Habitat Suitability of Danaus genutia Based on the Optimized MaxEnt Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
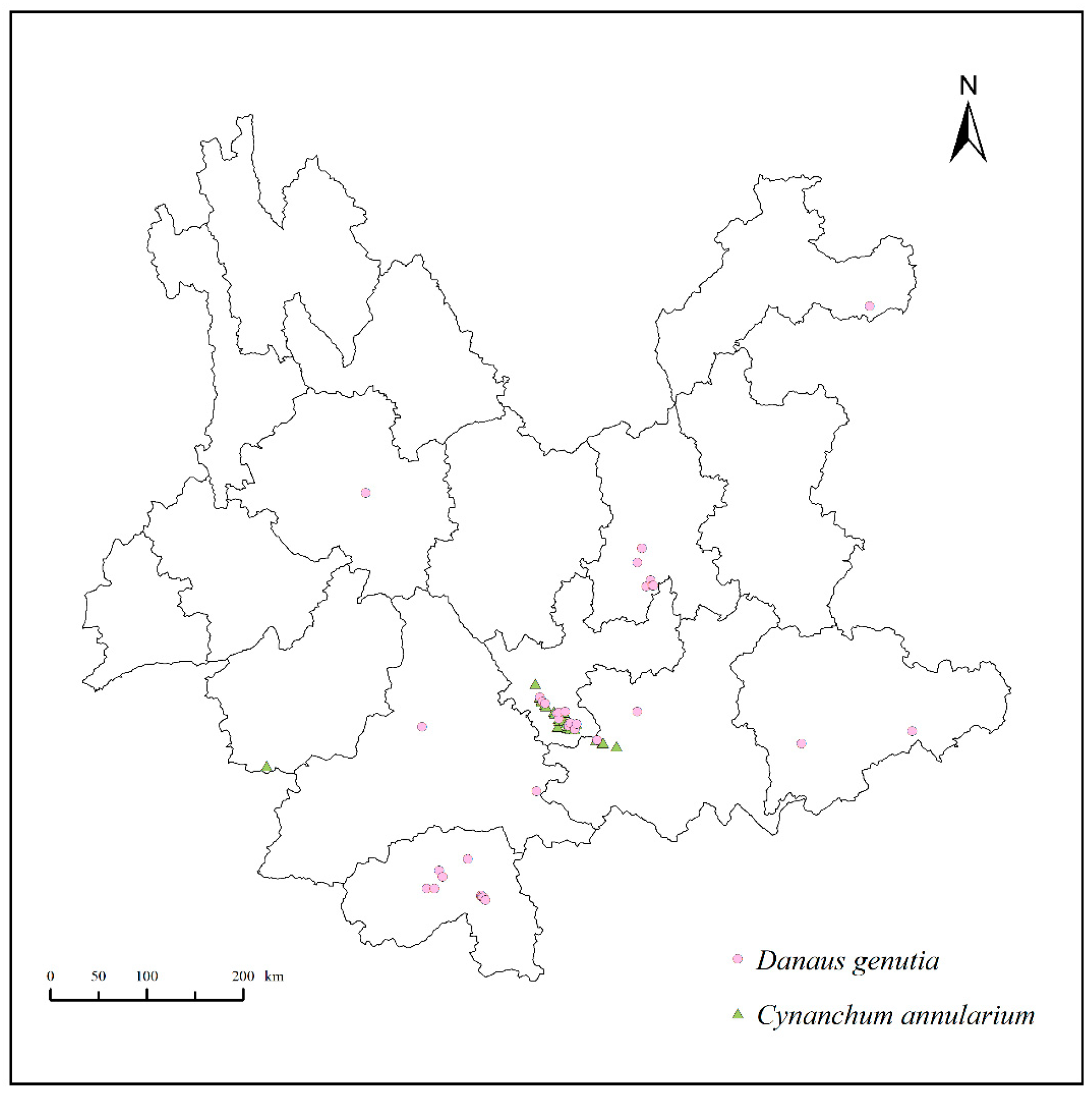
2.1. Collection and Processing of Distribution Data for D. genutia and Its Host Plant
2.2. Model Structure Design and Establishment
2.3. Acquisition and Screening of Environmental Factor Data
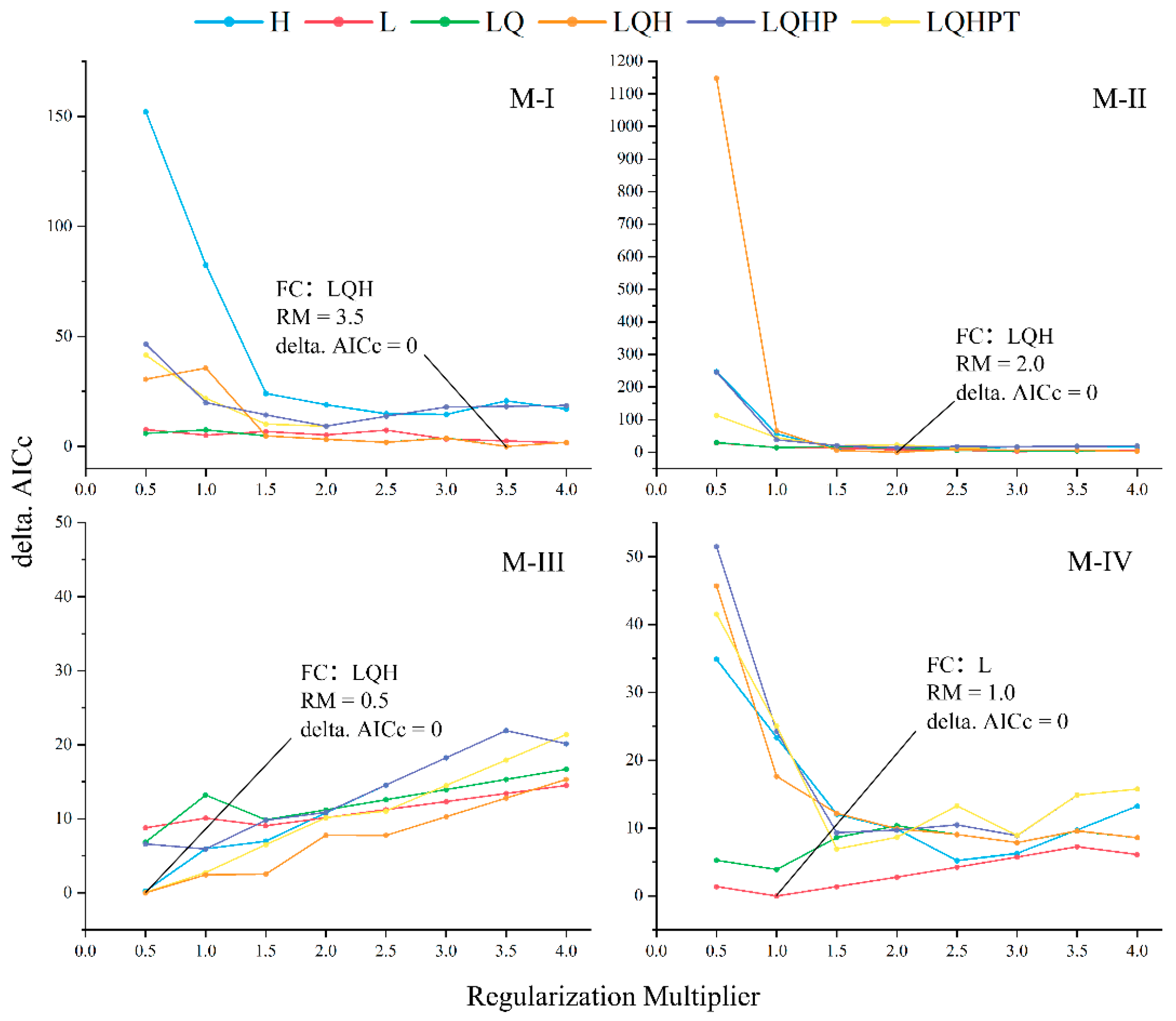
2.4. Model Optimization and Parameter Settings
2.5. Model Accuracy Assessment
2.6. Suitable Habitat Classification
3. Results
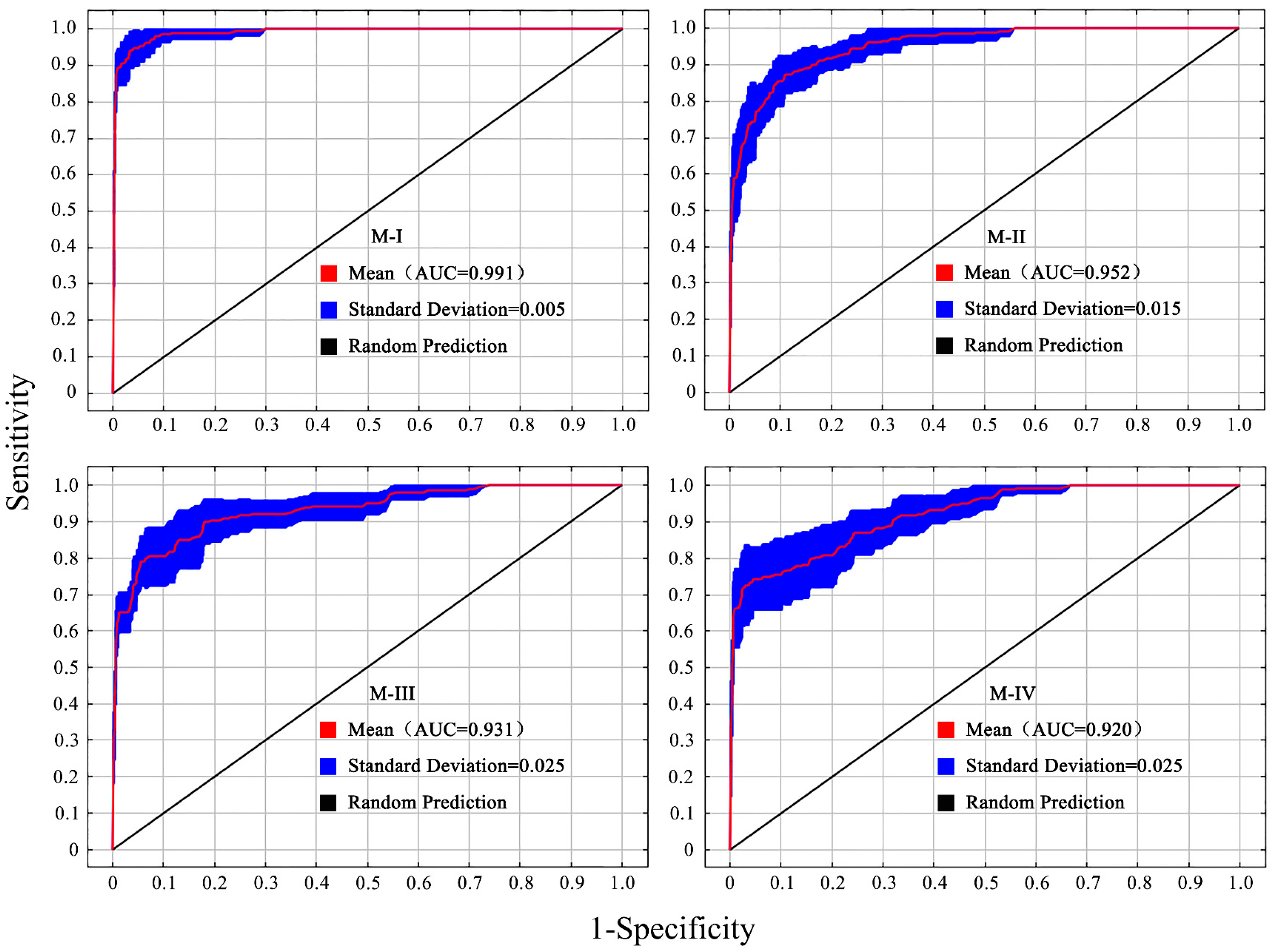
3.1. Model Accuracy Evaluation
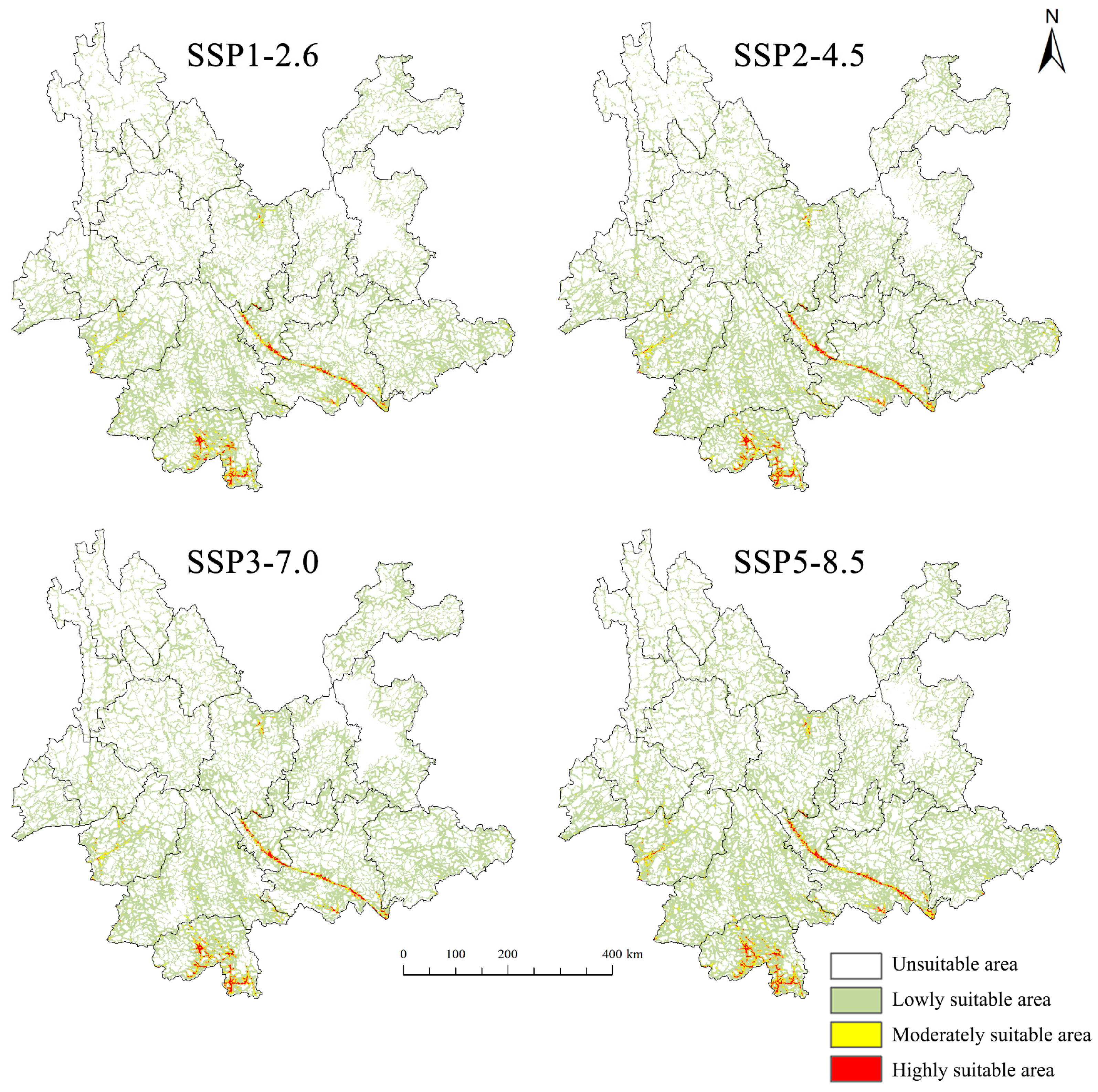
3.2. Potential Suitable Habitats for C. annularium and D. genutia Under Current Climate Conditions
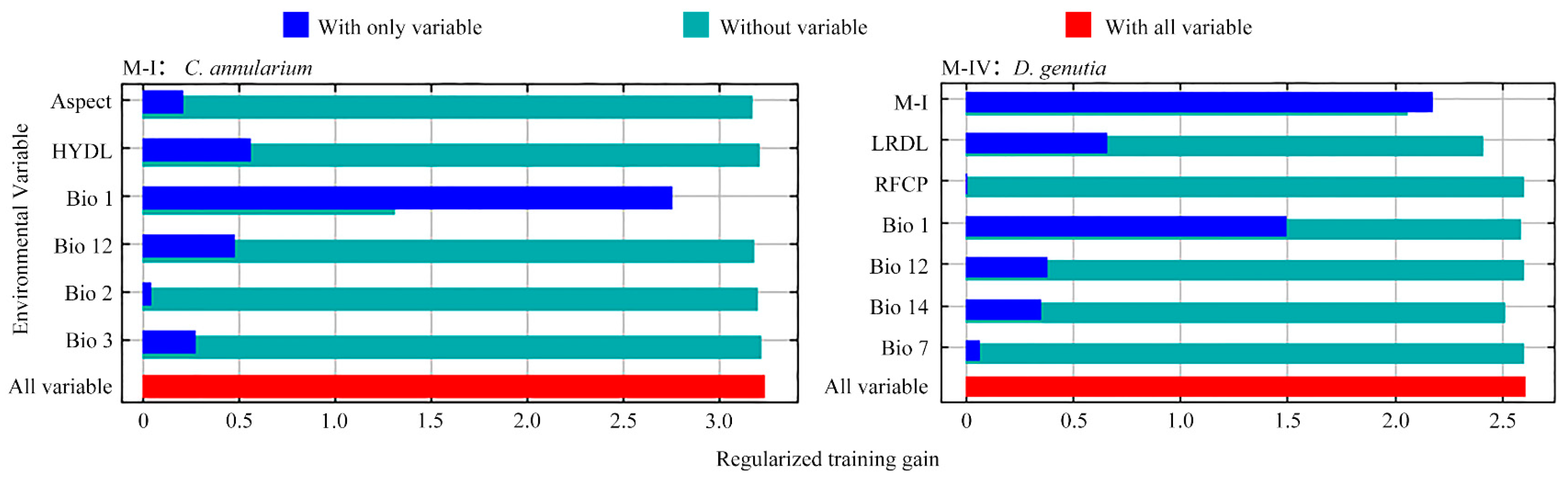
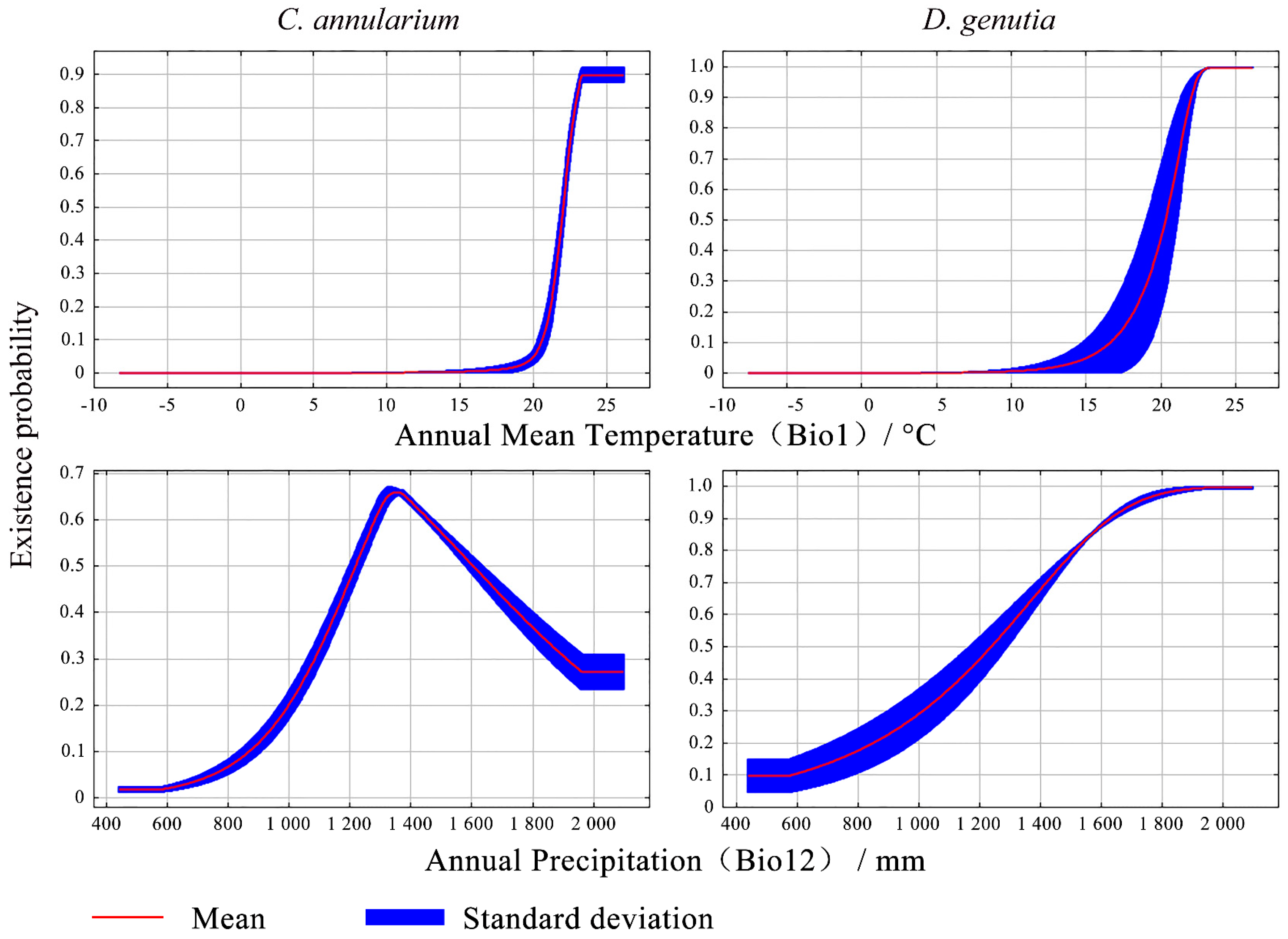
3.3. Main Environmental Factors Influencing the Distribution of D. genutia and Host Plant
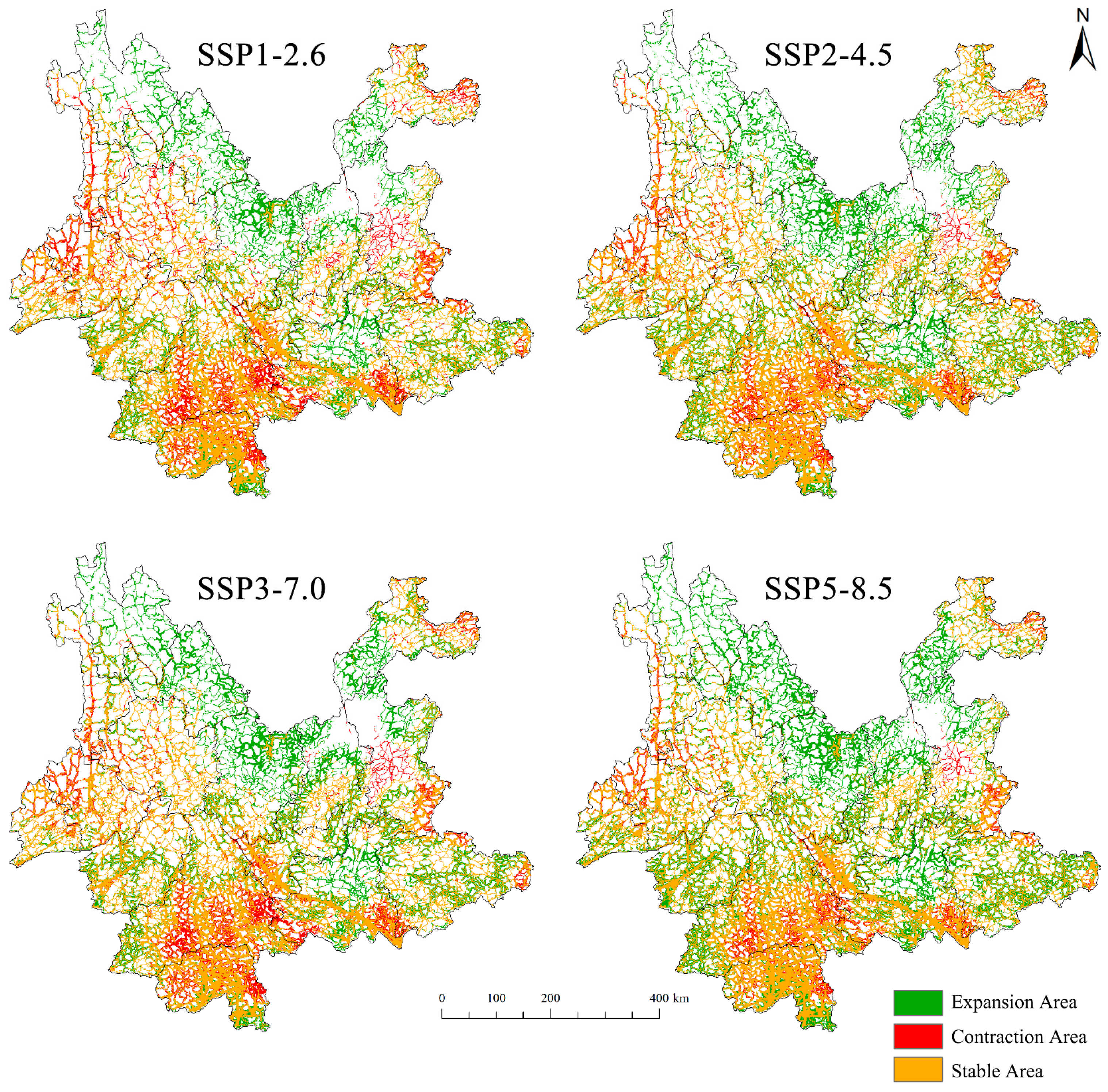
3.4. Changes in Potential Suitable Habitats for D. genutia in Yunnan Province Under Future Climate Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yunnan Province Three Parallel Rivers Administration Bureau. An Overview of the World Natural Heritage “Three Parallel Rivers” and the Protection Progress. Chin. Landsc. Archit. 2010, 26, 52–55. [Google Scholar]
- Xie, X.L. Thoughts on Comprehensively Strengthening Biodiversity Protection in Yunnan Province. Creation 2023, 31, 59–62. [Google Scholar]
- Yu, T.T.; Chang, Z.; Dong, Z.W.; Li, K.Q.; Ma, F.Z.; Wang, W.; Li, X.Y. A glimpse into the biodiversity of insects in Yunnan: An updated and annotated checklist of butterflies (Lepidoptera, Papilionoidea). Zool. Res. 2022, 43, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Burgess, K.S.; Gao, L.M.; Li, D.Z. Distributional responses to climate change for alpine species of Cyananthus and Primula endemic to the Himalaya-Hengduan Mountains. Plant Divers. 2019, 41, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Danaidae. In Monograph of Chinese Butterflies (Revised Edition), 2nd ed.; Henan Scientific and Technological Publishing House: Zhengzhou, China, 2000; pp. 270–271. [Google Scholar]
