, 12, 2252–2262. [Google Scholar] [CrossRef]
- Dong, H.L.; Zhang, S.X.; Chen, Z.H.; Tao, H.; Li, X.; Qiu, J.F.; Cui, W.Z.; Sima, Y.H.; Cui, W.Z.; Xu, S.Q. Differences in gut microbiota between silkworms (Bombyx mori) reared on fresh mulberry (Morus alba var. multicaulis) leaves or an artificial diet. RSC Adv. 2018, 8, 26188–26200. [Google Scholar] [CrossRef]
- Liu, R.; Wang, W.; Liu, X.; Lu, Y.; Xiang, T.; Zhou, W.; Wan, Y. Characterization of a lipase from the silkworm intestinal bacterium Bacillus pumilus with antiviral activity against Bombyx mori (Lepidoptera: Bombycidae) nucleopolyhedrovirus in vitro. J. Insect Sci. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Li, G.N.; Xia, X.J.; Tang, W.C.; Zhu, Y. Intestinal microecology associated with fluoride resistance capability of the silkworm (Bombyx mori L.). Appl. Microbiol. Biot. 2016, 100, 6715–6724. [Google Scholar] [CrossRef]
- Gunasekhar, V.; Somayaji, A. Effect of endophytic bacteria Burkholderia cepacia on growth, cocoon characters and enzyme activity of silkworm, Bombyx mori L. South Asian J. Res. Microbiol. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Sun, Z.L.; Kumar, D.; Cao, G.L.; Zhu, L.Y.; Liu, B.; Zhu, M.; Liang, Z.; Kuang, S.L.; Chen, F.; Gong, C.L.; et al. Effects of transient high temperature treatment on the intestinal flora of the silkworm Bombyx mori. Sci. Rep. 2017, 7, 3349. [Google Scholar] [CrossRef]
- Hou, C.; Shi, Y.; Wang, H.; Li, R.; Nartey, M.A.; Guo, X. Composition and diversity analysis of intestinal microbiota in the fifth instar silkworm, Bombyx mori L. Invertebr. Surviv. J. 2018, 15, 223–233. [Google Scholar] [CrossRef]
- Carrero-Colón, M.; Nakatsu, C.H.; Konopka, A. Effect of nutrient periodicity on microbial community dynamics. Appl. Environ. Microbiol. 2006, 72, 3175–3183. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Schloter, M.; et al. Microbiome definition revisited: Old concepts and new challenges. Microbiom 2020, 8, 103. [Google Scholar] [CrossRef]
- Knights, D.; Kuczynski, J.; Charlson, E.S.; Zaneveld, J.; Mozer, M.C.; Collman, R.G.; Bushman, F.D.; Knight, R.; Kelley, S.T. Bayesian community-wide culture-independent microbial source tracking. Nat. Methods 2011, 8, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.; Larson, J.; Meulemans, J.; Hillmann, B.; Lynch, J.; Sidiropoulos, D.; Spear, J.R.; Caporaso, G.; Blekhman, R.; Knight, R.; et al. Bugbase predicts organism level microbiome phenotypes. bioRxiv 2017, 1–19. [Google Scholar] [CrossRef]
- Suraporn, S.; Sangsuk, W.; Chanhan, P.; Promma, S. Effects of probiotic bacteria on the growth parameters of the Thai silkworm, Bombyx mori. Thai J. Agric. Sci. 2015, 48, 29–33. [Google Scholar]
- Wu, X.H.; Chen, X.D.; Ye, A.H.; Cao, J.R.; He, R.M.; Pan, M.L.; Jin, F.; Ma, H.Y.; Zhou, W.L. Multi-tissue metabolomic profiling reveals potential mechanisms of cocoon yield in silkworms (Bombyx mori) fed formula feed versus mulberry leaves. Front. Mol. Biosci. 2022, 9, 977047. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Ye, A.H.; Wu, X.H.; Qu, Z.G.; Xu, S.Q.; Sima, Y.H.; Wang, Y.J.; He, R.M.; Cao, J.R.; Zhou, W.L.; et al. Combined analysis of silk synthesis and hemolymph amino acid metabolism reveal key roles for glycine in increasing silkworm silk yields. Int. J. Biol. Macromol. 2022, 209, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.L.; Feng, P.; Mao, T.T.; Gu, H.Y.; Bian, D.D.; Sun, H.N.; Li, F.C.; Wei, J.; Li, B. Study of compensatory growth based on different nutrition conditions of Bombyx mori. J. Asia-Pac. Entomol. 2022, 25, 101948. [Google Scholar] [CrossRef]
