Flight Dispersal in Supratidal Rockpool Beetles
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Flight Behaviour
2.2. Wing Morphology
2.3. Microsatellites
3. Results
3.1. Flight Behaviour
3.2. Wing Morphology
3.3. Genetic Divergences
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lake, P.S. Drought and Aquatic Ecosystems: Effects and Responses|Wiley; Wiley-Blackwell: Chichester, UK, 2011; ISBN 978-1-4051-8560-8. [Google Scholar]
- Bogan, M.T.; Chester, E.T.; Datry, T.; Murphy, A.L.; Robson, B.J.; Ruhi, A.; Stubbington, R.; Whitney, J.E. Chapter 4.8—Resistance, Resilience, and Community Recovery in Intermittent Rivers and Ephemeral Streams. In Intermittent Rivers and Ephemeral Streams; Datry, T., Bonada, N., Boulton, A., Eds.; Academic Press: London, UK, 2017; pp. 349–376. ISBN 978-0-12-803835-2. [Google Scholar]
- Washko, S.; Bogan, M.T. Global Patterns of Aquatic Macroinvertebrate Dispersal and Functional Feeding Traits in Aridland Rock Pools. Front. Environ. Sci. 2019, 7, 106. [Google Scholar] [CrossRef]
- Prugnolle, F.; De Meeûs, T.; Prugnolle, F.; De Meeus, T. Inferring Sex-Biased Dispersal from Population Genetic Tools: A Review. Heredity 2002, 88, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Trochet, A.; Courtois, E.; Stevens, V.; Baguette, M.; Chaine, A.; Schmeller, D.; Jean, C.; Wiens, J. Evolution of Sex-Biased Dispersal. Q. Rev. Biol. 2016, 91, 297–320. [Google Scholar] [CrossRef] [PubMed]
- Longing, S.; Magoulick, D. Flight Capacity and Response to Habitat Drying of Endemic Diving Beetles (Coleoptera: Dytiscidae) in Arkansas (USA). Hydrobiology 2023, 2, 354–362. [Google Scholar] [CrossRef]
- Bogan, M.T.; Boersma, K.S. Aerial Dispersal of Aquatic Invertebrates along and Away from Arid-Land Streams. Freshw. Sci. 2012, 31, 1131–1144. [Google Scholar] [CrossRef]
- Strachan, S.; Chester, E.; Robson, B. Freshwater Invertebrate Life History Strategies for Surviving Desiccation. Springer Sci. Rev. 2015, 3, 57–75. [Google Scholar] [CrossRef]
- Zalom, F.G.; Grigarick, A.A.; Way, M.O. Diel Flight Periodicities of Some Dytiscidae (Coleoptera) Associated with California Rice Paddies. Ecol. Entomol. 1980, 5, 183–187. [Google Scholar] [CrossRef]
- Pallarés, S.; Arribas, P.; Céspedes, V.; Millán, A.; Velasco, J. Lethal and Sublethal Behavioural Responses of Saline Water Beetles to Acute Heat and Osmotic Stress. Ecol. Entomol. 2012, 37, 508–520. [Google Scholar] [CrossRef]
- Mirón-Gatón, J.M.; Botella-Cruz, M.; García-Meseguer, A.J.; Millán, A.; Velasco, J. Thermal Tolerance Differs between Co-Occurring Congeneric Beetle Species in Marine Supratidal Rockpools. Mar. Ecol. Prog. Ser. 2022, 681, 185–196. [Google Scholar] [CrossRef]
- Velasco, J.; Millan, A. Insect Dispersal in a Drying Desert Stream: Effects of Temperature and Water Loss. Southwest. Nat. 1998, 43, 80–87. [Google Scholar]
- Svensson, B.W. Local Dispersal and Its Life-History Consequences in a Rock Pool Population of a Gyrinid Beetle. Oikos 1998, 82, 111–122. [Google Scholar] [CrossRef]
- Leitch, K.J.; Ponce, F.V.; Dickson, W.B.; van Breugel, F.; Dickinson, M.H. The Long-Distance Flight Behavior of Drosophila Supports an Agent-Based Model for Wind-Assisted Dispersal in Insects. Proc. Natl. Acad. Sci. USA 2021, 118, e2013342118. [Google Scholar] [CrossRef]
- Martínez-Pérez, S.; Galante, E.; Micó, E. Sex Specificity of Dispersal Behaviour and Flight Morphology Varies among Tree Hollow Beetle Species. Mov. Ecol. 2022, 10, 41. [Google Scholar] [CrossRef]
