Chemical Composition and Nutritional Value of Royal Jelly Samples Obtained from Honey Bee (Apis mellifera) Hives Fed on Oak and Rapeseed Pollen Patties
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Analyses
2.2.1. Proximate and Amino Acid Analysis
2.2.2. 2D PAGE for Protein and MALDI-TOF
2.2.3. Fatty Acid and 10-HDA Analysis
2.2.4. Mineral Analysis
2.3. Statistics
3. Results
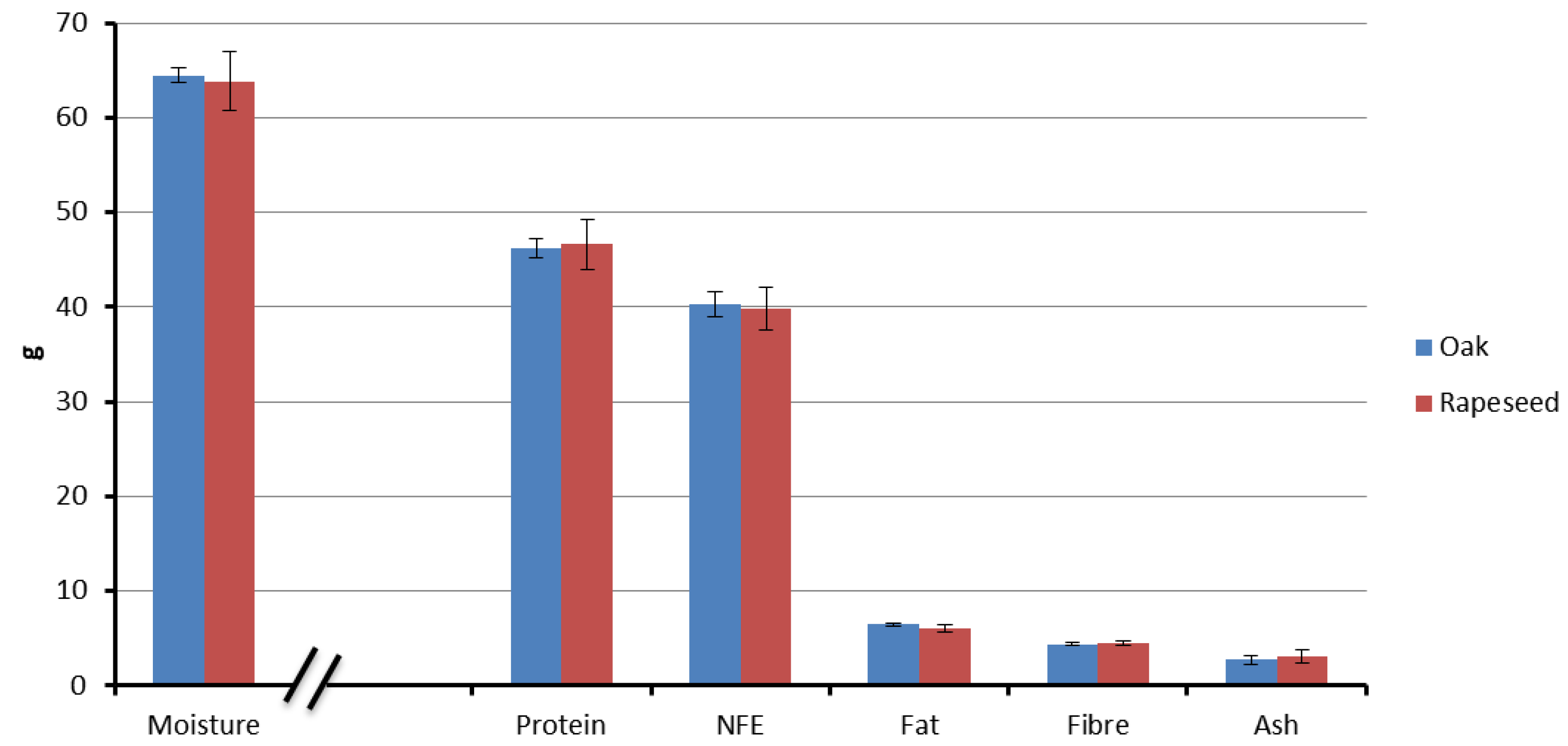
3.1. Proximate Composition
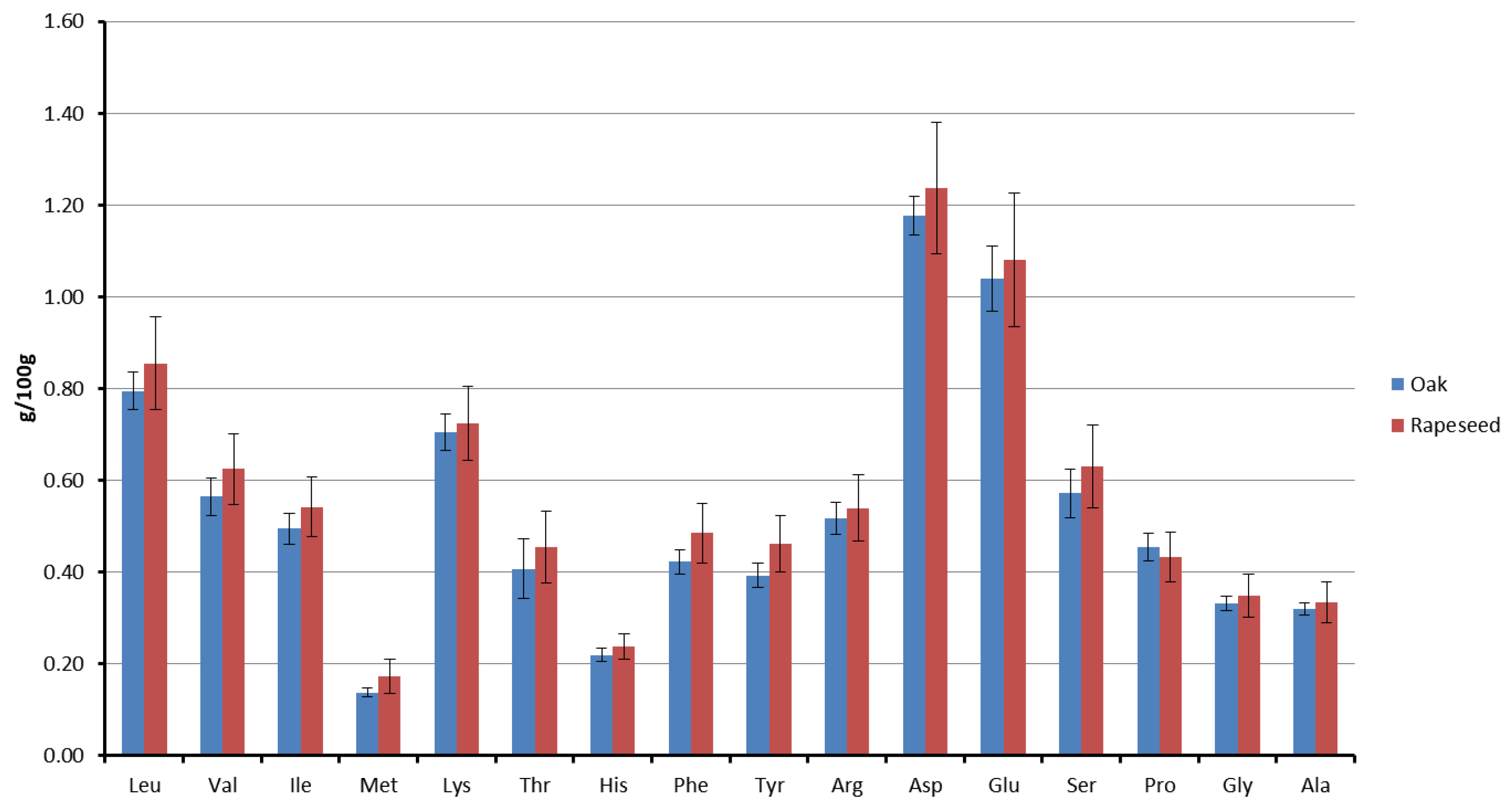
3.2. Amino Acid Composition and Peptide
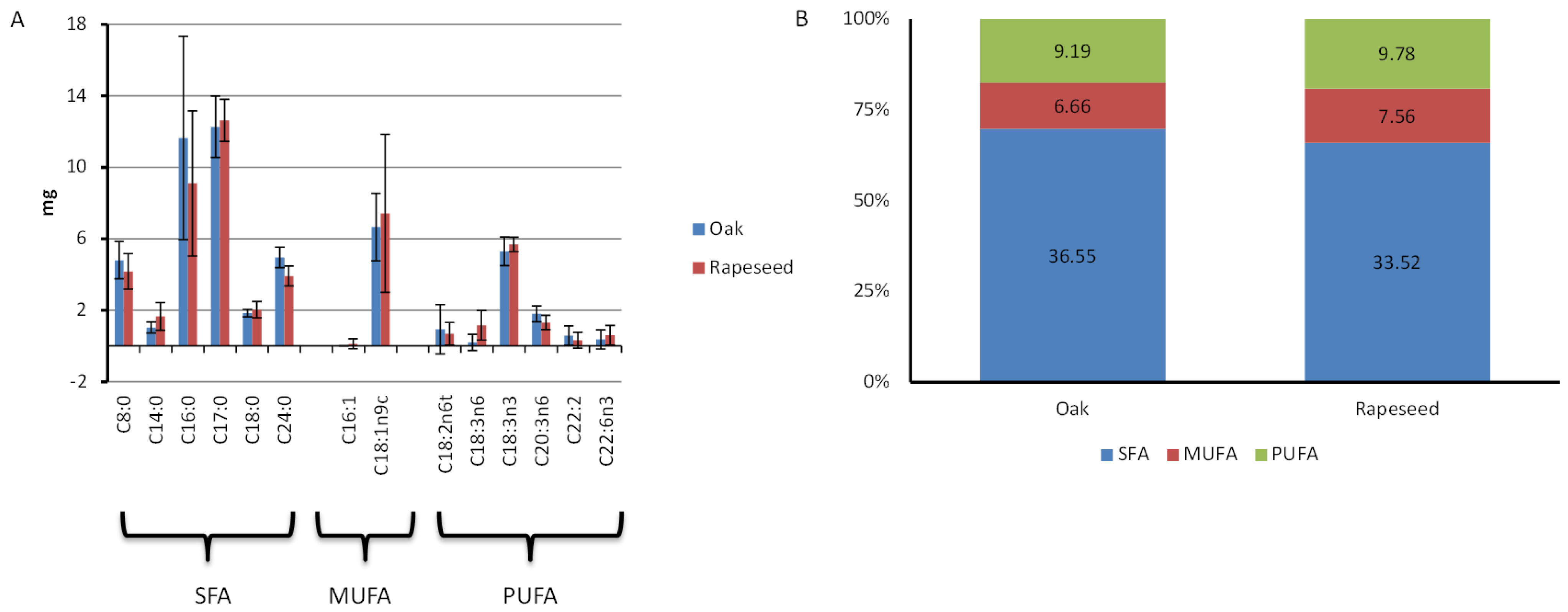
3.3. Fatty Acid Composition of 10-HDA Content
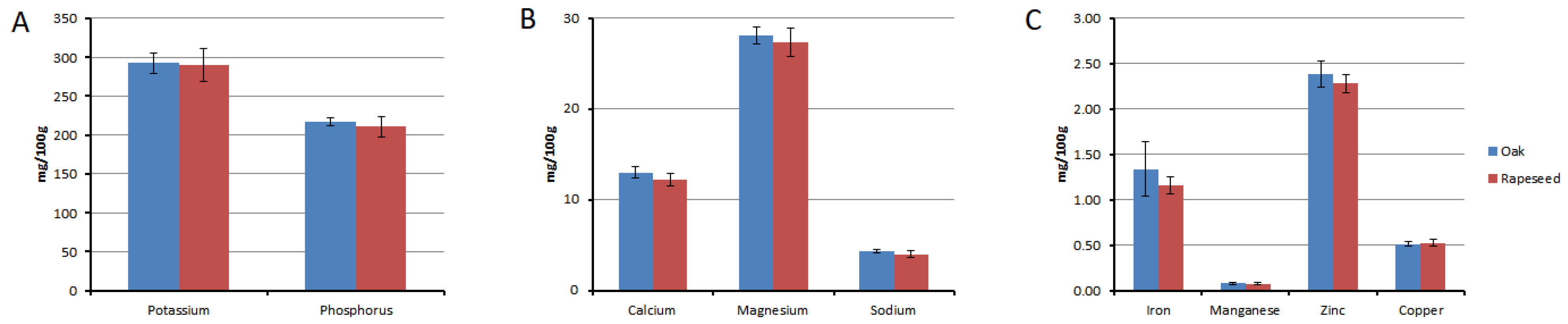
3.4. Mineral Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, F.-L.; Bíliková, K.; Casabianca, H.; Daniele, G.; Espindola, F.S.; Feng, M.; Guan, C.; Han, B.; Kraková, T.K.; Li, J.-K.; et al. Standard methods for Apis mellifera royal jelly research. J. Apic. Res. 2019, 58, 1–68. [Google Scholar] [CrossRef]
- Hora, Z.; Altaye, S.Z.; Wubie, A.J.; Li, J. Proteomics improves the new understanding of honeybee biology. J. Agric. Food Chem. 2018, 66, 3605–3615. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, R.; Maleszka, J.; Foret, S.; Maleszka, R. Nutritional control of reproductive status in honeybees via DNA methylation. Science 2008, 319, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, M.A.; Sabatini, A.G.; Grazia, L.; Tini, V.; Zambonelli, C. Il contenuto in vitamin come possible element di caratterizzazione della gelatine reale. Apicoltura 1998, 4, 139–146. [Google Scholar]
- Schmitzová, J.; Klaudiny, J.; Albert, Š.; Schröder, W.; Schreckengost, W.; Hanes, J.; Júdová, J.; Šimúth, J. A family of major royal jelly proteins of the honeybee Apis mellifera L. Cell. Mol. Life Sci. 1998, 54, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Inoue, R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Sabatini, A.G.; Marcazzan, G.L.; Caboni, M.F.; Bogdanov, S.; de Almeida-Muradian, L.B. Quality and standardisation of royal jelly. J. ApiProd. ApiMed. Sci. 2009, 1, 1–6. [Google Scholar] [CrossRef]
- Henschler, D. Hoher. Acetylcholingehalt von Bienenfuttersäften. Naturwissenschaften 1954, 41, 142. [Google Scholar] [CrossRef]
- Marko, P.; Pecháň, J.; Vittek, J. Some phosphorous compounds in royal jelly. Nature 1964, 202, 188–189. [Google Scholar] [CrossRef]
