Heat Shock Protein Genes Affect the Rapid Cold Hardening Ability of Two Invasive Tephritids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. RNA Extraction and Transcriptome Sequencing
2.3. Real-Time PCR and dsRNA Synthesis
2.4. Extreme Cold Stress and Hardening Response after dsHsp Feeding
3. Results
3.1. Low-Temperature Treatment
3.2. Transcriptome Data Analysis
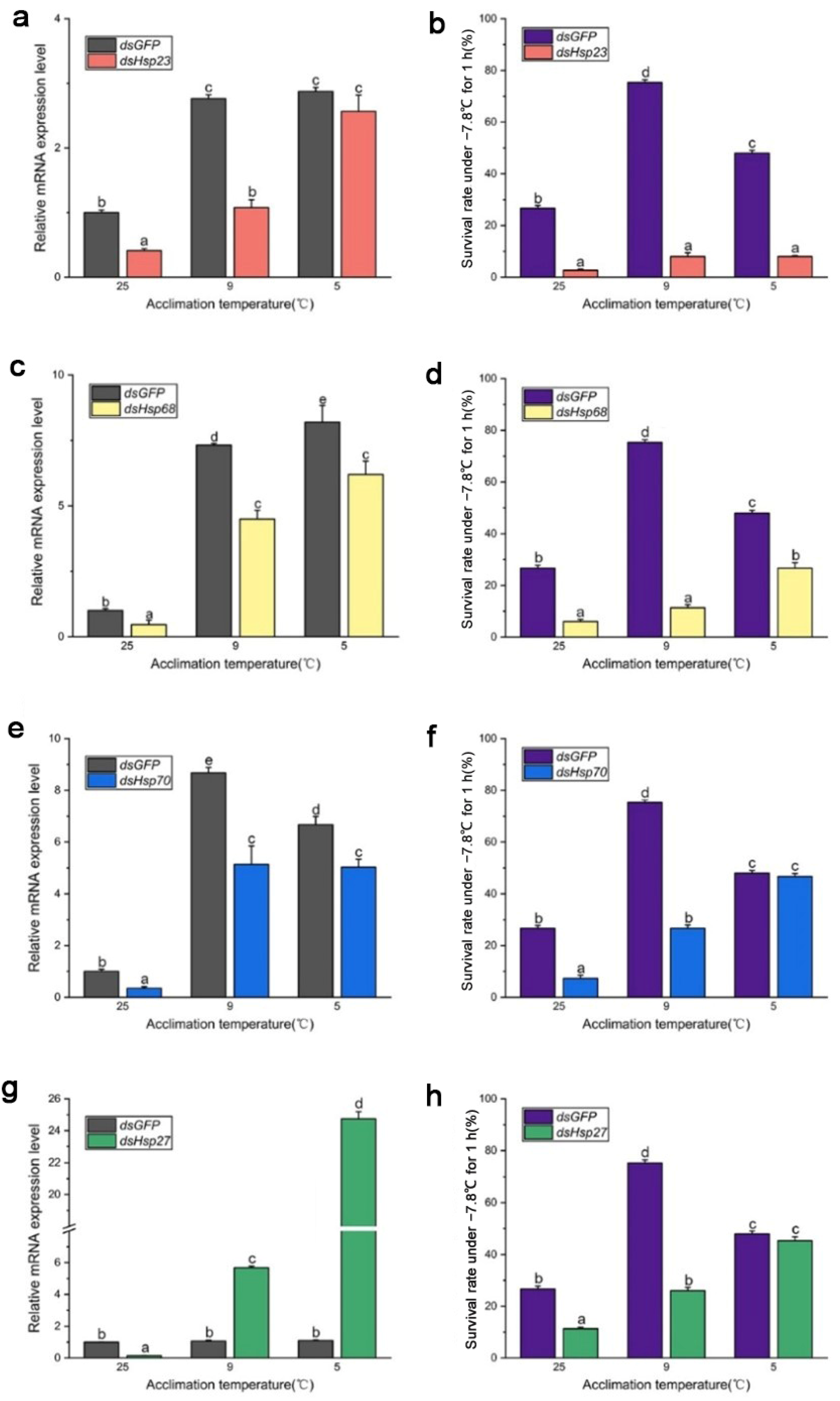
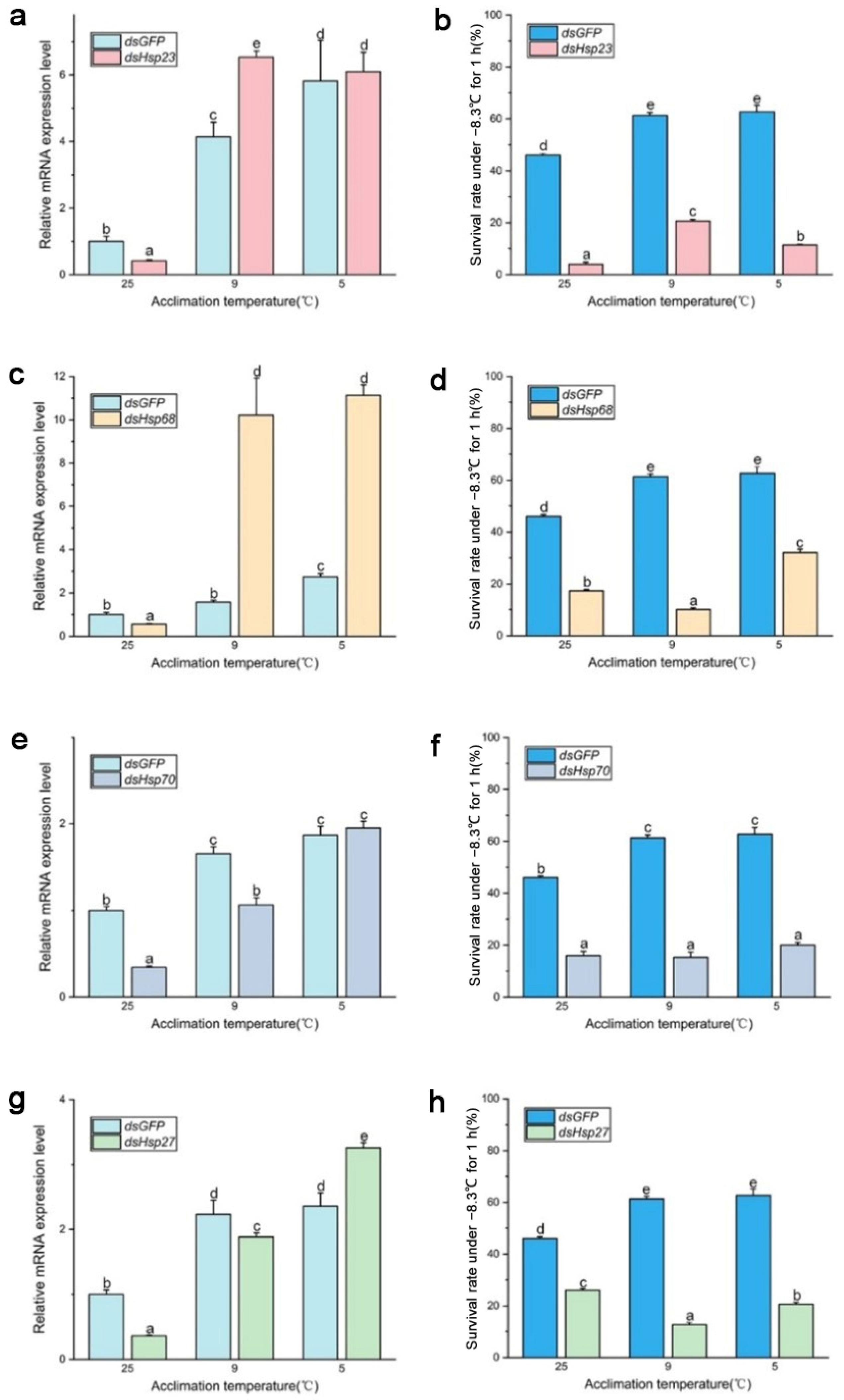
3.3. Expression Level of Selected Hsp Genes
3.4. Functions of Hsp Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabricius, J.C. Entomologia systematica emendata et aucta. Hafniae 1794, 3, 13–24. [Google Scholar] [CrossRef]
- Bezzi, M. On the fruit-flies of the genus Dacus occurring in India, Burma and Ceylon. Bull. Entomol. Res. 1916, 7, 99–121. [Google Scholar] [CrossRef]
- White, I.M.; Elson-Harris, M.M. Fruit flies of economic significance: Their identification and bionomics. Bull. Entomol. Res. 1992, 82, 433. [Google Scholar] [CrossRef]
- Guo, Z.; Lu, Y.; Yang, F.; Zeng, L.; Liang, G.W.; Xu, Y.J. Transmission modes of a pesticide-degrading symbiont of the oriental fruit fly Bactrocera dorsalis (Hendel). Appl. Microbiol. Biotechnol. 2017, 101, 8543–8556. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Ma, C.S.; Yan, Y.; David, R.; Hervé, C. The interspecific variations in molecular responses to various doses of heat and cold stress: The case of cereal aphids. J. Insect Physiol. 2023, 147, 104520. [Google Scholar] [CrossRef]
- Huang, Y.B.; Hong, C.J.; Chou, M.Y. Laboratory evaluation of interspecific mating between Zeugodacus fruit flies and Bactrocera dorsalis. Entomol. Exp. Appl. 2023, 1–8. [Google Scholar] [CrossRef]
- Jaffar, S.; Rizvi, S.A.H.; Lu, Y. Understanding the invasion, ecological adaptations, and management strategies of Bactrocera dorsalis in China: A review. Horticulturae 2023, 9, 1004. [Google Scholar] [CrossRef]
