Communities of Digger Wasps (Hymenoptera: Spheciformes) along a Tree Cover Gradient in the Cultural Landscape of River Valleys in Poland
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
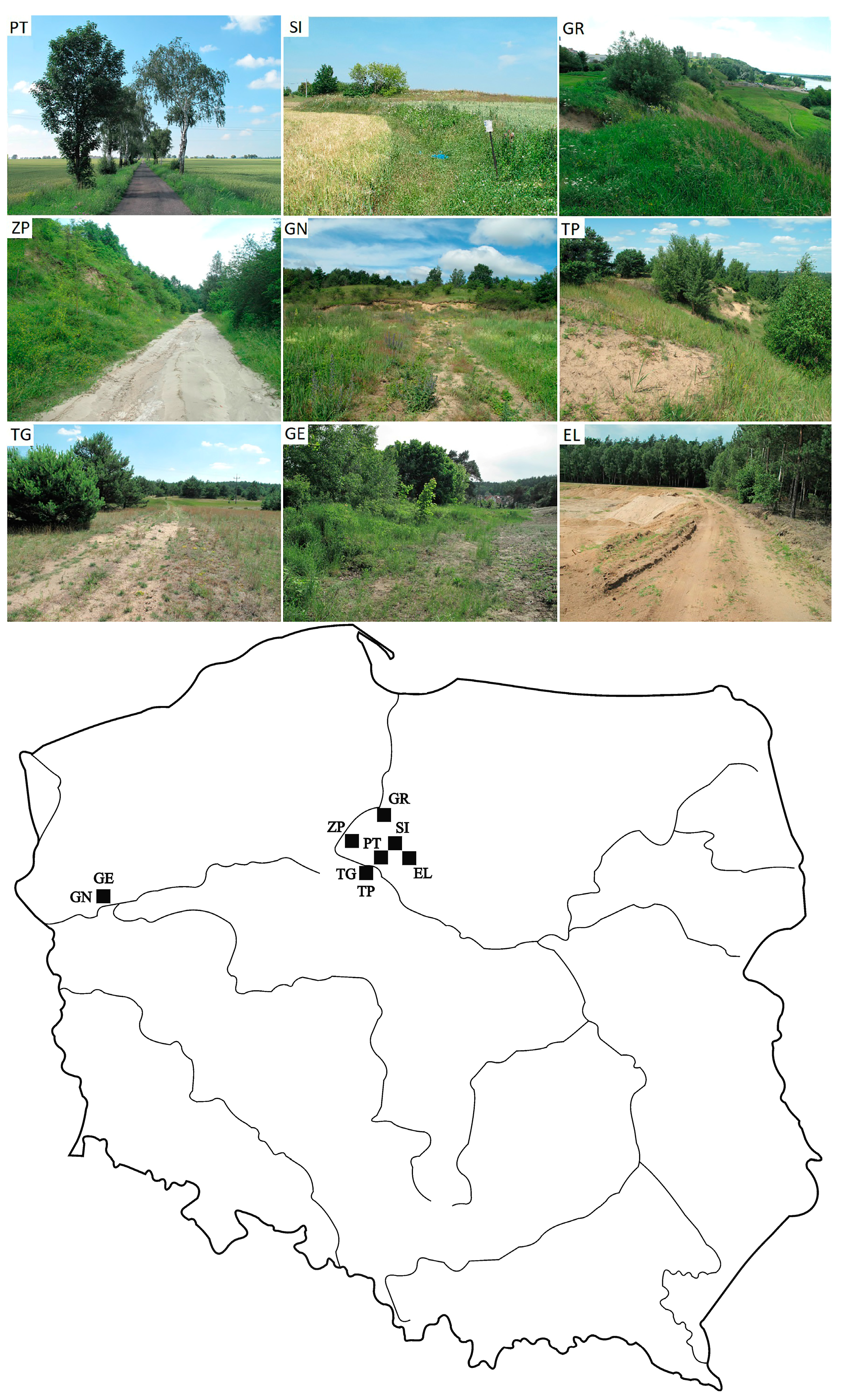
2.1. Study Area and Sites
- (i)
- endogeic species, digging nests in the soil and occupying existing cracks in the soil;
- (ii)
- hypergeic species, nesting above the ground in woods or plant shoots.