- Gibb, H.; Hjältén, J.; Ball, J.P.; Pettersson, R.B.; Landin, J.; Alvini, O.; Danell, K. Wing Loading and Habitat Selection in Forest Beetles: Are Red-Listed Species Poorer Dispersers or More Habitat-Specific than Common Congenerics? Biol. Conserv. 2006, 132, 250–260. [Google Scholar] [CrossRef]
- Hoffsten, P.-O. Site-Occupancy in Relation to Flight-Morphology in Caddisflies. Freshw. Biol. 2004, 49, 810–817. [Google Scholar] [CrossRef]
- Breuker, C.; Brakefield, P.; Gibbs, M. The Association between Wing Morphology and Dispersal Is Sex-Specific in the Glanville Fritillary Butterfly Melitaea Cinxia (Lepidoptera: Nymphalidae). Eur. J. Entomol. 2007, 104, 445–452. [Google Scholar] [CrossRef]
- Arribas, P.; Velasco, J.; Abellán, P.; Sánchez-Fernández, D.; Andújar, C.; Calosi, P.; Millán, A.; Ribera, I.; Bilton, D. Dispersal Ability Rather than Ecological Tolerance Drives Differences in Range Size between Lentic and Lotic Water Beetles (Coleoptera: Hydrophilidae). J. Biogeogr. 2012, 39, 984–994. [Google Scholar] [CrossRef]
- Carbonell, J.A.; Millán, A.; Green, A.J.; Céspedes, V.; Coccia, C.; Velasco, J. What Traits Underpin the Successful Establishment and Spread of the Invasive Water Bug Trichocorixa Verticalis Verticalis? Hydrobiologia 2016, 768, 273–286. [Google Scholar] [CrossRef]
- Wainwright, P.C.; Reilly, S.M. (Eds.) Ecological Morphology: Integrative Organismal Biology; University of Chicago Press: Chicago, IL, USA, 1994; ISBN 978-0-226-86995-7. [Google Scholar]
- Berwaerts, K.; Van Dyck, H.; Aerts, P. Does Flight Morphology Relate to Flight Performance? An Experimental Test with the Butterfly Pararge Aegeria. Funct. Ecol. 2002, 16, 484–491. [Google Scholar] [CrossRef]
- Grabow, K.; Rüppell, G. Wing Loading in Relation to Size and Flight Characteristics of European Odonata. Odonatologica 1995, 24, 175–186. [Google Scholar]
- Betts, C.R.; Wootton, R.J. Wing Shape and Flight Behaviour in Butterflies (Lepidoptera: Papilionoidea and Hesperioidea): A Preliminary Analysis. J. Exp. Biol. 1988, 138, 271–288. [Google Scholar] [CrossRef]
- Devries, P.; Penz, C.; Hill, R. Vertical Distribution, Flight Behaviour and Evolution of Wing Morphology in Morpho Butterflies. J. Anim. Ecol. 2010, 79, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, F.; Keena, M.A. Differences in Wing Morphometrics of Lymantria Dispar (Lepidoptera: Erebidae) Between Populations That Vary in Female Flight Capability. Ann. Entomol. Soc. Am. 2015, 108, 528–535. [Google Scholar] [CrossRef]
- Selkoe, K.A.; Toonen, R.J. Microsatellites for Ecologists: A Practical Guide to Using and Evaluating Microsatellite Markers. Ecol. Lett. 2006, 9, 615–629. [Google Scholar] [CrossRef]
- García-Meseguer, A.J.; Abellán, P.; Mirón-Gatón, J.M.; Botella-Cruz, M.; Guareschi, S.; Millán, A.; Velasco, J. Fine-Scale Niche Differences Allow the Co-Existence of Congeneric Aquatic Beetles in Supratidal Rockpools. Hydrobiologia 2024, 851, 471–485. [Google Scholar] [CrossRef]
- Hutchison, D.W.; Templeton, A.R. Correlation of Pairwise Genetic and Geographic Distance Measures: Inferring the Relative Influences of Gene Flow and Drift on the Distribution of Genetic Variability. Evolution 1999, 53, 1898–1914. [Google Scholar] [CrossRef] [PubMed]
- Margalef, R. Sobre la ecología de las larvas del mosquito Aëdes mariae. Publicaciones Inst. De Biol. Apl. 1949, 6, 83–101. [Google Scholar]