- Vargas, R.I.; Piñero, J.C.; Leblanc, L. An overview of pest species of Bactrocera fruit flies (Diptera: Tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the Pacific region. Insects 2015, 6, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hughes, A.; Zhao, Z.H.; Li, Z.H.; Qin, Y.J. Including climate change to predict the global suitable area of an invasive pest: Bactrocera correcta (Diptera: Tephritidae). Glob. Ecol. Conserv. 2022, 34, e02021. [Google Scholar] [CrossRef]
- Wee, S.L.; Chinvinijkul, S.; Tan, K.H.; Nishida, R. A new and highly effective male lure for the guava fruit fly Bactrocera correcta. J. Pest Sci. 2018, 91, 691–698. [Google Scholar] [CrossRef]
- Lee, R.E.; Denlinger, D.L. Insects at Low Temperature; Chapman and Hall: New York, NY, USA, 1991; pp. 174–197. [Google Scholar] [CrossRef]
- Sunil, S.; Laird, R.A.; Floate, K.D.; Fields, P.G. Cross-tolerance to desiccation and cold in khapra beetle (Coleoptera: Dermestidae). J. Econ. Entomol. 2019, 113, 695–699. [Google Scholar] [CrossRef]
- Zhao, Z.H.; James, C.R.; Li, Z.H. The global epidemic of Bactrocera pests: Mixed-species invasions and risk assessment. Annu. Rev. Entomol. 2024, 69, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Sgro, C.M.; Terblanche, J.S.; Hoffmann, A.A. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 2016, 61, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Kang, L. Adaptation and population differentiation of insects to environmental temperature stress. Prog. Nat. Sci. 2005, 3, 11–17. [Google Scholar]