- Nye, M.J.; Shuel, R.W.; Dixon, S.E. Gluconic acid in the food of larval honeybees. J. Apic. Res. 1973, 12, 9–15. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Sugiyama, T.; Takahashi, K.; Mori, H. Royal jelly acid, 10-hydroxy-trans-2-decenoic acid, as a modulator of the innate immune responses. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 368–376. [Google Scholar] [CrossRef]
- Izuta, H.; Chikaraishi, Y.; Shimazawa, M.; Mishima, S.; Hara, H. 10-Hydroxy-2-decenoic acid, a major fatty acid from royal jelly, inhibits VEGF-induced angiogenesis in human umbilical vein endothelial cells. eCAM 2009, 6, 489–494. [Google Scholar] [CrossRef]
- Najafi, G.; Nejati, V.; Jalali, A.S.; Zahmatkesh, E. Protective role of royal jelly in oxymetholone-induced oxidative injury in mouse testis. Iran. J. Toxicol. 2014, 8, 1073–1080. [Google Scholar]
- Taavoni, S.; Barkhordari, F.; Goushegir, A.; Haghani, H. Effect of royal jelly on premenstrual syndrome among Iranian medical sciences students: A randomized, triple-blind, placebo-controlled study. Complement. Ther. Med. 2014, 22, 601–606. [Google Scholar] [CrossRef]
- Seyyedi, F.; Rafiean-Kopaei, M.; Miraj, S. Comparison of the effects of vaginal royal jelly and vaginal estrogen on quality of life, sexual and urinary function in postmenopausal women. J. Clin. Diagn. Res. 2016, 10, QC01–QC05. [Google Scholar] [CrossRef] [PubMed]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. 