- Tao, S.S.; Wang, J.; Liu, M.H.; Sun, F.; Li, B.; Ye, C.J. Haemolymph metabolomic differences in silkworms (Bombyx mori L.) under mulberry leaf and two artificial diet rearing methods. Arch. Insect Biochem. Physiol. 2021, 109, e21851. [Google Scholar] [CrossRef]
- Yin, X.C.; Zhang, Y.L.; Yu, D.L.; Li, G.L.; Wang, X.L.; Wei, Y.T.; He, C.H.; Liu, Y.W.; Xu, K.Z.; Zhang, G.Z.; et al. Effects of artificial diet rearing during all instars on silk secretion and gene transcription in Bombyx mori (Lepidoptera: Bombycidae). J. Econ. Entomol. 2023, 116, 1–12. [Google Scholar] [CrossRef]
- Anantha Raman, K.V.; Magadum, S.B.; Datta, R.K. Feed efficiency of the silkworm Bombyx mori l. hybrid (nb4d2 x ka). Int. J. Trop. Insect Sci. 1994, 15, 111–116. [Google Scholar] [CrossRef]
- Horie, Y.; Sudo, M.; Fujiwara, Y. Daily consumption and utilization of dietary dry matter by the silkworm, Bombyx mori, reared on the artificial pellet diet. Rep. Silk Sci. Res. Inst. 1997, 45, 7–12. [Google Scholar]
- Qin, L.J.; Qi, J.P.; Shen, G.W.; Qin, D.Y.; Wu, J.X.; Song, Y.W.; Cao, Y.; Zhao, P.; Xia, Q.Y. Effects of Microbial Transfer during Food-Gut-Feces Circulation on the Health of Bombyx mori. Microbiol. Spectr. 2022, 10, e0235722. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sun, Z.L.; Cao, G.L.; Xue, R.Y.; Hu, X.L.; Gong, C.L. Bombyx mori bidensovirus infection alters the intestinal microflora of fifth instar silkworm (bombyx mori) larvae. J. Invertebr. Pathol. 2019, 163, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.L.; Lu, Y.H.; Zhang, H.; Kumar, D.; Liu, B.; Gong, Y.C.; Zhu, M.; Zhu, L.Y.; Liang, Z.; Gong, C.L.; et al. Effects of BmCPV infection on silkworm Bombyx mori intestinal bacteria. PLoS ONE 2016, 11, e0146313. [Google Scholar] [CrossRef]
- Zhang, X.C.; Feng, H.H.; He, J.T.; Muhammad, A.; Zhang, F.; Lu, X.M. Features and colonization strategies of Enterococcus faecalis in the gut of Bombyx mori. Front. Microbiol. 2022, 13, 921330. [Google Scholar] [CrossRef]
- Liang, X.L.; He, J.T.; Zhang, N.; Muhammad, A.; Lu, X.M.; Shao, Y.Q. Probiotic potentials of the silkworm gut symbiont Enterococcus casseliflavus ECB140, a promising L- tryptophan producer living inside the host. J. Appl. Microbiol. 2022, 133, 1620–1635. [Google Scholar] [CrossRef]
- Anand, A.A.P.; Vennison, S.J.; Sankar, S.G.; Prabhu, D.I.G.; Vasan, P.T.; Raghuraman, T.; Geoffrey, C.J.; Vendan, S.E. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J. Insect Sci. 2010, 10, 1–20. [Google Scholar] [CrossRef]
- Kim, B.-C.; Deshpande, T.R.; Chun, J.; Yi, S.C.; Kim, H.; Um, Y.; Sang, B.-I. Production of hydrogen and volatile fatty acid by Enterobacter sp. T4384 using organic waste materials. J. Microbiol. Biotechnol. 2013, 23, 189–194. [Google Scholar] [CrossRef]
- Subudhi, S.; Nayak, T.; Kumar, N.R.; Vijayananth, P.; Lal, B. Impact of regulated pH on proto scale hydrogen production from xylose by an alkaline tolerant novel bacterial strain, Enterobacter cloacae DT-1. Int. J. Hydrogen Energy 2013, 38, 2728–2737. [Google Scholar] [CrossRef]
- Bandaiphet, C.; Prasertsan, P. Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kla in exopolysaccharide production from enterobacter cloacae wd7. Carbohydr. Polym. 2006, 66, 216–228. [Google Scholar] [CrossRef]