2.2. Digger Wasp Collection
2.3. Statistical Analysis
3. Results
3.1. Dominant Species
3.2. Habitat Preferences
3.3. Rare Species
- CR (critically endangered): Stizus perrisi Dufour;
- EN category (Endangered): Mimumesa littoralis (Bond.);
- VU category (vulnerable): Ectemnius fossorius (L.) and Miscophus postumus Bisch.;
- NT category (near threatened): Lestica subterranea (Fabr.), Mimumesa beaumonti Lith, Harpactus pulchellus (Costa), Harpactus leavis (Latr.), Cerceris flavilabris (Fabr.);
- LC category (Least Concern): Tachysphex fulvitarsis (Costa), Tachysphex psammobius (Kohl), Oxybelus variegatus Wesm., O. argentatus Curt., Nysson niger Chevr., Miscophus spurius (Dahlb.), Miscophus niger Dahlb., M. concolor Dahlb., L. alata (Panz.), Harpactus elegans (Lep.), Gorytes fallax Handl., Dryudella pinguis (Dahlb.), Crossocerus tarsatus Shuck., C. cinxius Dahlb., Bembix rostrata (L.) and Bembecinus tridens (Fabr.);
- DD category (data-deficient): Astata kashmirensis Nurse, Crossocerus styrius Kohl., Miscophus ater Lep., Tachysphex tarsinus (Lep.) and Passaloecus brevilabris Wolf.
3.4. The Influence of Woodland Cover
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bohart, R.M.; Menke, A.S. Digger Wasps of the World. A Generic Revision; University of California Press: Berkeley, CA, USA, 1976; 696p. [Google Scholar]
- Blösch, M. Die Grabwespen Deutschlands—Lebensweise, Verhalten, Verbreitung; Goecke & Evers: Keltern, Germany, 2000; 480p. [Google Scholar]
- Heneberg, P.; Bogusch, P.; Rehounek, J. Sandpits provide critical refuge for bees and wasps (Hymenoptera: Apocrita). J. Insect Conserv. 2013, 17, 473–490. [Google Scholar] [CrossRef]
- Heneberg, P.; Bogusch, P.; Řezáč, M. Roadside verges can support spontaneous establishment of steppe-like habitats hosting diverse assemblages of bees and wasps (Hymenoptera: Aculeata) in an intensively cultivated central European landscape. Biodivers. Conserv. 2017, 26, 843–864. [Google Scholar] [CrossRef]
- Szczepko, K. Ekologia Grzebaczowatych (Hymenoptera, Apoidea, Spheciformes) Odłogów w Kampinoskim Parku Narodowym [Ecology of Digger Wasps (Hymenoptera, Apoidea, Spheciformes) of Fallow Land in Kampinos National Park]; Wydawnictwo Uniwersytetu Łódzkiego: Łódź, Poland, 2013; 168p. [Google Scholar]
- Bogusch, P.; Macek, J.; Janšta, P.; Kubík, Š.; Řezáč, M.; Holý, K.; Malenovský, I.; Baňař, P.; Mikát, M.; Astapenková, A.; et al. Post-industrial habitats serve as critical refuges for pioneer species of newly identified arthropod assemblages associated with reed galls. Biodivers. Conserv. 2016, 25, 827–863. [Google Scholar] [CrossRef]
- Wallis de Vries, M.F.; Poschlod, P.; Willems, J.H. Challenges for the conservation of calcareous grasslands in northwestern Europe: Integrating the requirements of flora and fauna. Biol. Conserv. 2002, 104, 265–273. [Google Scholar] [CrossRef]
- Barańska, K.; Żmihorski, M. Occurrence of rare and protected plant species related to species richness in calcareous xerothermic grassland. Pol. J. Ecol. 2008, 56, 343–350. [Google Scholar]