2014, 89, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Dobritzch, D.; Aumer, D.; Fuszard, M.; Erler, S.; Buttstedt, A. The rise and fall of major royal jelly proteins during a honeybee (Apis mellifera) workers’ life. Ecol. Evol. 2019, 9, 8771–8782. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Bieri, K.; Gremaud, G.; Iff, D.; Känzig, A.; Seiler, K.; Stöckli, H.; Zürcher, K. Swiss Food Manual: Gelée Royale; Bienenprodukte, BAG (Swiss Federal Office for Public Health): Berne, Switzerland, 2004. [Google Scholar]
- Park, B.-S.; Choi, Y.-S.; Kang, E.-J.; Park, H.; Frunze, O.; Kim, D. Analysis of royal jelly production status against establishment of smart farm system in Korea. J. Knowl. Inf. Technol. Syst. 2020, 15, 845–853. [Google Scholar]
- Kim, E.; Jang, M.; Jeong, H.; Kim, Y.; Shin, Y.; Park, J.; Lee, J.; Cho, S.; Hwang, I.; Shin, Y. Changes in the chemical composition of royal jelly produced through artificial bee-feeding in response to seasonal variations during non-migratory beekeeping. J. Food Comp. Anal. 2023, 115, 104982. [Google Scholar] [CrossRef]
- TRASS. 2020. Available online: https://www.bandtrass.or.kr (accessed on 20 December 2023).
