Knockdown of the Expression of Two Trehalase Genes with RNAi Disrupts the Trehalose and Chitin Metabolism Pathways in the Oriental Armyworm, Mythimna separata
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Identification of MsTre1 and MsTre2
2.3. Bioinformatic Analysis and Phylogenetic Tree Construction of MsTre1 and MsTre2
2.4. Spatial-Temporal Expression Patterns of MsTre1 and MsTre2
2.5. Double-Stranded RNA (dsRNA) Preparation and RNAi of MsTre1 and MsTre2
2.6. Determination of the MsTre1 and MsTre2 Activity, Sugar, and Chitin Content
2.7. Microsectioning and Hematoxylin and Eosin (H&E) Staining of the Cuticle
2.8. Transmission Electron Microscopy (TEM) of the Cuticle
2.9. Effects on Growth and Development after MsTre1 and MsTre2 Knockdown
2.10. Statistical Analysis
3. Results
3.1. Bioinformatic Analysis of MsTre1 and MsTre2
3.2. Spatio-Temporal Expression Patterns of MsTre1 and MsTre2
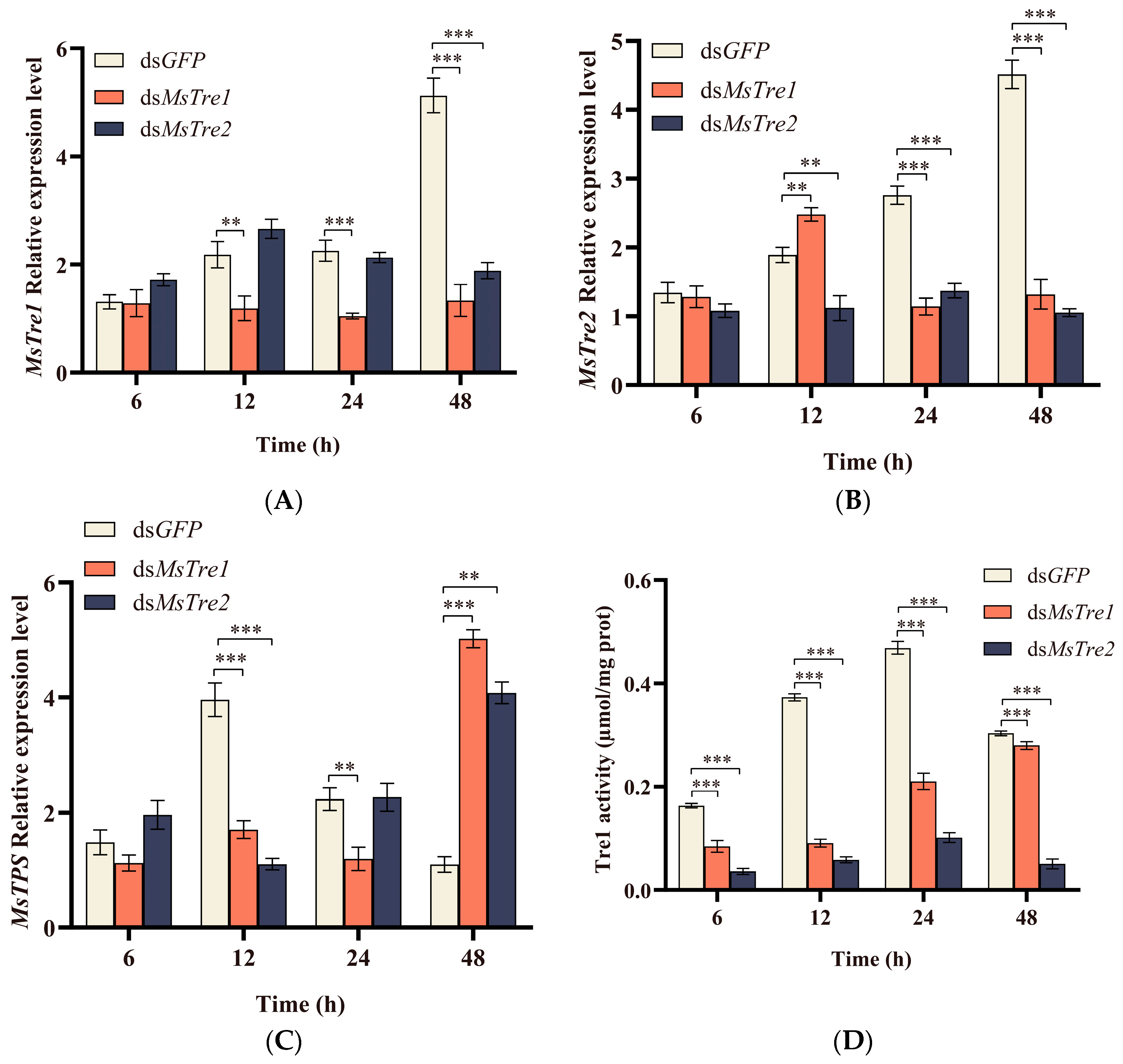
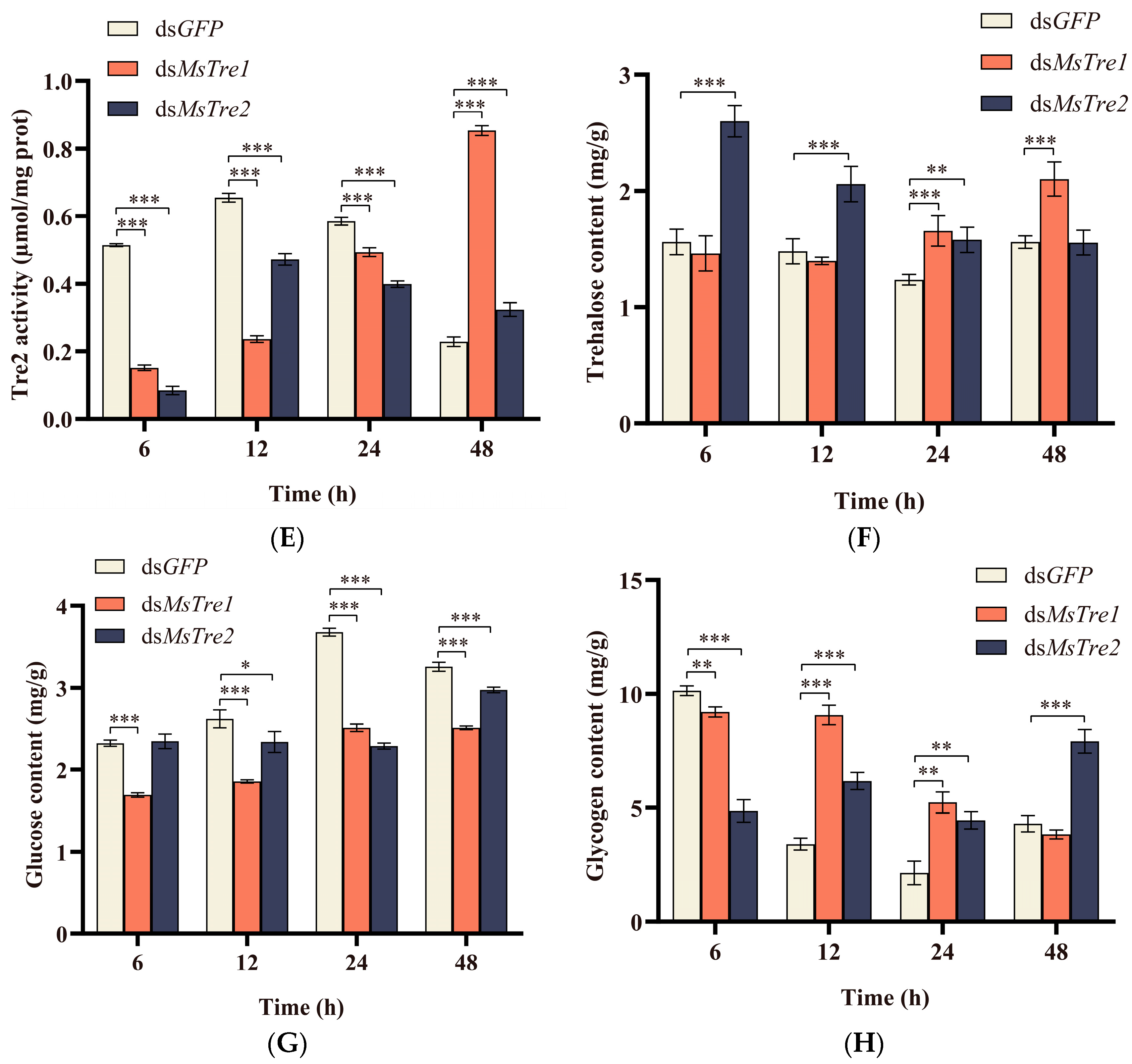
3.3. The Expression of MsTre1, MsTre2, and MsTPS, Trehalase Activity and Sugar Content after RNAi
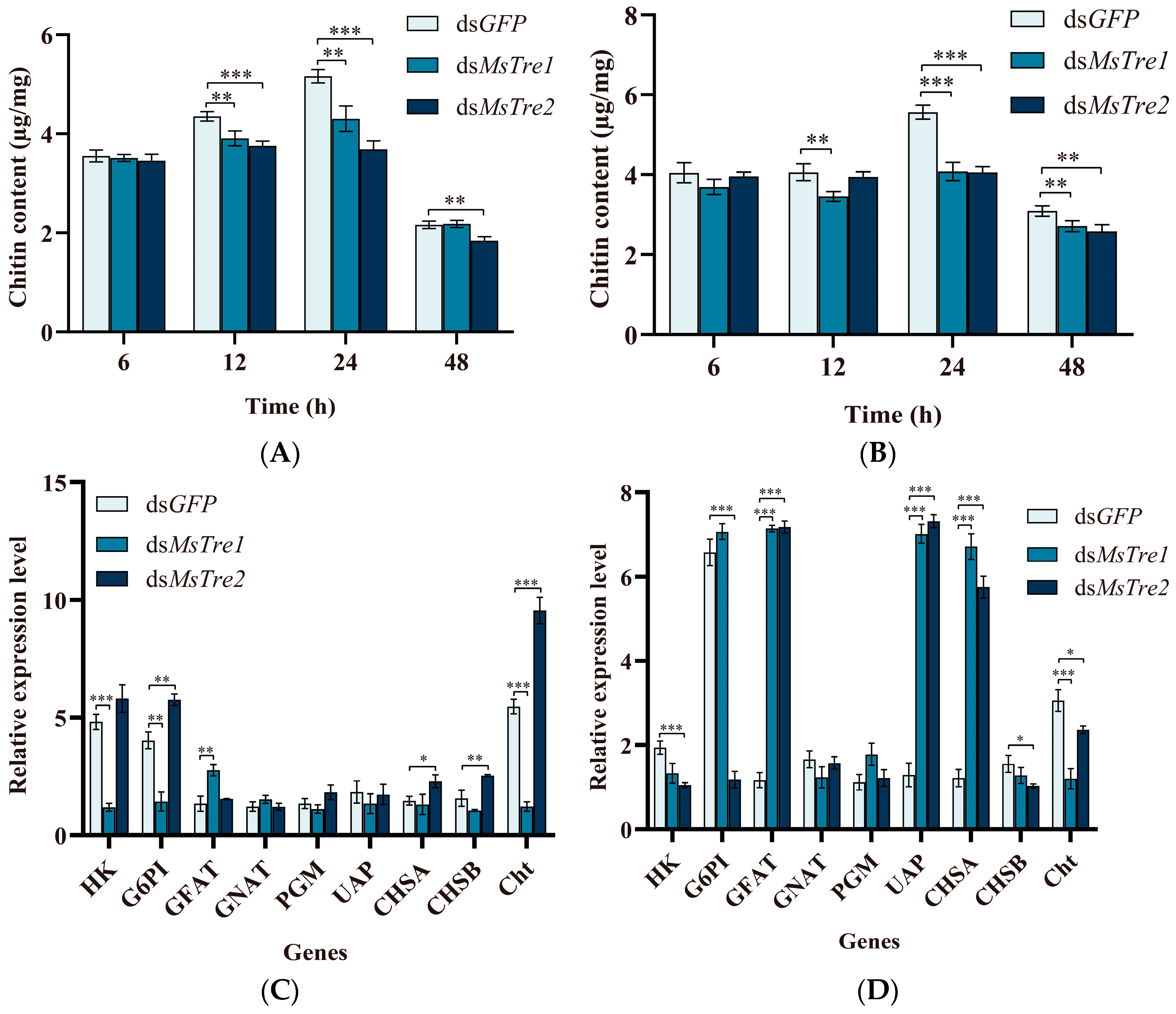
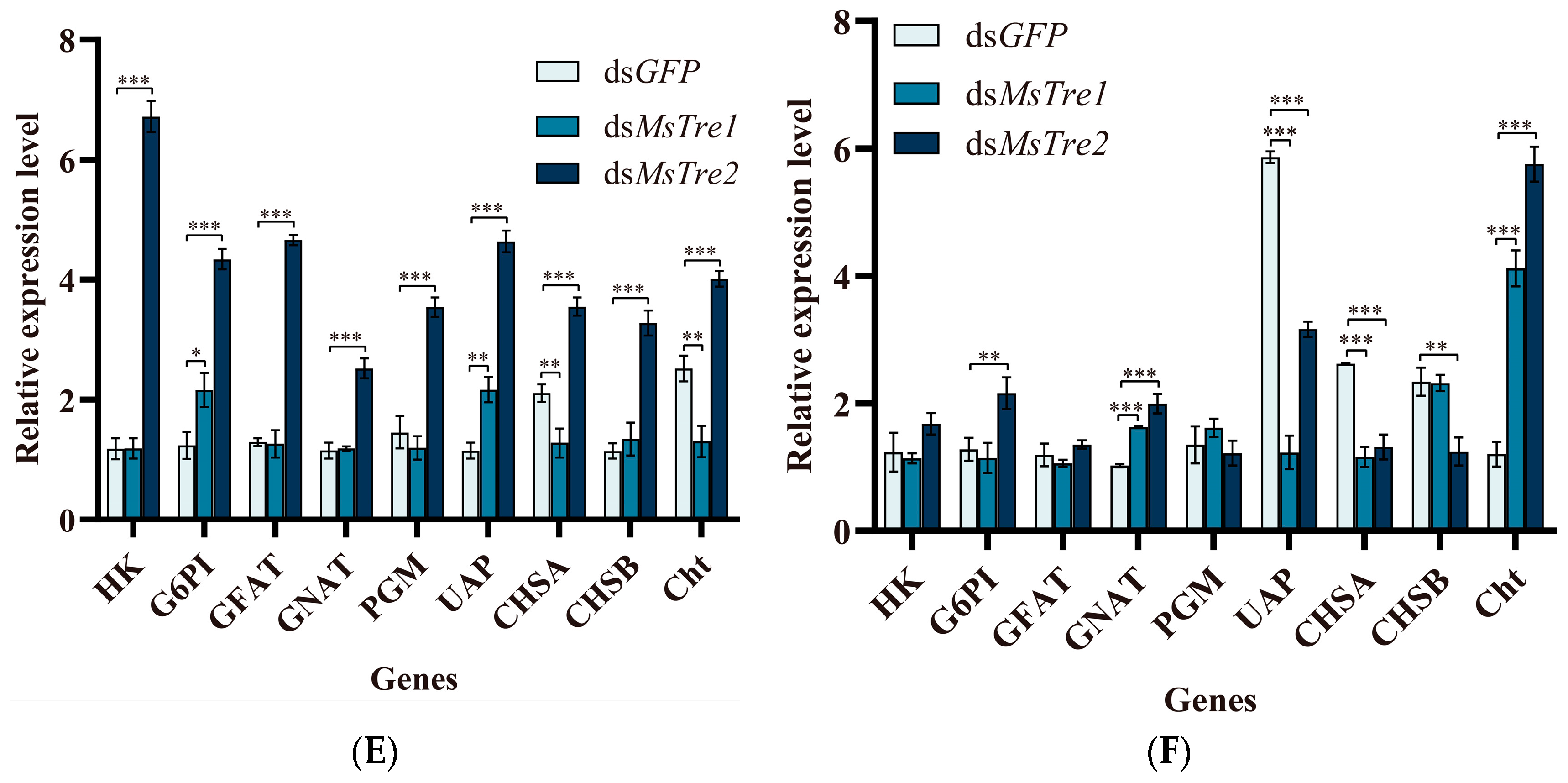
3.4. Alteration in the Chitin Content and Expression of Genes in the Chitin Metabolism Pathway after RNAi
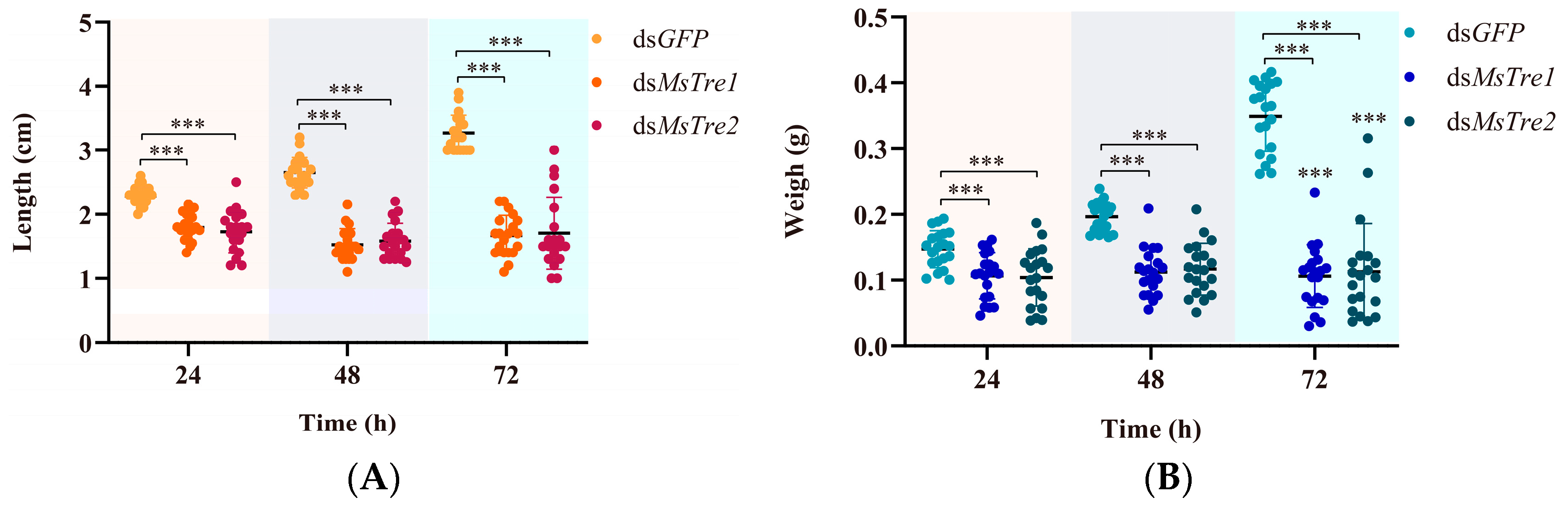
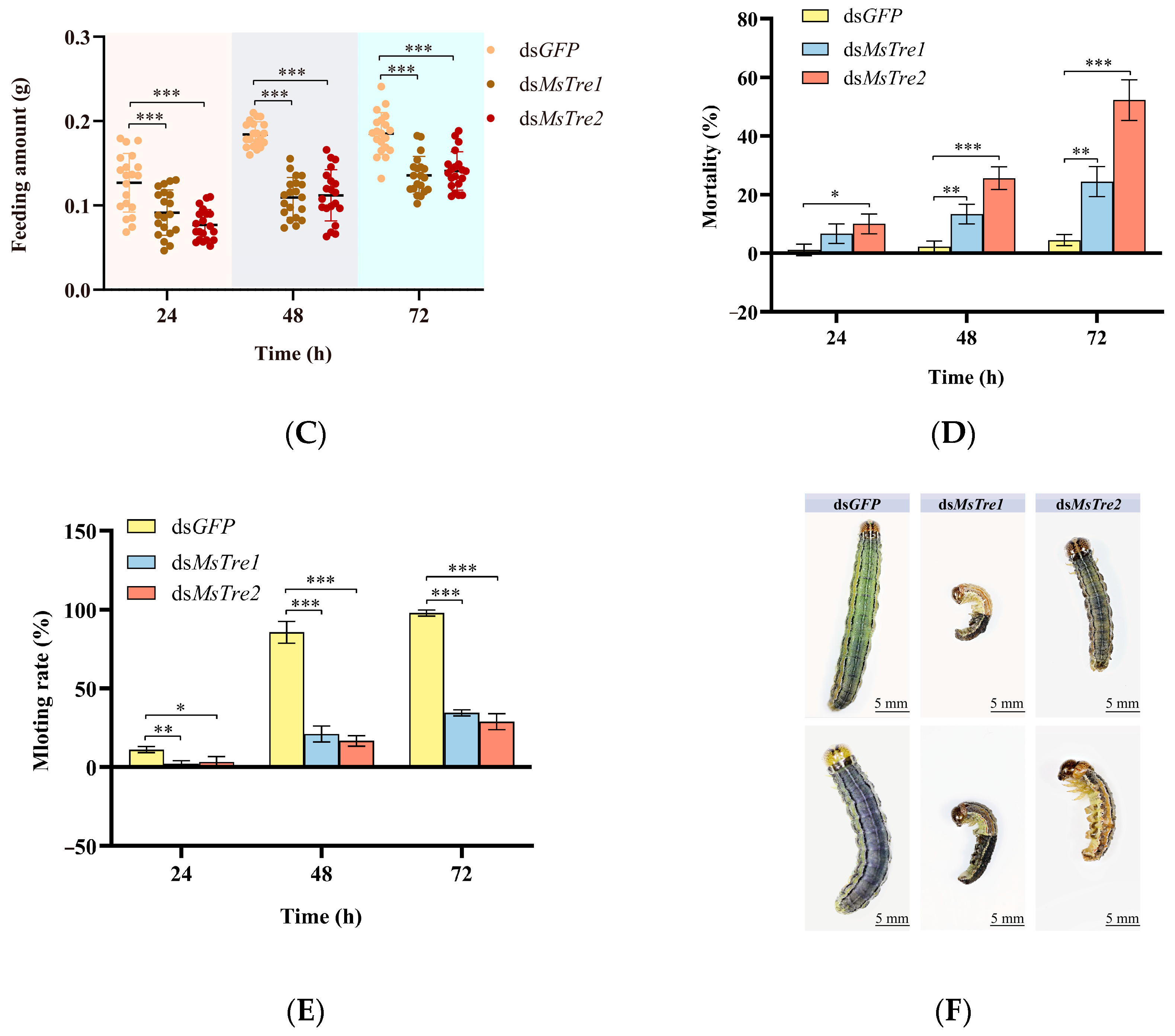
3.5. Effect on M. separata Growth and Development after RNAi
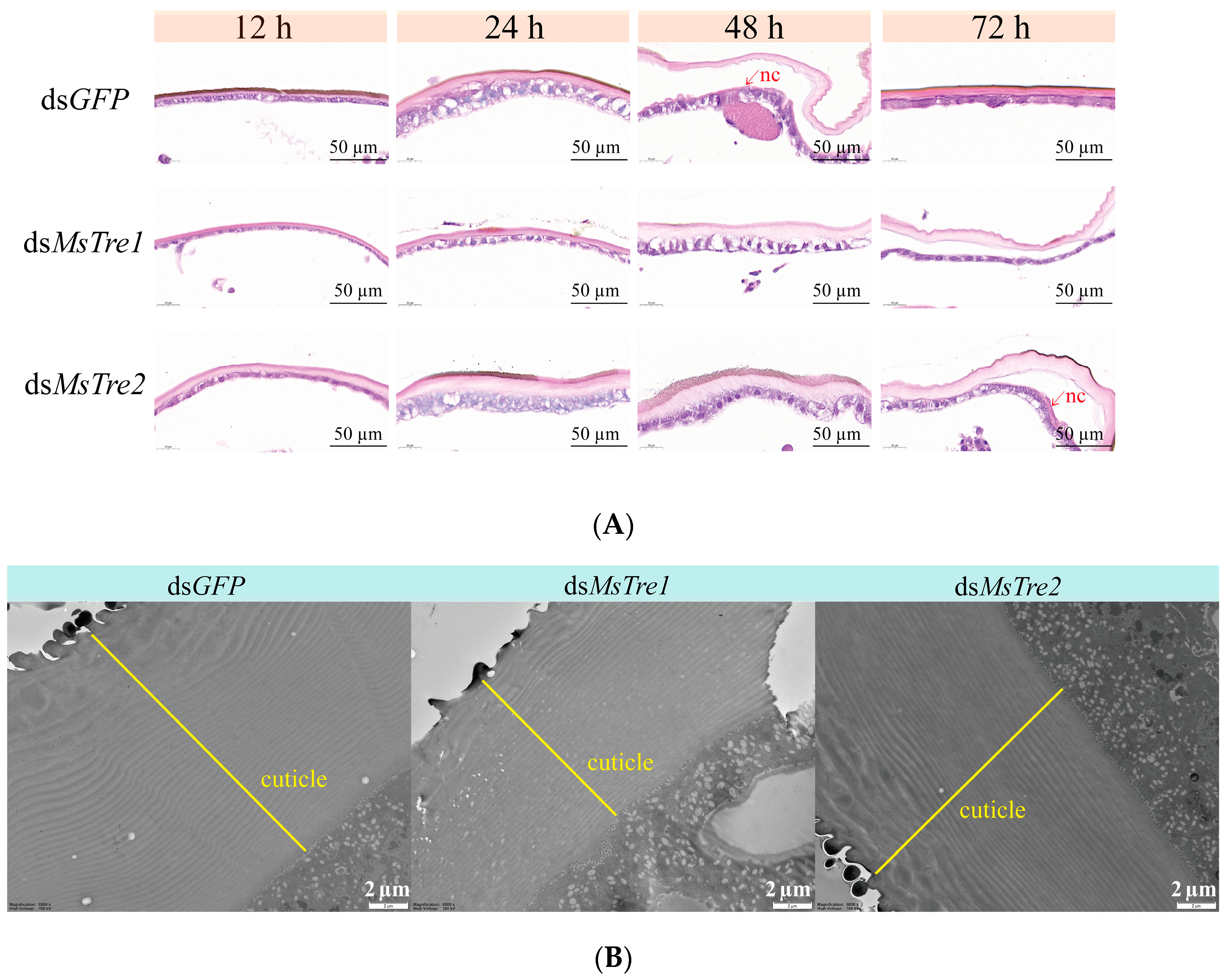
3.6. Effects on Cuticle Formation after MsTre1 and MsTre2 Knockdown
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feofilova, E.P.; Usov, A.I.; Mysyakina, I.S.; Kochkina, G.A. Trehalose: Chemical structure, biological functions, and practical application. Microbiology 2014, 83, 184–194. [Google Scholar] [CrossRef]