- Li, G.Q.; Liu, C.C.; Liu, Y.G.; Yang, J.; Zhang, S.X.; Guo, K. Advances in theoretical issues of species distribution models. Acta Ecol. Sin. 2013, 33, 4827–4835. [Google Scholar]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Zimmermann, N.E.; Edwards Jr, T.C.; Graham, C.H.; Pearman, P.B.; Svenning, J.C. New trends in species distribution modelling. Ecography 2010, 33, 985–989. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Zhang, L. The application of MAXENT maximum entropy model in predicting the potential distribution range of species. Bull. Biol. 2015, 50, 9–12. [Google Scholar]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Grazer, V.M.; Martin, O.Y. Investigating climate change and reproduction: Experimental tools from evolutionary biology. Biology 2012, 1, 411–438. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, L.; Rohr, R.P.; Ndiribe, C.; Pradervand, J.N.; Salamin, N.; Guisan, A.; Wisz, M. Combining food web and species distribution models for improved community projections. Ecol. Evol. 2013, 3, 4572–4583. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Li, X.S.; Zhao, C.Y.; Li, F.F.; Zhu, J.F.; Ji, W.J. Simulation of potential suitable distribution of Bhutanitis thaidina and its gap analysis of National Nature Reserves in China under climate change scenarios. J. Environ. Entomol. 2021, 43, 1168–1177. [Google Scholar]
- Zhang, H.F. Prediction of the potential suitable area of the rare and endangered species, Teinopalpus aureus in China. J. Jinggangshan Univ. (Nat. Sci. Ed.) 2023, 44, 56–62. [Google Scholar]
- Yan, M.Y.; Peng, X.C.; Dai, G.H.; Zhang, Z.M. Effects of interaction between host plants and environmental factors on potential distribution analysis of Byasa daemonius. J. West China For. Sci. 2021, 50, 118–123, 131. [Google Scholar]