- Catalán, J.; Ballesteros, E. Contribución al estudio de las cubetas supralitorales (Tossa, Costa Brava). Limnetica 1984, 1, 43–50. [Google Scholar] [CrossRef]
- Ganning, B. Studies on Chemical, Physical and Biological Conditions in Swedish Rockpool Ecosystems. Ophelia 1971, 9, 51–105. [Google Scholar] [CrossRef]
- Denny, M.W.; Gaines, S.D. Encyclopedia of Tidepools and Rocky Shores, 1st ed.; Encyclopedias of the Natural World; University of California Press: Berkeley, CA, USA, 2007; ISBN 978-0-520-25118-2. [Google Scholar]
- Williams, D.D. The Biology of Temporary Waters; Oxford University Press: Oxford, UK; New York, NY, USA, 2005; ISBN 978-0-19-852812-8. [Google Scholar]
- Villastrigo, A.; Hernando, C.; Millán, A. The Ochthebius (Coleoptera, Hydraenidae) from Western Palaearctic Supratidal Rockpools. Bol.—Asoc. Esp. Entomol. 2022, 4, 100–108. [Google Scholar]
- Hase, A. Zur Kenntnis Der Lebensgewohnheiten Und Der Umwelt Des Marinen Käfers Ochthebius Quadricollis Mulsant (Hydrophilidae). Int. Rev. Gesamten Hydrobiol. Hydrogr. 1926, 16, 141–179. [Google Scholar] [CrossRef]
- Jacquin, A. Recherches biologiques sur Ochthebius quadricollis Mulsant (Coléoptère: Hydrophilide). Bull. Société D’histoire Nat. L’afrique Nord. 1956, 47, 270–290. [Google Scholar]
- Villastrigo, A.; Orenes-Salazar, V.; García-Meseguer, A.J.; Mirón-Gatón, J.; Mourre, B.; Millán, A.; Velasco, J. Oceanic Currents Maintain the Genetic Structure of Non-Marine Coastal Taxa in the Western Mediterranean Sea. NPJ Biodivers. 2023, 2, 25. [Google Scholar] [CrossRef]
- Villastrigo, A.; Bilton, D.T.; Abellán, P.; Millán, A.; Ribera, I.; Velasco, J. Cryptic Lineages, Cryptic Barriers: Historical Seascapes and Oceanic Fronts Drive Genetic Diversity in Supralittoral Rockpool Beetles (Coleoptera: Hydraenidae). Zool. J. Linn. Soc. 2022, 196, zlac032. [Google Scholar] [CrossRef]
- Bilton, D.T. Differentiation of South African Coastal Rock Pool Ochthebius Is Associated with Major Ocean Currents (Coleoptera: Hydraenidae). Acta Entomol. Musei Natl. Pragae 2021, 61, 253–260. [Google Scholar] [CrossRef]
- Kelly, L.C.; Bilton, D.T.; Rundle, S.D. Population Structure and Dispersal in the Canary Island Caddisfly Mesophylax Aspersus (Trichoptera, Limnephilidae). Heredity 2001, 86, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Bertin, A.; Alvarez, E.; Gouin, N.; Gianoli, E.; Montecinos, S.; Lek, S.; Gascoin, S.; Lhermitte, S. Effects of Wind-Driven Spatial Structure and Environmental Heterogeneity on High-Altitude Wetland Macroinvertebrate Assemblages with Contrasting Dispersal Modes. Freshw. Biol. 2015, 60, 297–310. [Google Scholar] [CrossRef]
- Juračka, P.J.; Dobiáš, J.; Boukal, D.S.; Šorf, M.; Beran, L.; Černý, M.; Petrusek, A. Spatial Context Strongly Affects Community Composition of Both Passively and Actively Dispersing Pool Invertebrates in a Highly Heterogeneous Landscape. Freshw. Biol. 2019, 64, 2093–2106. [Google Scholar] [CrossRef]
- Epele, L.B.; Dos Santos, D.A.; Sarremejane, R.; Grech, M.G.; Macchi, P.A.; Manzo, L.M.; Miserendino, M.L.; Bonada, N.; Cañedo-Argüelles, M. Blowin’ in the Wind: Wind Directionality Affects Wetland Invertebrate Metacommunities in Patagonia. Glob. Ecol. Biogeogr. 2021, 30, 1191–1203. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2023. Available online: https://www.R-project.org (accessed on 2 February 2024).