- Behar, A.; Yuval, B.; Jurkevitch, E. Enterobacteria-mediated nitrogen fixation in natural populations of the fruit fly Ceratitis capitate. Mol. Ecol. 2005, 14, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Azis, K.; Zerva, I.; Melidis, P.; Caceres, C.; Bourtzis, K.; Ntougias, S. Biochemical and nutritional characterization of the medfly gut symbiont Enterobacter sp. AA26 for its use as probiotics in sterile insect technique applications. BMC Biotechnol. 2019, 19 (Suppl. S2), 1–10. [Google Scholar] [CrossRef]
- Suzuki, J.; Uda, A.; Watanabe, K.; Shimizu, T.; Watarai, M. Symbiosis with Francisella tularensis provides resistance to pathogens in the silkworm. Sci. Rep. 2016, 6, 31476. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, X.Q.; Zhou, W.; Liu, G.Y.; Wan, Y.J. Isolation and characterization of lipase-producing bacteria in the intestine of the silkworm, Bombyx mori, reared on different forage. J. Insect Sci. 2011, 11, 135. [Google Scholar] [CrossRef]
- Anjum, S.I.; Shah, A.H.; Aurongzeb, M.; Kori, J.; Azim, M.K.; Ansari, M.J.; Bin, L. Characterization of gut bacterial flora of Apis mellifera from north-west Pakistan. Saudi J. Biol. Sci. 2017, 25, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Toma´s, A.A.; Andersen, M.A.; Suen, G.; Stevenson, D.M.; Chu, F.S.T.; Cleland, W.W.; Weimer, P.J.; Currie, C.R. Symbiotic nitrogen fixation in the fungus gardens of leaf-cutter ants. Science 2009, 326, 1120–1123. [Google Scholar] [CrossRef]
- Suen, G.; Scott, J.J.; Aylward, F.O.; Adams, S.M.; Tringe, S.G.; Pinto-Toma´s, A.A.; Foster, C.E.; Pauly, M.; Weimer, P.J.; Currie, C.R.; et al. An insect herbivore microbiome with high plant biomass-degrading capacity. PLoS Genet. 2010, 6, e1001129. [Google Scholar] [CrossRef]
- Ling, Y.; Li, W.J.; Li, F.F.; Xue, X.B.; Gao, Y.Y.; Wang, L.; Liang, K.; Li, X.J. Microbial gut diversity in four grasshopper species and its correlation with cellulose digestibility. Front. Microbiol. 2022, 13, 1002532. [Google Scholar] [CrossRef]
- Dillon, R.J.; Vennard, C.T.; Charnley, A.K. A note: Gut bacteria produce components of a locust cohesion pheromone. J. Appl. Microbiol. 2002, 92, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.R.; Ray, L.; Panda, A.N.; Sahu, N.; Xess, S.S.; Jadhao, S.; Suar, M.; Adhya, T.K.; Rastogi, G.; Pattnaik, A.K.; et al. Draft genome sequence of Acinetobacter sp. Strain BMW17, a cellulolytic and plant growth-promoting bacterium isolated from the rhizospheric region of Phragmites karka of chilika lake, India. Genome Announc. 2016, 4, e00395-16. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, S.; He, X.H.; Ma, H.J.; Zhou, X.D.; Lin, H.P.; Zhang, S.K. Specific enriched Acinetobacter in Camellia weevil gut facilitate the degradation of tea saponin: Inferred from bacterial genomic and transcriptomic analyses. Microbiol. Spectr. 2022, 10, e0227222. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Zhang, N.; He, J.T.; Shen, X.Q.; Zhu, X.Y.; Xiao, J.; Qian, Z.Y.; Sun, C.; Shao, Y.Q. Multiomics analysis reveals the molecular basis for increased body weight in silkworms (Bombyx mori) exposed to environmental concentrations of polystyrene micro- and nanoplastics. J. Adv. Res. 2024, 57, 43–57. [Google Scholar] [CrossRef]
- Briones-Roblero, C.I.; Rodríguez-Díaz, R.; Santiago-Cruz, J.A.; Zúñiga, G.; Rivera-Orduña, F.N. Degradation capacities of bacteria and yeasts isolated from the gut of Dendroctonus rhizophagus (Curculionidae: Scolytinae). Folia Microbiol. 2017, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Yokoe, M. A novel protein-deamidating enzyme from Chryseobacterium proteolyticum sp. nov., a newly isolated bacterium from soil. Appl. Environ. Microbiol. 2000, 66, 3337–3343. [Google Scholar] [CrossRef]