- Chen, Z.; Cao, Y.; Zhou, Y.Q.; Zhou, C.L.; Chen, X.M.; Shi, L. Biological characteristics of an experimental population of the common tiger butterfly, Danaus genutia (Lepidoptera: Nymphalidae). Chin. J. Appl. Entomol. 2017, 54, 279–291. [Google Scholar]
- Chen, Z.; Zhou, C.L.; Chen, X.M.; Shi, L. Effects of Temperature and Photoperiod on the Development of Immature Stages of Danaus genutia. Sichuan J. Zool. 2018, 37, 420–425. [Google Scholar]
- Chen, Z.; Zhou, C.L. Effects of photoperiod and temperature on the survival of immature stages of Danaus genutia. J. Environ. Entomol. 2020, 42, 938–943. [Google Scholar]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Zhang, L.X.; Chen, X.L.; Xin, X.G. Short commentary on CMIP6 Scenario Model Intercomparison Project (ScenarioMIP). Clim. Change Res. 2019, 15, 519–525. [Google Scholar]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for MAXENT ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Zhu, G.P.; Qiao, H.J. Effect of the MaxEnt model’s complexity on the prediction of species potential distributions. Biodivers. Sci. 2016, 24, 1189–1196. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Jaynes, E.T. Information theory and statistical mechanics. Phys. Rev. 1957, 106, 620–630. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; ACM Press: New York, NY, USA, 2004; pp. 655–662. [Google Scholar]
- Wang, C.; Cai, P.M.; Yi, C.D.; Chen, J.H. Bibliometric based analysis of the risk assessment of alien invasive pest from 2007 to 2017 and introduction of several risk assessment models. J. China Agric. Univ. 2018, 23, 225–238. [Google Scholar]
- Roshani; Rahaman, M.H.; Masroor, M.; Sajjad, H.; Saha, T.K. Assessment of Habitat Suitability and Potential Corridors for Bengal Tiger (Panthera tigris tigris) in Valmiki Tiger Reserve, India, Using MaxEnt Model and Least-Cost Modeling Approach. Environ. Model. Assess. 2024, 29, 405–422. [Google Scholar] [CrossRef]