- Ibrahim, Y.Y.M. Studies on Some Activities of Honeybee Colonies under Giza City Conditions. Master’s Thesis, Cairo University, Giza, Egypt, 2017; 116p. [Google Scholar]
- Altaye, S.Z.; Meng, L.; Li, J. Molecular insights into the enhanced performance of royal jelly secretion by a stock of honeybee (Apis mellifera ligustica) selected for increasing royal jelly production. Apidologie 2019, 50, 436–453. [Google Scholar] [CrossRef]
- Standifer, L.N. A comparison of the protein quality of pollens for growth-stimulation of the hypopharyngeal glands and longevity of honey bees, Apis mellifera L. (Hymenoptera: Apidae). Insectes Sociaux 1967, 14, 415–425. [Google Scholar] [CrossRef]
- Hrassnigg, N.; Crailsheim, K. Adaptation of hypopharyngeal gland development to the brood status of honeybee (Apis mellifera L.) colonies. J. Insect Physiol. 1998, 44, 929–939. [Google Scholar] [CrossRef]
- Bitondi, M.M.G.; Simões, Z.L.P. The relationship between level of pollen in the diet, vitellogenin and juvenile hormone titres in Africanized Apis mellifera workers. J. Apic. Res. 1996, 35, 27–36. [Google Scholar] [CrossRef]
- Engels, W. Occurrence and significance of vitellogenins in female castes of social hymenoptera. Am. Zool. 1974, 14, 1229–1237. [Google Scholar]
- Winkler, P.; Sieg, F.; Buttstedt, A. Transcriptional control of honey bee (Apis mellifera) major Royal Jelly proteins by 20-Hydroxyecdysone. Insects 2018, 9, 122. [Google Scholar] [CrossRef]
- Amdam, G.V.; Norberg, K.; Hagen, A.; Omholt, S.W. Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. USA 2003, 100, 1799–1802. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C. Nutritional value of bee-collected pollens of hardy kiwi, Actinidia arguta (Actinidiaceae) and oak, Quercus sp. (Fagaceae). J. Asia-Pac. Entomol. 2017, 20, 245–251. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C. Changes in nutritional composition from bee pollen patty used in bumblebee rearing. J. Asia-Pac. Entomol. 2020, 23, 701–708. [Google Scholar] [CrossRef]
- A.O.A.C. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.R.; Kirsch, D.R.; Morris, N.R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal. Biochem. 1980, 105, 361–363. [Google Scholar] [CrossRef]
- Wang, J.; Kliks, M.M.; Qu, W.; Jun, S.; Shi, G.; Li, Q.X. Rapid determination of the geographical origin of honey based on protein fingerprinting and barcoding using MALDI TOF MS. J. Agric. Food Chem. 2009, 57, 10081–10088. [Google Scholar] [CrossRef]
- Korean Food Standard Codex; Ministry of Food and Drug Safety: Chungcheongbuk-do, Republic of Korea, 2010.