- Teets, N.M.; Gantz, J.D.; Kawarasaki, Y. Rapid cold hardening: Ecological relevance, physiological mechanisms and new perspectives. J. Exp. Biol. 2020, 223, jeb203448. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.W.; Cancio-Martinez, E.; Hallman, G.J.; Fontenot, E.A.; Vreysen, M.J. Relative tolerance of six Bactrocera (Diptera: Tephritidae) species to phytosanitary cold treatment. J. Econ. Entomol. 2016, 109, 2341–2347. [Google Scholar] [CrossRef]
- Raza, M.F.; Wang, Y.C.; Cai, Z.H.; Bai, S.; Yao, Z.C.; Awan, U.A.; Zhang, Z.Y.; Zheng, W.W.; Zhang, H.Y. Gut microbiota promotes host resistance to low-temperature stress by stimulating its arginine and proline metabolism pathway in adult Bactrocera dorsalis. PLoS Pathog. 2020, 16, e1008441. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, H.B.; Liang, C.H.; Ma, Y.P.; Dou, W.; Wang, J.J. Chromosome-level genome assembly reveals potential epigenetic mechanisms of the thermal tolerance in the oriental fruit fly, Bactrocera dorsalis. Int. J. Biol. Macromol. 2023, 225, 430–441. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.L.; Meyer, M.D.; Liao, Z.X.; Zhao, Y.; Virgilio, M.; Feng, S.Q.; Qin, Y.J.; Singh, S.; Wee, S.L.; et al. Genomes of the cosmopolitan fruit pest Bactrocera dorsalis (Diptera: Tephritidae) reveal its global invasion history and thermal adaptation. J. Adv. Res. 2022, 53, 61–74. [Google Scholar] [CrossRef]
- Singh, V.; Braddick, D.; Dhar, P.K. Exploring the potential of genome editing CRISPR-Cas9 technology. Gene 2017, 599, 1–18. [Google Scholar] [CrossRef]
- Garcia, M.J.; Littler, A.S.; Sriram, A.; Teets, N.M. Distinct cold tolerance traits independently vary across genotypes in Drosophila melanogaster. Evolution 2020, 74, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.Y.; Zhao, Y.; Su, Y.; Wu, J.J.; Wang, Z.Y.; Hu, J.T.; Liu, L.J.; Zhao, Z.H.; Hoffmann, A.A.; Chen, B.; et al. A transcriptional and functional analysis of heat hardening in two invasive fruit fly species, Bactrocera dorsalis and Bactrocera correcta. Evol. Appl. 2019, 12, 1147–1163. [Google Scholar] [CrossRef] [PubMed]