- Mori, N.; Yamashita, M.; Inoue, M.N. Integration of satellite remote sensing and MaxEnt modeling for improved detection and management of forest pests. Environ. Monit. Assess. 2024, 196, 616. [Google Scholar] [CrossRef]
- Huan, Z.Q.; Geng, X.M.; Xu, X.R.; Liu, W.; Zhu, Z.L.; Tang, M. Potential Geographical Distribution of Michelia martinii under Different Climate Change Scenarios Based on Max-Ent Model. J. Ecol. Rural Environ. 2023, 39, 1277–1287. [Google Scholar]
- Jiménez-Valverde, A.; Peterson, A.T.; Soberón, J.; Overton, J.M.; Aragón, P.; Lobo, J.M. Use of niche models in invasive species risk assessments. Biol. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.; Guisan, A.; et al. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef]
- James, D.G.; James, T.A. Migration and overwintering in australian monarch butterflies (Danaus plexippus (L.) (Lepidoptera: Nymphalidae): A review with new observations and research needs. J. Lepid. Soc. 2019, 73, 177–190. [Google Scholar] [CrossRef]
- Chen, X.M.; Zhou, C.L.; Shi, J.Y.; Shi, L.; Yi, C.H. Ornamental Butterflies in China; China Forestry Publishing House: Beijing, China, 2008; pp. 43, 209. [Google Scholar]
- Tsiang, Y.; Li, P.T. Delectis Florae Reipublicae Popularis Sinicae, Agendae Academiae Sinicae Edita. In Flora Reipublicae Popularis Sinicae (Tomus 63); Science Press: Beijing, China, 1977; pp. 410–412. [Google Scholar]
- Liu, F.Y.; Zhu, H. Numerical classification and diversity analysis for the vegetation in the dry-hot valley of Yuanjiang, Yunnan Province. Guihaia 2005, 25, 22–25, 92. [Google Scholar]
- Shen, R.; Zhang, J.L.; He, B.; Li, F.; Zhang, Z.M.; Zhou, R.; Ou, X.K. The structure characteristic and analysis on similarity of grassland community in dry-hot valley of Yuanjiang River. Ecol. Environ. Sci. 2010, 19, 2821–2825. [Google Scholar]
- Chen, M.Y.; Li, Z.L.; Wang, A.M.; Liu, Z.Q. The Diversity of Butterflies in Xishuangbanna; Yunnan Fine Arts Publishing House: Kunming, China, 2012; pp. 1, 66. [Google Scholar]