- Gancedo, C.; Flores, C.L. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 2004, 4, 351–359. [Google Scholar] [CrossRef]
- Tang, B.; Wang, S.; Wang, S.G.; Wang, H.J.; Zhang, J.Y.; Cui, S.Y. Invertebrate trehalose-6-phosphate synthase gene: Genetic architecture, biochemistry, physiological function, and potential applications. Front. Physiol. 2018, 9, 30. [Google Scholar] [CrossRef]
- Friedman, S. Treholose regulation, one aspect of metabolic homeostasis. Annu. Rev. Entomol. 1978, 23, 389–407. [Google Scholar] [CrossRef]
- Wyatt, G.R. The biochemistry of sugars and polysaccharides in insects. Adv. Insect Phys. 1967, 4, 287–360. [Google Scholar]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17–27. [Google Scholar] [CrossRef]
- Chang, Y.P.; Zhang, B.; Du, M.F.; Geng, Z.C.; Wei, J.Z.; Guan, R.B.; An, S.H.; Zhao, W.L. The vital hormone 20-hydroxyecdysone controls ATP production by upregulating binding of trehalase 1 with ATP synthase subunit α in Helicoverpa armigera. J. Biol. Chem. 2022, 298, 101565. [Google Scholar] [CrossRef]
- Thompson, S.N. Trehalose—The insect ‘blood’ sugar. Adv. Insect Phys. 2003, 31, 205–285. [Google Scholar]
- Becker, A.; Schlöder, P.; Steele, J.E.; Wegener, G. The regulation of trehalose metabolism in insects. Experientia 1996, 52, 433–439. [Google Scholar] [CrossRef]
- Clegg, J.S.; Evans, D.R. Blood trehalose and flight metabolism in the blowfly. Science 1961, 134, 54–55. [Google Scholar] [CrossRef]
- Candy, D.J.; Becker, A.; Wegener, G. Coordination and integration of metabolism in insect flight. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 117, 497–512. [Google Scholar] [CrossRef]