- Tscharntke, T.; Gathmann, A.; Steffan-Dewenter, I. Bioindication using trap-nesting bees and wasps and their natural enemies: Community structure and interactions. J. Appl. Ecol. 1998, 35, 708–719. [Google Scholar] [CrossRef]
- Szczepko, K.; Kowalczyk, J.K. Digger wasps (Hymenoptera: Sphecidae) in habitats of abandoned village in forest territory of Kampinos National Park (Poland). Pol. Pismo Entomol. 2001, 70, 185–193. [Google Scholar]
- Saure, C. Bienen und Wespen eines ehemaligen militärischen Übungsgeländes in Berlin-Lichterfelde (Hymenoptera). Märkische Entomol. Nachrichten 2015, 17, 1–36. [Google Scholar]
- Shlyakhtenok, A.S.; Skibińska, E. Contribution to the knowledge of Aculeate Hymenoptera of Byelorus. Family Digger Wasps (Sphecidae). Vestn. Zool. 2002, 36, 31–40. [Google Scholar]
- Steffan-Dewenter, I. Landscape context affects trap-nesting bees, wasps, and their natural enemies. Ecol. Entomol. 2002, 27, 631–637. [Google Scholar] [CrossRef]
- Kazenas, V.L. Fauna and Biology of Sphecid Wasps (Hymenoptera, Sphecidae) of Kazakhstan and Central Asia; Kazgos INTI: Almaty, Kazakhstan, 2001; 333p. [Google Scholar]
- Pulawski. 2023. Available online: https://www.calacademy.org/scientists/projects/catalog-of-sphecidae (accessed on 24 October 2023).
- Skibińska, E. Structure of Sphecidae (Hymenoptera) communities in urban green areas in Warsaw. Memorab. Zool. 1986, 41, 141–202. [Google Scholar]
- Skibińska, E. Sphecidae (Aculeata) of subcontinental pine forest stands (Peucedano-Pinetum) of various ages in Puszcza Białowieska. Fragm. Faun. 1995, 38, 419–433. [Google Scholar] [CrossRef]
- Skibińska, E. Aculeata (Hymenoptera) of linden-oak-hornbeam and thermophilous oak forests of the Mazovian Lowland. Fragm. Faun. 1989, 32, 197–224. [Google Scholar] [CrossRef]
- Skibińska, E. Predatory Aculeata (Hymenoptera) of moist meadows on the Mazovian Lowland. Memorab. Zool. 1989, 43, 289–296. [Google Scholar]
- Skibińska, E. Sphecidae (Hymenoptera) of Warsaw and Mazovia. Memorab. Zool. 1982, 36, 103–127. [Google Scholar]
- Trojan, P.; Bańkowska, R.; Chudzicka, E.; Pilipiuk, I.; Skibińska, E.; Sterzyńska, M.; Wytwer, J. Secondary succession of fauna in the pine forests of Puszcza Białowieska. Fragm. Faun. 1994, 37, 3–104. [Google Scholar] [CrossRef]
- Kowalczyk, J.K. Żądłówki (Aculeata, Hymenoptera) Wyżyny Łódzkiej. Część I—Okolice Rogowa (woj. skierniewickie) [Aclueates (Aculeata, Hymenoptera) of Łódź Upland. Part one—Rogów vicinity (Skierniewice voivodship)]. Acta Univ. Lodz. Folia Zool. Anthropol. 1988, 6, 39–55. [Google Scholar]
- Kowalczyk, J.K. Materiały do znajomości żądłówek (Hymenoptera, Aculeata) Łodzi. Acta Universitatis Lodziensis [Contribution to the knowledge of Aculeata (Hymenoptera) of the city of Łódź]. Folia Zool. Anthropol. 1991, 7, 67–114. [Google Scholar]
- Kowalczyk, J.K. Materiały do znajomości żądłówek Puszczy Augustowskiej [Contribution to the knowledge of Aculeata (Hymenoptera) of Augustów Forest]. Acta Universitatis Lodziensis. Folia Zool. Anthropol. 1991, 7, 115–129. [Google Scholar]