- Zhang, X.; Gao, S.T.; Lu, Z.X.; Chen, Y.Q. Effects of different habitat types and environmental factors on butterfly diversity in Xishuangbanna, southwestern China on varying regional scale. Acta Entomol. Sin. 2022, 65, 73–83. [Google Scholar]
- Wang, S.; Bao, F.Y.; Mei, B.M.; Ding, S.C. Vertical distribution and community diversity of butterflies in Yaoluoping National Nature Reserve, Anhui, China. Chin. J. Appl. Ecol. 2009, 20, 2262–2270. [Google Scholar]
- Zhang, Y.X.; Liu, Z.X.; Yu, G.Q. Faunistic Structure and Vertical Distribution of Butterflies in Hupingshan National Nature Reserve, Hunan. Sichuan J. Zool. 2007, 26, 892–897. [Google Scholar]
- Wettstein, W.; Schmid, B. Conservation of arthropod diversity in montane wetlands: Effect of altitude, habitat quality and habitat fragmentation on butterflies and grasshoppers. J. Appl. Ecol. 1999, 36, 363–373. [Google Scholar] [CrossRef]
- Xie, Z.P.; Ni, Y.Q.; Li, Z.Z.; Li, X.M. Vertical distribution and diversity of butterflies in the northern slopes of Qilian Mountains and Hexi Corridor. Acta Prataculturae Sin. 2009, 18, 195–201. [Google Scholar]
- Yu, F.; Wang, H.; Wang, S.K.; Zhang, Q.; Ji, R. Response of Parnassius apollo population and vertical distribution to climate warming. Acta Ecol. Sin. 2012, 32, 6203–6209. [Google Scholar]
- Yang, F.C.; Ma, X.H.; He, Q.; Dai, F.W.; Huang, H.P.; Li, Y.; Gou, J.Y.; Kritana, P.; Chaya, S.; Wen, H.T. Processing of the Major Climate Factors in Yuanjiang-Red River (China section) in the Past Fifty-seven Years (1962–2018). Environ. Impact Assess. 2023, 45, 115–121. [Google Scholar]
- Yao, P.; Kou, W.L.; Wang, Q.H.; Han, Y.T. Relationship between Climate Change and Rubber Planting in Xishuangbanna in Recent 60 years. For. Inventory Plan. 2020, 45, 17–23. [Google Scholar]
- Ji, W.J.; Zhang, L.C.; Zhang, J.Y.; Yang, X.P. Characteristics of Agricultural Climate Resources in Xishuangbanna Tea Area under Global Climate Change. Southwest China J. Agric. Sci. 2016, 29, 2988–2993. [Google Scholar]