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2018. Available online: https://imagej.net/ij/ (accessed on 2 February 2024).
- García-Meseguer, A.J.; Villastrigo, A.; Mirón-Gatón, J.M.; Millán, A.; Velasco, J.; Muñoz, I. Novel Microsatellite Loci, Cross-Species Validation of Multiplex Assays, and By-Catch Mitochondrial Genomes on Ochthebius Beetles from Supratidal Rockpools. Insects 2023, 14, 881. [Google Scholar] [CrossRef]
- Applied Biosystems. GeneMapper Software v.5. Applied Biosystems™. Waltham, MA, USA. Available online: https://www.thermofisher.com/order/catalog/product/es/es/4370784 (accessed on 2 February 2024).
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- Goudet, J. Hierfstat, a Package for r to Compute and Test Hierarchical F-Statistics. Mol. Ecol. Notes 2005, 5, 184–186. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package 2022. Available online: https://github.com/vegandevs/vegan (accessed on 16 February 2024).
- Rousset, F. Genetic Differentiation and Estimation of Gene Flow from F-Statistics under Isolation by Distance. Genetics 1997, 145, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Use R! Springer: Cham, Switzerland, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Aphalo, P. Learn R…as You Learnt Your Mother Tongue; Leanpub: Victoria, BC, Canada, 2017; Available online: https://www.researchgate.net/publication/304581791 (accessed on 16 February 2024).
- Dingle, H.; Arora, G. Experimental Studies of Migration in Bugs of the Genus Dysdercus. Oecologia 1973, 12, 119–140. [Google Scholar] [CrossRef]
- Dingle, H.; Blakley, N.R.; Miller, E.R. Variation in Body Size and Flight Performance in Milkweed Bugs (Oncopeltus). Evolution 1980, 34, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Angelo, M.J.; Slansky, F. Body Building by Insects: Trade-Offs in Resource Allocation with Particular Reference to Migratory Species. Fla. Entomol. 1984, 67, 22–41. [Google Scholar] [CrossRef]