- Zhou, J.; Xue, X.; Li, Y.; Zhang, J.; Zhao, J. Optimized determination method for trans-10-Hydroxy-2-Decenoic Acid content in Royal Jelly by High-Performance Liquid Chromatography with an internal standard. J. AOAC Int. 2007, 90, 244–249. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C. Temporal changes of nutrient composition from pollen patty to bee bread with special emphasis on amino and fatty acids composition. J. Asia-Pac. Entomol. 2022, 25, 101873. [Google Scholar] [CrossRef]
- Human, H.; Nicolson, S.W. Nutritional content of fresh, bee-collected and stored pollen of Aloe greateadii var. davyana (Asphodelaceae). Phytochemistry 2006, 67, 1486–1492. [Google Scholar] [CrossRef]
- Wongchai, V.; Ratanavalachai, T. Seasonal variation of chemical composition of royal jelly produced in Thailand. Thammasat Int. J. Sci. Technol. 2002, 7, 1–8. [Google Scholar]
- Wang, Y.; Ma, L.; Zhang, W.; Cui, X.; Wang, H.; Xu, B. Comparison of the nutrient composition of royal jelly and worker jelly of honey bees (Apis mellifera). Apidologie 2016, 47, 48–56. [Google Scholar] [CrossRef]
- Xue, X.; Wu, L.; Wang, K. Chemical composition of royal jelly. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar] [CrossRef]
- Millward, D.J.; Fereday, A.; Gibson, N.; Pacy, P.J. Aging, protein requirements, and protein turnover. Am. J. Clin. Nutr. 1997, 66, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Paoli, P.P.; Donley, D.; Stabler, D.; Saseendranath, A.; Nicolson, S.W.; Simpson, S.J.; Wright, G.A. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids 2014, 46, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Crailsheim, K. The protein balance of the honey bee worker. Apidologie 1990, 21, 417–429. [Google Scholar] [CrossRef]
- Howe, S.R.; Dimick, P.S.; Benton, A.W. Composition of freshly harvested and commercial royal jelly. J. Apic. Res. 1985, 24, 52–61. [Google Scholar] [CrossRef]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health promoting properties of bee royal jelly: Food of the queen. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef]
- Albert, S.; Klaudiny, J.; Simuth, J. Molecular characterization of MRJP3, highly polymorphic protein of honeybee (Apis mellifera) Royal Jelly. Insect Biochem. Mol. Biol. 1999, 29, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Amano, S.; Kono, T.; Kondoh, J.; Yamaguchi, K.; Kobayashi, S.; Ayabe, T.; Moriyama, T. Molecular characteristics and physiological functions of major royal jelly protein 1 oligomer. Proteomics 2009, 9, 5534–5543. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, R.; Tamura, S.; Ito, A.; Moriyama, T.; Yamaguchi, K.; Kono, T. A rapid method to isolate soluble royal jelly proteins. Food Chem. 2012, 134, 2332–2337. [Google Scholar] [CrossRef] [PubMed]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. More than royal food- major royal jelly protein genes in sexuals and workers of the honeybee Apis mellifera. Front. Zool. 2013, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Bíliková, K.; Mirgorodskaya, E.; Bukovská, G.; Gobom, J.; Lehrach, H.; Šimúth, J. Towards functional proteomics of minority component of honeybee royal jelly: The effect of post-translational modifications on the antimicrobial activity of apalbumin2. Proteomics 2009, 9, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Vezeteu, T.V.; Bobiş, O.; Moritz, R.F.A.; Buttstedt, A. Food to some, poison to others—Honeybee royal jelly and its growth inhibiting effect on European foulbrood bacteria. Microbiol. Open 2017, 6, e00397. [Google Scholar] [CrossRef] [PubMed]
- Šimúth, J.; Bíliková, K.; Kováčová, E.; Kuzmová, Z.; Schroder, W. Immunochemical approach to detection of adulteration in honey: Physiologically active royal jelly protein stimulating TNF-α release is a regular component of honey. J. Agric. Food Chem. 2004, 52, 2154. [Google Scholar] [CrossRef] [PubMed]