- Riffel, A.; Brandelli, A.; Bellato, C.D.M.; Souza, G.H.; Eberlin, M.N.; Tavares, F.C. Purification and characterization of a keratinolytic metalloprotease from Chryseobacterium sp. kr6. J. Biotechnol. 2007, 128, 693–703. [Google Scholar] [CrossRef]
- Wang, S.L.; Hsu, W.T.; Liang, T.W.; Yen, Y.H.; Wang, C.L. Purification and characterization of three novel keratinolytic metalloproteases produced by Chryseobacterium indologenes TKU014 in a shrimp shell powder medium. Bioresour. Technol. 2007, 99, 5679–5686. [Google Scholar] [CrossRef]
- Kshetri, P.; Roy, S.S.; Sharma, S.K.; Singh, T.S.; Ansari, M.A.; Prakash, N.; Ngachan, S.V. Transforming chicken feather waste into feather protein hydrolysate using a newly isolated multifaceted keratinolytic bacterium Chryseobacterium sediminis RCM-SSR-7. Waste Biomass Valorization 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Yang, M.K.; Luo, F.H.; Song, Y.C.; Ma, S.L.; Ma, Y.D.; Fazal, A. The host niches of soybean rather than genetic modification or glyphosate application drive the assembly of root-associated microbial communities. Microb. Biotechnol. 2022, 15, 2942–2957. [Google Scholar] [CrossRef]
- Skowronek, M.; Sajnaga, E.; Pleszczyńska, M.; Kazimierczak, W.; Lis, M.; Wiater, A. Bacteria from the midgut of common cockchafer (Melolontha melolontha L.) larvae exhibiting antagonistic activity against bacterial symbionts of entomopathogenic nematodes: Isolation and molecular identification. Int. J. Mol. Sci. 2020, 21, 580. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Zhao, S.; Wu, X. Bmnpv-induced hormone metabolic disorder in silkworm leads to enhanced locomotory behavior. Dev. Comp. Immunol. 2021, 121, 104036. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Son, J.S.; Chung, J.H.; Lee, S.; Kim, J.S.; Ryu, C. Population dynamics of intestinal enterococcus modulate galleria mellonella metamorphosis. Microbiol. Spectrum. 2023, 11, e0278022. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Nannini, G.; Cianchi, F.; Coratti, F.; Amedei, A. The Impact of Microbiota-Immunity-Hormone Interactions on Autoimmune Diseases and Infection. Biomedicines 2024, 12, 616. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Li, M.; Li, F.; Liang, X.; Zhang, H.; Gu, Y.; Guo, G. Dynamic Alterations of the Intestinal Microbiota of Fifth-Instar Silkworms (Bombyx mori) Fed an Artificial Diet or Mulberry Leaves. Insects 2024, 15, 970. https://doi.org/10.3390/insects15120970
Chen C, Li M, Li F, Liang X, Zhang H, Gu Y, Guo G. Dynamic Alterations of the Intestinal Microbiota of Fifth-Instar Silkworms (Bombyx mori) Fed an Artificial Diet or Mulberry Leaves. Insects. 2024; 15(12):970. https://doi.org/10.3390/insects15120970
Chicago/Turabian StyleChen, Chuanjie, Meng Li, Feng Li, Xiaoyan Liang, Haiyang Zhang, Yinyu Gu, and Guang Guo. 2024. "Dynamic Alterations of the Intestinal Microbiota of Fifth-Instar Silkworms (Bombyx mori) Fed an Artificial Diet or Mulberry Leaves" Insects 15, no. 12: 970. https://doi.org/10.3390/insects15120970
APA StyleChen, C., Li, M., Li, F., Liang, X., Zhang, H., Gu, Y., & Guo, G. (2024). Dynamic Alterations of the Intestinal Microbiota of Fifth-Instar Silkworms (Bombyx mori) Fed an Artificial Diet or Mulberry Leaves. Insects, 15(12), 970. https://doi.org/10.3390/insects15120970