- Cheng, X.; Sun, M. Wing-Kinematics Measurement and Aerodynamics in a Small Insect in Hovering Flight. Sci. Rep. 2016, 6, 25706. [Google Scholar] [CrossRef]
- Li, X.-Y.; Kokko, H. Sex-Biased Dispersal: A Review of the Theory. Biol. Rev. Camb. Philos. Soc. 2019, 94, 721–736. [Google Scholar] [CrossRef]
- Hirota, T. The Evolution of Sex-Biased Dispersal by Pre-Dispersal Copulation and Fluctuating Environment. J. Anim. Ecol. 2004, 73, 1115–1120. [Google Scholar] [CrossRef]
- Hirota, T. The Effect of Female Polyandry and Sperm Precedence on the Evolution of Sexual Difference in Dispersal Timing. J. Evol. Biol. 2005, 18, 1395–1402. [Google Scholar] [CrossRef]
- Wild, G.; Taylor, P. Kin Selection Models for the Co-Evolution of the Sex Ratio and Sex-Specific Dispersal. Evol. Ecol. Res. 2004, 6, 481–502. [Google Scholar]
- Shaw, A.K.; Kokko, H. Mate Finding, Allee Effects and Selection for Sex-Biased Dispersal. J. Anim. Ecol. 2014, 83, 1256–1267. [Google Scholar] [CrossRef]
- Henry, R.C.; Coulon, A.; Travis, J.M.J. The Evolution of Male-Biased Dispersal under the Joint Selective Forces of Inbreeding Load and Demographic and Environmental Stochasticity. Am. Nat. 2016, 188, 423–433. [Google Scholar] [CrossRef]
- Rocha, É.; Brigatti, E.; Niebuhr, B.; Ribeiro, M.; Vieira, M. Dispersal Movement through Fragmented Landscapes: The Role of Stepping Stones and Perceptual Range. Landsc. Ecol. 2021, 36, 3249–3267. [Google Scholar] [CrossRef]


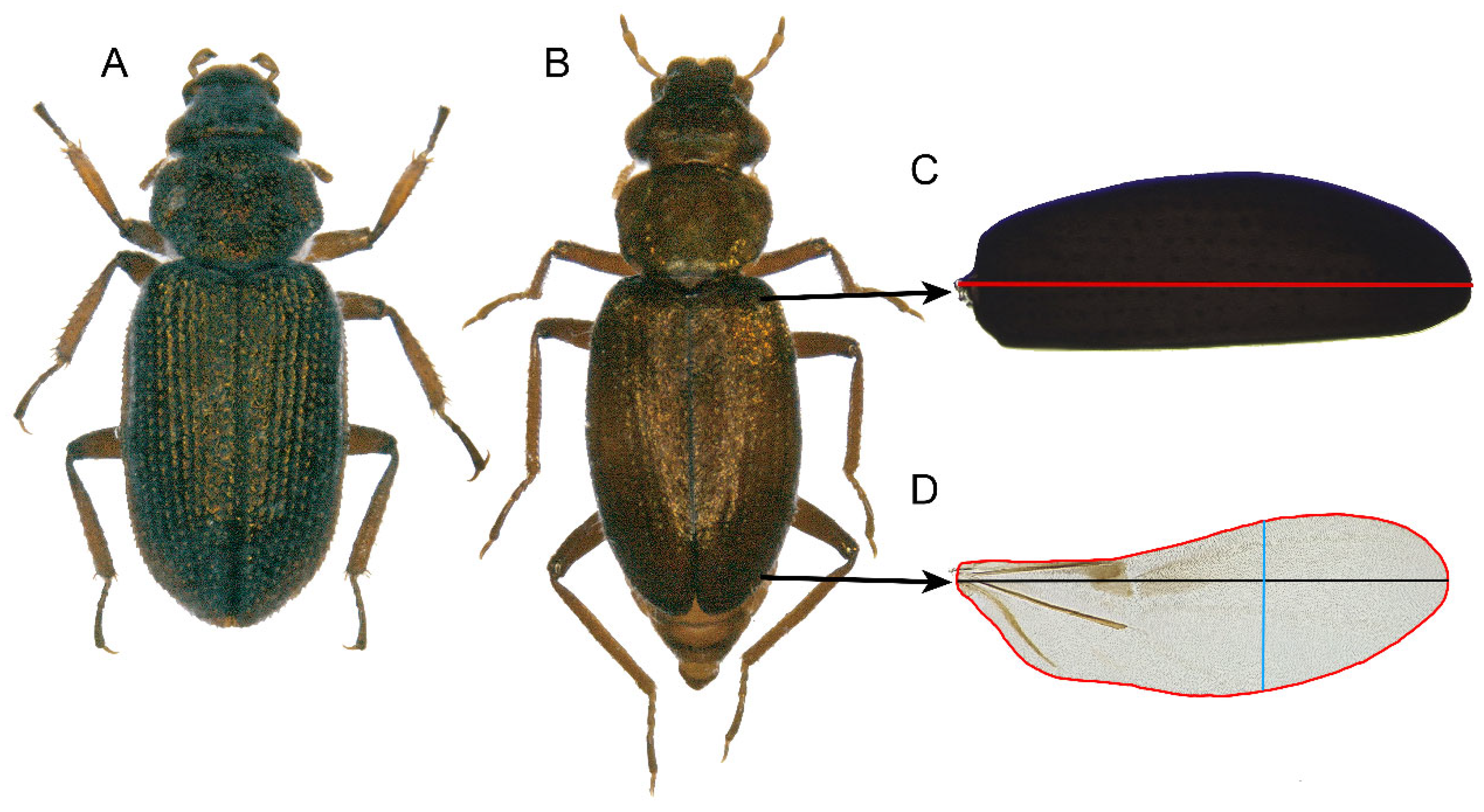


| Df | Sum | Sq Mean | Sq F value | Pr(>F) | |
|---|---|---|---|---|---|
| temperature interval | 5 | 14.22 | 2.844 | 1.384 | 0.265 |