- Ma, R.J.; Jiang, Z.G. Impact of global climate change on wildlife. Acta Ecol. Sin. 2005, 25, 3061–3066. [Google Scholar]
- Hill, J.K.; Thomas, C.D.; Fox, R.; Telfer, M.G.; Willis, S.G.; Asher, J.; Huntley, B. Responses of butterflies to twentieth century climate warming: Implications for future ranges. Proc. R. Soc. London. Ser. B Biol. Sci. 2002, 269, 2163–2171. [Google Scholar] [CrossRef]
- Mikkola, K. Population trends of Finnish Lepidoptera during 1961–1996. Entomol. Fenn. 1997, 8, 121–143. [Google Scholar] [CrossRef]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.D.; Descimon, H.; Huntley, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Crozier, L.G. Field transplants reveal summer constraints on a butterfly range expansion. Oecologia 2004, 141, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Crozier, L. Winter warming facilitates range expansion: Cold tolerance of the butterfly Atalopedes campestris. Oecologia 2003, 135, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Pollard, E. Population ecology and change in range of the white admiral butterfly Ladoga camilla L. in England. Ecol. Entomol. 1979, 4, 61–74. [Google Scholar] [CrossRef]
- Li, H.; Qin, Y.; Yuan, X.M. Investigation on the traditional production techniques of sheep milk pickled vegetable in Jianshui County. Rural Econ. Sci. -Technol. 2018, 29, 105–106. [Google Scholar]
- Li, Y.Y.; Li, H.L.; Li, J.Y.; Bai, Y.Y.; Yan, G.Y.; Zhang, M.; Li, T.; You, J.P. Study on Pharmacognosy of Yao Medicine Cynanchum corymbosum Wight. Asia-Pac. Tradit. Med. 2024, 20, 56–60. [Google Scholar]


| Model | Species | Biotic Factor | Abiotic Factor |
|---|---|---|---|
| M-I | Cynanchum annularium | / | Climate, Terrain, Natural environment, Human activities |
| M-II | Danaus genutia | / | Climate, Terrain, Natural environment, Human activities |
| M-III | Danaus genutia | Vegetation, M-I | / |