- Wiśniowski, B. Żądłówki z rodzin Tiphiidae, Sapygidae, Mutillidae, Pompilidae, Eumenidae, Vespidae i Sphecidae (Hymenoptera: Aculeata) Ojcowskiego Parku Narodowego. Część II. Analiza zgrupowań [Aculeata of the families Tiphiidae, Sapygidae, Mutillidae, Pompilidae, Eumenidae, Vespidae and Sphecidae (Hymenoptera: Aculeata) of Ojców National Park. Part 2: Analyses of insect assemblages]. Prądnik Pr. Mater. Muz. Szafera 2005, 15, 311–338. [Google Scholar]
- Kowalczyk, J.K. Wybrane rodziny żądłówek (Hymenoptera, Aculeata) Świętokrzyskiego Parku Narodowego [Selected families of Aculeata (Hymenoptera) of Świętokrzyski National Park]. Fragm. Faun. 1990, 33, 285–306. [Google Scholar] [CrossRef]
- Pawlikowski, T.; Kruszyński, T. Materiały do studiów nad strukturą zespołów żądłówek (Hymenoptera, Aculeata) Polski. Wiadomości Entomol. 1996, 16, 165–176. [Google Scholar]
- Twerd, L.; Krzyżyński, M.; Waldon-Rudzionek, B.; Olszewski, P. Can soda ash dumping grounds provide replacement habitats for digger wasps (Hymenoptera, Apoidea, Spheciformes)? PLoS ONE 2017, 12, e0175664. [Google Scholar] [CrossRef] [PubMed]
- Twerd, L.; Szefer, P.; Sobieraj-Betlińska, A.; Olszewski, P. The conservation value of Aculeata communities in sand quarries changes during ecological succession. Glob. Ecol. Conserv. 2021, 28, e01693. [Google Scholar] [CrossRef]
- Witt, R. Wespen, 2nd ed.; Vademecum: Oldenburg, Germany, 2009; 400p. [Google Scholar]
- Skibińska, E. Sphecidae Grzebaczowate. W: Czerwona lista zwierząt ginących i zagrożonych w Polsce [Apoidea Bees]. In Red List of Threatened Animals in Poland; Głowaciński, Z., Ed.; Instytut Ochrony Przyrody Polskiej Akademii Nauk: Kraków, Poland, 2002; pp. 66–68. [Google Scholar]
- Olszewski, P.; Wiśniowski, B.; Ljubomirov, T. Current list of the Polish digger wasps (Hymenoptera: Spheciformes). Spixiana 2021, 44, 81–107. [Google Scholar]
- Lomholdt, O. The Sphecidae (Hymenoptera) of Fennoscandia and Denmark. In Fauna Entomologica Scandinavica (Klampenbork); Scandinavian Science Press: Klampenborg, Denmark, 1984; Volume 4, pp. 1–452. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering plants and pteridophytes of Poland. A checklist. In Biodiversity of Poland; Mirek, Z., Ed.; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002; Volume 1, 442p. [Google Scholar]
- Dollfuss, H. Bestimmungsschlüssel der Grabwespen Nord- und Zentraleuropas. (Hymenoptera, Sphecidae) mit speziellen Angaben zur Grabwespenfauna Österreichs. Stapfia 1991, 24, 1–247. [Google Scholar]
- Bitsch, J.; Leclercq, J. Faune de France. France et Régions Limitrophes. 79. Hyménoptères Sphecidae d’Europe Occidentale; Généralités—Crabroninae; Fédération Française des Sociétés de Sciences Naturelles: Paris, France, 1993; Volume 1. [Google Scholar]
- Bitsch, J.; Barbier, Y.; Gayubo, S.F.; Schmidt, K.; Ohl, M. Faune de France. France et Régions Limitrophes. 82. Hyménoptères Sphecidae d’Europe Occidentale; Fédération Française des Sociétés de Sciences Naturelles: Paris, France, 1997; Volume 2. [Google Scholar]