| species | 1 | 0.11 | 0.111 | 0.054 | 0.818 |
| temperature × species | 5 | 17.89 | 3.578 | 1.741 | 0.164 |
| Residuals | 24 | 49.33 | 2.056 |
| Df | Sum Sq | Mean Sq | F Value | p Value | |
|---|---|---|---|---|---|
| Elytron length | |||||
| species | 1 | 0.18266 | 0.18266 | 34.569 | 2.38 × 10−7 |
| sex | 2 | 0.09368 | 0.04684 | 8.864 | 0.000452 |
| residuals | 56 | 0.29590 | 0.00528 | ||
| Wing area | |||||
| species | 1 | 0.0516 | 0.05155 | 2.76 | 0.102 |
| sex | 2 | 0.5178 | 0.25891 | 13.86 | 1.29 × 10−5 |
| residuals | 56 | 1.0460 | 0.01868 | ||
| Aspect ratio | |||||
| species | 1 | 0.00087 | 0.00086 | 0.186 | 0.668 |
| sex | 2 | 0.00335 | 0.00160 | 0.359 | 0.700 |
| residuals | 56 | 0.26105 | 0.00466 | ||
| Wing loading | |||||
| species | 1 | 0.01803 | 0.01803 | 4.109 | 0.047416 |
| sex | 2 | 0.07427 | 0.03713 | 8.462 | 0.000615 |
| residuals | 56 | 0.24576 | 0.00439 |
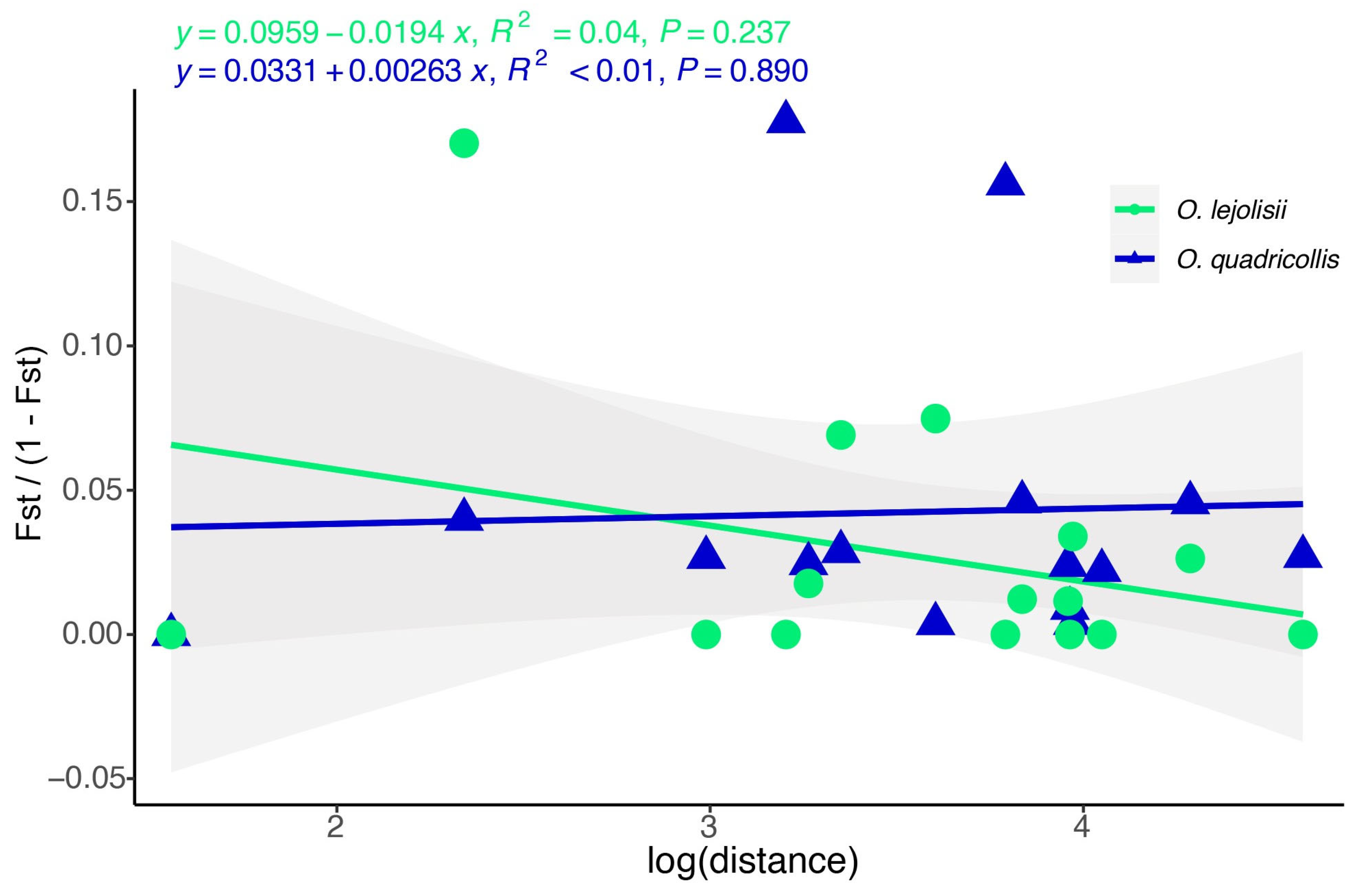
| Pairwise FST Estimates | ||||
|---|---|---|---|---|
| Locality | Locality | Geographic Distance (km) | Ochthebius quadricollis | Ochthebius lejolisii |
| Moraira | La Illeta | 52.40 | 0.02339 (0.01107–0.03412) | 0.01145 (−0.00155–0.03216) |
| La Illeta | Santa Pola | 28.51 | 0.02796 (−0.00371–0.05866) | 0.06467 (0.00333–0.11443) |
| Santa Pola | Punta del Cocedor | 53.07 | 0.00384 (−0.01610–0.02746) | 0.03286 (0.00993–0.05025) |
| Punta del Cocedor | Cala de las Pulgas | 72.69 | 0.04370 (0.01784–0.07344) | 0.02569 (−0.01268–0.08342) |
| Punta del Cocedor | Cala Panizo | 98.31 | 0.02638 (−0.00920–0.08267) | −0.02076 (−0.03394–−0.01145) |
| Cala Panizo | Percheles | 36.74 | 0.00377 (−0.03225–0.03935) | 0.06962 (−0.05322–0.17334) |
| Percheles | Cala de las Pulgas | 10.39 | 0.03841 (0.00829–0.07813) | 0.14545 (0.04615–0.23690) |