| M-IV | Danaus genutia | Vegetation, M-I | Climate, Terrain, Natural environment, Human activities |
| Code | Environmental Factor | M-I (6) | M-I (9) | M-III (2) | M-IV (7) |
|---|---|---|---|---|---|
| Bio 1 | Annual Mean Temperature | √ | √ | × | √ |
| Bio 2 | Mean Diurnal Range | √ | √ | × | × |
| Bio 3 | Isothermality | √ | × | × | × |
| Bio 4 | Temperature Seasonality | × | √ | × | × |
| Bio 5 | Max Temperature of Warmest Month | × | × | × | × |
| Bio 6 | Min Temperature of Coldest Month | × | × | × | × |
| Bio 7 | Temperature Annual Range | × | √ | × | √ |
| Bio 8 | Mean Temperature of Wettest Quarter | × | × | × | × |
| Bio 9 | Mean Temperature of Driest Quarter | × | × | × | × |
| Bio 10 | Mean Temperature of Warmest Quarter | × | × | × | × |
| Bio 11 | Mean Temperature of Coldest Quarter | × | × | × | × |
| Bio 12 | Annual Precipitation | √ | × | × | √ |
| Bio 13 | Precipitation of Wettest Month | × | × | × | × |
| Bio 14 | Precipitation of Driest Month | × | × | × | √ |
| Bio 15 | Precipitation Seasonality | × | × | × | × |
| Bio 16 | Precipitation of Wettest Quarter | × | × | × | × |
| Bio 17 | Precipitation of Driest Quarter | × | × | × | × |
| Bio 18 | Precipitation of Warmest Quarter | × | √ | × | × |
| Bio 19 | Precipitation of Coldest Quarter | × | × | × | × |
| Altitude | Altitude | × | × | × | × |
| Slope | Slope | × | × | × | × |
| Aspect | Aspect | √ | √ | × | × |
| HYDL | Water system | √ | × | × | × |
| RESP | Residential area | × | √ | × | × |
| RFCP | Establishment points | × | √ | × | √ |
| LRDL | Road | × | √ | × | √ |
| VEGA | Vegetation type | × | × | √ | × |
| M-I | Potential distribution of host plant | × | × | √ | √ |
| Suitable Level | M-I | M-II | M-III | M-IV | ||||
|---|---|---|---|---|---|---|---|---|
| Area | Proportion (%) | Area | Proportion (%) | Area | Proportion (%) | Area | Proportion (%) | |
| Highly suitable area | 0.061 | 2.35 | 0.167 | 0.86 | 0.143 | 2.77 | 0.160 | 1.52 |