- Avonce, N.; Mendoza-Vargas, A.; Morett, E.; Iturriaga, G. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 2006, 6, 109. [Google Scholar] [CrossRef]
- Shukla, E.; Thorat, L.; Bhavnani, V.; Bendre, A.D.; Pal, J.K.; Nath, B.B.; Gaikwad, S.M. Molecular cloning and in silico studies of physiologically significant trehalase from Drosophila melanogaster. Int. J. Biol. Macromol. 2016, 92, 282–292. [Google Scholar] [CrossRef]
- Takiguchi, M.; Niimi, T.; Su, Z.H.; Yaginuma, T. Trehalase from male accessory gland of an insect, Tenebrio molitor cDNA sequencing and developmental profile of the gene expression. Biochem. J. 1992, 288, 19–22. [Google Scholar] [CrossRef]
- Mitsumasu, K.; Azuma, M.; Niimi, T.; Yamashita, O.; Yaginuma, T. Membrane-penetrating trehalase from silkworm Bombyx mori Molecular cloning and localization in larval midgut. Insect Mol. Biol. 2005, 14, 501–508. [Google Scholar] [CrossRef]
- Shukla, E.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2015, 25, 357–367. [Google Scholar] [CrossRef]
- Tang, B.; Wei, P.; Chen, J.; Wang, S.G.; Zhang, W.Q. Progress in gene features and functions of insect trehalases. Acta. Entomol. Sin. 2012, 55, 1315–1321. [Google Scholar]
- Bansal, R.; Mian, M.A.R.; Mittapalli, O.; Michel, A.P. Molecular characterization and expression analysis of soluble trehalase gene in Aphis glycines, a migratory pest of soybean. Bull. Entomol. Res. 2013, 103, 286–295. [Google Scholar] [CrossRef]