- Bitsch, J.; Dollfuss, H.; Bouček, Z.; Schmidt, K.; Schmid-Egger, C.; Gayubo, S.F.; Antropov, A.V.; Barbier, Y. Faune de France. France et Régions Limitrophes. 86. Hyménoptères Sphecidae d’Europe Occidentale; Fédération Française des Sociétés de Sciences Naturelles: Paris, France, 2007; Volume 3. [Google Scholar]
- Bitsch, J.; Dollfuss, H.; Bouček, Z.; Schmidt, K.; Schmid-Egger, C.; Gayubo, S.F.; Antropov, A.V.; Barbier, Y. Faune de France. France et Régions Limitrophes. 86. Hyménoptères Sphecidae d’Europe Occidentale, 2nd ed.; Compléments à la Première Edition; Fédération Française des Sociétés de Sciences Naturelles: Paris, France, 2007. [Google Scholar]
- Jacobs, H.J. Die Grabwespen Deutschlands. Ampulicidae, Sphecidae, Crabronidae; Goecke & Evers: Keltern, Germany, 2007; 207p. [Google Scholar]
- Hammer, Ř.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Tropek, R.; Cerna, I.; Straka, J.; Kadlec, T.; Pech, P.; Tichanek, F.; Sebek, P. Restoration management of fly ash deposits crucially influence their conservation potential for terrestrial arthropods. Ecol. Eng. 2014, 73, 45–52. [Google Scholar] [CrossRef]
- Moroń, D.; Skórka, P.; Lenda, M.; Rożej-Pabijan, E.; Wantuch, M.; Kajzer-Bonk, J.; Celary, W.; Mielczarek, Ł.E.; Tryjanowski, P. Railway embankments as new habitat for pollinators in an agricultural landscape. PLoS ONE 2014, 9, e101297. [Google Scholar] [CrossRef]
- Saure, C. Bienen und Wespen des Forts Hahneberg in Berlin-Spandau (Hymenoptera). Märkische Entomol. Nachrichten 2011, 13, 189–219. [Google Scholar]
- Saure, C. Beitrag zur Stechimmenfauna von Sachsen-Anhalt, Teil 1: Das FFH Gebiet ‘Heide südlich Burg’ (Hymenoptera: Aculeata). Entomol. Z. Stuttg. 2011, 121, 195–208. [Google Scholar]
- Saure, C. Bienen und Wespen der Gosener Wiesen in Berlin, Bezirk Treptow-Köpenick (Hymenoptera). Märkische Entomol. Nachrichten 2013, 15, 1–54. [Google Scholar]
- Szczepko, K.; Kruk, A.; Bartos, M. The role of mosaicity of the post-agriculture area of the Kampinos National Park in determining the diversity of species of spider wasps (Hymenoptera: Pompilidae). Eur. J. Entomol. 2012, 109, 35–46. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewski, P.; Sparks, T.; Twerd, L.; Wiśniowski, B. Communities of Digger Wasps (Hymenoptera: Spheciformes) along a Tree Cover Gradient in the Cultural Landscape of River Valleys in Poland. Insects 2024, 15, 88. https://doi.org/10.3390/insects15020088
Olszewski P, Sparks T, Twerd L, Wiśniowski B. Communities of Digger Wasps (Hymenoptera: Spheciformes) along a Tree Cover Gradient in the Cultural Landscape of River Valleys in Poland. Insects. 2024; 15(2):88. https://doi.org/10.3390/insects15020088
Chicago/Turabian StyleOlszewski, Piotr, Tim Sparks, Lucyna Twerd, and Bogdan Wiśniowski. 2024. "Communities of Digger Wasps (Hymenoptera: Spheciformes) along a Tree Cover Gradient in the Cultural Landscape of River Valleys in Poland" Insects 15, no. 2: 88. https://doi.org/10.3390/insects15020088
APA StyleOlszewski, P., Sparks, T., Twerd, L., & Wiśniowski, B. (2024). Communities of Digger Wasps (Hymenoptera: Spheciformes) along a Tree Cover Gradient in the Cultural Landscape of River Valleys in Poland. Insects, 15(2), 88. https://doi.org/10.3390/insects15020088