| Moderately suitable area | 0.201 | 7.73 | 0.465 | 2.39 | 0.266 | 5.15 | 0.223 | 2.13 |
| Lowly suitable area | 2.339 | 89.92 | 18.841 | 96.75 | 4.752 | 92.08 | 10.111 | 96.35 |
| Total suitable area | 2.601 | 100.00 | 19.473 | 100.00 | 5.161 | 100.00 | 10.494 | 100.00 |
| Model | Species | Environmental Factor | Percent Contribution (%) | Permutation Importance (%) |
|---|---|---|---|---|
| M-I | C. annularium | Bio 1 | 85.8 | 94.1 |
| Bio 3 | 4.5 | 0.6 | ||
| Aspect | 3.3 | 1.7 | ||
| HYDL | 3.1 | 1.7 | ||
| Bio 12 | 2.6 | 1.4 | ||
| Bio 2 | 0.6 | 0.5 | ||
| M-IV | D. genutia | M-II | 81.3 | 26.7 |
| LRDL | 9.7 | 51.6 | ||
| Bio 14 | 5.1 | 15.1 | ||
| Bio 12 | 2.2 | 0 | ||
| Bio 1 | 1.4 | 4.8 | ||
| RFCP | 0.2 | 0.1 | ||
| Bio 7 | 0.2 | 1.6 |
| Suitable Level | Current | 2040s | |||
|---|---|---|---|---|---|
| SSP1-2.6 | SSP2-4.5 | SSP3-7.0 | SSP5-8.5 | ||
| Highly suitable area | 0.160 | 0.138 | 0.183 | 0.163 | 0.202 |
| Moderately suitable area | 0.223 | 0.292 | 0.348 | 0.297 | 0.426 |
| Lowly suitable area | 10.111 | 11.475 | 13.315 | 12.803 | 14.663 |
| Total suitable area | 10.494 | 11.905 | 13.846 | 13.263 | 15.291 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Zhou, C.; Wang, W.; Li, Y.; Du, T.; Shi, L. Habitat Suitability of Danaus genutia Based on the Optimized MaxEnt Model. Insects 2024, 15, 971. https://doi.org/10.3390/insects15120971
Yao J, Zhou C, Wang W, Li Y, Du T, Shi L. Habitat Suitability of Danaus genutia Based on the Optimized MaxEnt Model. Insects. 2024; 15(12):971. https://doi.org/10.3390/insects15120971
Chicago/Turabian StyleYao, Jun, Chengli Zhou, Wenquan Wang, Yangyang Li, Ting Du, and Lei Shi. 2024. "Habitat Suitability of Danaus genutia Based on the Optimized MaxEnt Model" Insects 15, no. 12: 971. https://doi.org/10.3390/insects15120971
APA StyleYao, J., Zhou, C., Wang, W., Li, Y., Du, T., & Shi, L. (2024). Habitat Suitability of Danaus genutia Based on the Optimized MaxEnt Model. Insects, 15(12), 971. https://doi.org/10.3390/insects15120971