- Tan, Y.; Xiao, L.B.; Sun, Y.; Zhao, J.; Bai, L.X.; Xiao, Y.F. Molecular characterization of soluble and membrane-bound trehalases in the cotton mirid bug, Apolygus lucorum. Arch. Insect Biochem. Physiol. 2014, 86, 107–121. [Google Scholar] [CrossRef]
- Tatun, N.; Singtripop, T.; Tungjitwitayakul, J.; Sakurai, S. Regulation of soluble and membrane-bound trehalase activity and expression of the enzyme in the larval midgut of the bamboo borer Omphisa fuscidentalis. Insect Biochem. Mol. Biol. 2008, 38, 788–795. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, D.H.; Pu, J.; Wu, M.; Han, Z.J. Cloning and RNA interference effects of trehalase genes in Laodelphax striatellus (Homoptera: Delphacidae). Acta. Entomol. Sin. 2012, 55, 911–920. [Google Scholar]
- Tang, B.; Yang, M.M.; Shen, Q.D.; Xu, Y.X.; Wang, H.J.; Wang, S.G. Suppressing the activity of trehalase with validamycin disrupts the trehalose and chitin biosynthesis pathways in the rice brown planthopper, Nilaparvata lugens. Pestic. Biochem. Physiol. 2017, 137, 81–90. [Google Scholar] [CrossRef]
- Chen, J.; Tang, B.; Chen, H.X.; Yao, Q.; Huang, X.F.; Chen, J.; Zhang, D.W.; Zhang, W.Q. Different functions of the insect soluble and membrane-bound trehalase genes in chitin biosynthesis revealed by RNA interference. PLoS ONE 2010, 5, e10133. [Google Scholar] [CrossRef]
- Wang, J.; He, W.B.; Su, Y.L.; Bing, X.L.; Liu, S.S. Molecular characterization of soluble and membrane-bound trehalases of the whitefly, Bemisia tabaci. Arch. Insect Biochem. Physiol. 2014, 85, 216–233. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Zhang, X.; Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Ma, E.; Zhu, K.Y. Identification and characterization of a novel chitinase-like gene cluster (AgCht5) possibly derived from tandem duplications in the African malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol. 2011, 41, 521–528. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Chen, X.F.; Tang, B.; Tian, H.G.; Chen, J.; Yao, Q. Insect chitin biosynthesis and its regulation. Chin. J. Appl. Entomol. 2011, 48, 475–479. [Google Scholar]
- Zhang, X.; Zhang, J.Z.; Park, Y.; Zhu, K.Y. Identification and characterization of two chitin synthase genes in African malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol. 2012, 42, 674–682. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.Q.; Zhang, J.Z.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Cohen, E. Chitin synthesis and inhibition: A revisit. Pest Manag. Sci. 2001, 57, 946–950. [Google Scholar] [CrossRef]
- Nakabachi, A.; Shigenobu, S.; Miyagishima, S. Chitinase-like proteins encoded in the genome of the pea aphid, Acyrthosiphon pisum. Insect Mol. Biol. 2010, 19, 175–185. [Google Scholar] [CrossRef]
- Quan, G.X.; Ladd, T.; Duan, J.; Wen, F.Y.; Doucet, D.; Cusson, M.; Krell, P.J. Characterization of a spruce budworm chitin deacetylase gene: Stage- and tissue-specific expression, and inhibition using RNA interference. Insect Biochem. Mol. Biol. 2013, 43, 683–691. [Google Scholar] [CrossRef]
- Xi, Y.; Pan, P.L.; Ye, Y.X.; Yu, B.; Zhang, C.X. Chitin deacetylase family genes in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Mol. Biol. 2014, 23, 695–705. [Google Scholar] [CrossRef]
- Yu, H.Z.; Huang, Y.L.; Lu, Z.J.; Zhang, Q.; Su, H.N.; Du, Y.M.; Yi, L.; Zhong, B.L.; Chen, C.X. Inhibition of trehalase affects the trehalose and chitin metabolism pathways in Diaphorina citri (Hemiptera: Psyllidae). Insect Sci. 2021, 28, 718–734. [Google Scholar] [CrossRef]