| Cala de las Pulgas | Cala Panizo | 26.14 | 0.02399 (−0.00029–0.04641) | 0.01738 (−0.02107–0.06049) |
| Cala Panizo | Cala Conchas | 4.74 | −0.01105 (−0.02868–0.01554) | −0.03440 (−0.11121–0.02991) |
| Cala Conchas | El Playazo | 52.66 | 0.00901 (−0.01176–0.04150) | 0.00000 (0.00000–0.00000) |
| Cala Panizo | El Playazo | 57.36 | 0.02174 (−0.00796–0.05468) | −0.04519 (−0.07066–−0.02616) |
| Cala Rijana | Velilla | 24.61 | 0.15088 (0.11918–0.18392) | −0.00709 (−0.03338–0.01618) |
| Cala Rijana | Nerja | 44.30 | 0.13505 (0.09631–0.17350) | −0.01727 (−0.05207–0.01285) |
| Velilla | Nerja | 19.87 | 0.02605 (−0.00001–0.05432) | −0.00816 (−0.02670–0.00801) |
| Cala Milla | Isla de las Palomas | 46.33 | 0.04396 (0.00614–0.08026) | 0.01212 (−0.02171–0.03797) |
| Mean FST value | 0.03780 | 0.01642 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza-Buendía, J.; Mirón-Gatón, J.M.; García-Meseguer, A.J.; Villastrigo, A.; Millán, A.; Velasco, J. Flight Dispersal in Supratidal Rockpool Beetles. Insects 2024, 15, 140. https://doi.org/10.3390/insects15030140
Plaza-Buendía J, Mirón-Gatón JM, García-Meseguer AJ, Villastrigo A, Millán A, Velasco J. Flight Dispersal in Supratidal Rockpool Beetles. Insects. 2024; 15(3):140. https://doi.org/10.3390/insects15030140
Chicago/Turabian StylePlaza-Buendía, Jorge, Juana María Mirón-Gatón, Antonio José García-Meseguer, Adrián Villastrigo, Andrés Millán, and Josefa Velasco. 2024. "Flight Dispersal in Supratidal Rockpool Beetles" Insects 15, no. 3: 140. https://doi.org/10.3390/insects15030140
APA StylePlaza-Buendía, J., Mirón-Gatón, J. M., García-Meseguer, A. J., Villastrigo, A., Millán, A., & Velasco, J. (2024). Flight Dispersal in Supratidal Rockpool Beetles. Insects, 15(3), 140. https://doi.org/10.3390/insects15030140