- Welma, P.; John, S.T.; Pia, A. Do thermal tolerances and rapid thermal responses contribute to the invasion potential of Bactrocera dorsalis (Diptera: Tephritidae)? J. Insect Physiol. 2017, 98, 1–6. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Lin, F.; Raychowdhury, R.; Zeng, Q.D. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using diamond. Nat. Biotechnol. 2015, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat. Methods 2008, 5, 621. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139. [Google Scholar] [CrossRef]
- Chen, B.; Wagner, A. Hsp90 is important for fecundity, longevity, and buffering of cryptic deleterious variation in wild fly populations. BMC Evol. Biol. 2012, 12, 25. [Google Scholar] [CrossRef]
- Jang, E.B. Thermal death kinetics and heat tolerance in early and late third instars of the oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 1991, 84, 1298–1303. [Google Scholar] [CrossRef]
- Jiang, F.; Liang, L.; Wang, J.; Zhu, S.F. Chromosome-level genome assembly of Bactrocera dorsalis reveals its adaptation and invasion mechanisms. Commun. Biol. 2022, 5, 25. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, Y.; Zhang, J.; Li, Z. Heat Shock Protein Genes Affect the Rapid Cold Hardening Ability of Two Invasive Tephritids. Insects 2024, 15, 90. https://doi.org/10.3390/insects15020090
Wang Y, Zhao Y, Zhang J, Li Z. Heat Shock Protein Genes Affect the Rapid Cold Hardening Ability of Two Invasive Tephritids. Insects. 2024; 15(2):90. https://doi.org/10.3390/insects15020090
Chicago/Turabian StyleWang, Yuning, Yan Zhao, Junzheng Zhang, and Zhihong Li. 2024. "Heat Shock Protein Genes Affect the Rapid Cold Hardening Ability of Two Invasive Tephritids" Insects 15, no. 2: 90. https://doi.org/10.3390/insects15020090