- Gong, C.; Yang, Z.Z.; Hu, Y.; Wu, Q.J.; Wang, S.L.; Guo, Z.J.; Zhang, Y.J. Silencing of the BtTPS genes by transgenic plant-mediated RNAi to control Bemisia tabaci MED. Pest Manag. Sci. 2021, 78, 1128–1137. [Google Scholar] [CrossRef]
- Shi, J.F.; Xu, Q.Y.; Sun, Q.K.; Meng, Q.W.; Mu, L.L.; Guo, W.C.; Li, G.Q. Physiological roles of trehalose in Leptinotarsa larvae revealed by RNA interference of trehalose-6-phosphate synthase and trehalase genes. Insect Biochem. Mol. Biol. 2016, 77, 52–68. [Google Scholar] [CrossRef]
- Chen, L.; Pan, Q.J.; Waqas, M.S.; Liu, T.X. Morphological traits for sex identification of the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae). J. Integr. Agric. 2020, 19, 1458–1463. [Google Scholar] [CrossRef]
- Chen, G.Q.; Yang, J.M.; Sun, D.; Han, X.X.; Tian, Y.; Liu, S.M.; Jiang, J.; Che, Z.P. Syntheses and insecticidal activities of some paeonol-based phenylsulfonylhydrazone derivatives against Mythimna separata in vivo. Chemistry 2020, 83, 139–143. [Google Scholar]
- Wang, J.D.; Chen, L.F.; Lin, D.J.; Zhang, J.S.; Zhao, J.H.; Xiao, D.; Wang, R.; Wang, R.; Gao, S.J. Molecular cloning, characterization and functional analysis of GluCl from the oriental armyworm, Mythimna separata Walker. Pestic. Biochem. Physiol. 2019, 156, 56–62. [Google Scholar] [CrossRef]
- Wang, D.M.; Dai, A.M.; Liu, X.L.; Li, C.H. Assessment on control effect of veratridine on cotton aphid. Xinjiang Agric. Sci. 2019, 56, 46–51. [Google Scholar]