- Rosmilah, M.; Patel, M.S.G.; Lock, J.; Rahman, D.; Masita, A.; Noormalin, A. Characterization of major allergens of royal jelly Apis mellifera. Trop. Biomed. 2008, 25, 243–251. [Google Scholar]
- Knecht, D.; Kaatz, H.H. Patterns of larval food production by hypopharyngeal glands in adult worker honey bees. Apidologie 1990, 21, 457–468. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salingnon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzchmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed]
- Antinelli, J.-F.; Zeggane, S.; Davico, R.; Ragnone, C.; Faucon, J.-P.; Lizzani, L. Evaluation of (E)-10-hydroxydec-2-enoic acid as a freshness parameter for royal jelly. Food Chem. 2003, 80, 85–89. [Google Scholar] [CrossRef]
- Crailsheim, K.; Riessberger-Gallé, U. Honey bee age-dependent resistance against American foulbrood. Apidologie 2001, 32, 91–103. [Google Scholar] [CrossRef]
- Šedivá, M.; Laho, M.; Kohútová, L.; Mojžišová, A.; Majtán, J.; Klaudiny, J. 10-HDA, a major fatty acid of royal jelly, exhibits pH dependent growth-inhibitory activity against different strains of Paenibacillus larvae. Molecules 2018, 23, 3236. [Google Scholar] [CrossRef] [PubMed]
- Arien, Y.; Dag, A.; Zarchin, S.; Masci, T.; Shafir, S. Omega-3 deficiency impairs honey bee learning. Proc. Natl. Acad. Sci. USA 2015, 112, 15761–15766. [Google Scholar] [CrossRef] [PubMed]
- Arien, Y.; Dag, A.; Yona, S.; Tietel, Z.; Lapidot Cohen, T.; Shafir, S. Effect of diet lipids and omega-6:3 ratio on honey bee brood development, adult survival and body composition. J. Insect Physiol. 2020, 124, 104074. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Amoedo, L.H.; de Almeida-Muradian, L.B. Physicochemical composition of pure and adulterated royal jelly. Química Nova 2007, 30, 257–259. [Google Scholar] [CrossRef]
- Benfenati, L.; Sabatini, A.G.; Nanetti, A. Composizione in Sali minerali della gelatine reale. Apicoltura 1986, 2, 129–143. [Google Scholar]

| Standard Spot | Molecular Weight | Isoelectric Point | Spot Intensity | Protein Identified | O/R * | |
|---|---|---|---|---|---|---|
| Oak | Rapeseed | |||||
| 2001 | 17.50 | 6.25 | 55.2 ± 39.5 | 26.2 ± 23.2 | 2.1 | |
| 2201 | 45.40 | 5.89 | 474.4 ± 480.9 | 75.6 ± 166.8 | 6.3 | |
| 4606 | 78.04 | 6.91 | 3971.4 ± 5532.4 | 1519.0 ± 1097.8 | 2.6 | |
| 6101 | 29.17 | 7.21 | 461.9 ± 297.8 | 127.8 ± 231.8 | 3.6 | |
| 6802 | 109.36 | 7.28 | 50.7 ± 54.0 | 17.3 ± 36.5 | 2.9 | |
| 7101 | 29.34 | 7.54 | 461.9 ± 297.8 | 41.8 ± 39.6 | MRJP3 precursor | 11.0 |
| 7503 | 68.26 | 7.84 | 1084.4 ± 591.0 | 416.3 ± 280.9 | MRJP3 | 2.6 |
| 7701 | 87.58 | 7.48 | 341.1 ± 251.9 | 131.1 ± 105.0 | Glucose oxidase | 2.6 |
| 7702 | 79.52 | 7.63 | 1704.3 ± 877.1 | 843.5 ± 451.1 | 2.0 | |
| 8103 | 34.82 | 8.13 | 301.0 ± 389.0 | 45.3 ± 27.2 | 6.6 | |
| 8106 | 44.39 | 8.26 | 470.0 ± 415.1 | 76.0 ± 42.0 | MRJP3 like | 6.2 |
| 8801 | 80.69 | 7.91 | 107.6 ± 98.7 | 34.0 ± 73.7 | 3.2 | |
| 8803 | 88.11 | 7.67 | 30.9 ± 34.1 | 14.0 ± 17.9 | 2.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Jung, C. Chemical Composition and Nutritional Value of Royal Jelly Samples Obtained from Honey Bee (Apis mellifera) Hives Fed on Oak and Rapeseed Pollen Patties. Insects 2024, 15, 141. https://doi.org/10.3390/insects15030141
Ghosh S, Jung C. Chemical Composition and Nutritional Value of Royal Jelly Samples Obtained from Honey Bee (Apis mellifera) Hives Fed on Oak and Rapeseed Pollen Patties. Insects. 2024; 15(3):141. https://doi.org/10.3390/insects15030141
Chicago/Turabian StyleGhosh, Sampat, and Chuleui Jung. 2024. "Chemical Composition and Nutritional Value of Royal Jelly Samples Obtained from Honey Bee (Apis mellifera) Hives Fed on Oak and Rapeseed Pollen Patties" Insects 15, no. 3: 141. https://doi.org/10.3390/insects15030141
APA StyleGhosh, S., & Jung, C. (2024). Chemical Composition and Nutritional Value of Royal Jelly Samples Obtained from Honey Bee (Apis mellifera) Hives Fed on Oak and Rapeseed Pollen Patties. Insects, 15(3), 141. https://doi.org/10.3390/insects15030141