- Lin, D.J.; Zhang, Y.X.; Fang, Y.; Gao, S.J.; Wang, R.; Wang, J.D. The effect of chlorogenic acid, a potential botanical insecticide, on gene transcription and protein expression of carboxylesterases in the armyworm (Mythimna separata). Pestic. Biochem. Physiol. 2023, 195, 105575. [Google Scholar] [CrossRef]
- Azhar, M.; Freed, S.; Sabir, H.; Rafique, S.; Naeem, A.; Ahmed, R. Effect of sub-lethal and lethal concentrations of the entomopathogenic fungus Metarhizium anisopliae Sorokin on detoxification enzymes and demographic parameters of Mythimna separata (Walker). Crop Prot. 2023, 172, 106323. [Google Scholar] [CrossRef]
- Chen, X.D.; Neupane, S.; Gill, T.A.; Gossett, H.; Pelz-Stelinski, K.S.; Stelinski, L.L. Comparative transcriptome analysis of thiamethoxam susceptible and resistant Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), using RNA-sequencing. Insect Sci. 2021, 28, 1708–1720. [Google Scholar] [CrossRef]
- He, C.; Liang, J.J.; Yang, J.; Xue, H.; Huang, M.J.; Fu, B.L.; Wei, X.G.; Liu, S.N.; Du, T.H.; Ji, Y.; et al. Over-expression of CP9 and CP83 increases whitefly cell cuticle thickness leading to imidacloprid resistance. Int. J. Biol. Macromol. 2023, 233, 123647. [Google Scholar] [CrossRef]
- Yang, H.J.; Cui, M.Y.; Zhao, X.H.; Zhang, C.Y.; Hu, Y.S.; Fan, D. Trehalose-6-phosphate synthase regulates chitin synthesis in Mythimna separata. Front. Physiol. 2023, 14, 1109661. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 0034.1. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, J.; Sun, Y.W.; Liang, X.Y.; Zhang, R.; Zhao, X.M.; Zhang, M.; Zhang, J.Z. A nuclear receptor HR4 is essential for the formation of epidermal cuticle in the migratory locust, Locusta migratoria. Insect Biochem Mol. Biol. 2022, 143, 103740. [Google Scholar] [CrossRef]
- He, W.; Xu, W.; Fu, K.; Guo, W.; Zhang, J. Low Mismatch Rate between Double-Stranded RNA and Target mRNA Does Not Affect RNA Interference Efficiency in Colorado Potato Beetle. Insects 2020, 11, 449. [Google Scholar] [CrossRef]
- Tatun, N.; Singtripop, T.; Sakurai, S. Dual control of midgut trehalase activity by 20-hydroxyecdysone and an inhibitory factor in the bamboo borer Omphisa fuscidentalis Hampson. J. Insect Physiol. 2008, 54, 351–357. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, M.M.; Shen, Q.D.; Liu, X.J.; Shi, Z.K.; Wang, S.G.; Tang, B. Functional characterization of three trehalase genes regulating the chitin metabolism pathway in rice brown planthopper using RNA interference. Sci. Rep. 2016, 6, 27841. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.E. Occurrence of a hyperglycæmic factor in the corpus cardiacum of an insect. Nature 1961, 192, 680–681. [Google Scholar] [CrossRef]
- Ge, L.Q.; Zhao, K.F.; Huang, L.J.; Wu, J.C. The effects of triazophos on the trehalose content, trehalase activity and their gene expression in the brown planthopper Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Pestic. Biochem. Physiol. 2011, 100, 172–181. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Specht, C.A.; Tomoyasu, Y.; Lorenzen, M.D.; Kanost, M.; Beeman, R.W. The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol. Biol. 2005, 14, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Reissig, J.L.; Strominger, J.L.; Leloir, L.F. A modified colorimetric method for the estimation of N-acetylamino sugars. J. Biol. Chem. 1955, 217, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.M.; Xie, Y.P.; Xue, J.L.; Gao, Y.; Zhang, Y.F.; Zhang, X.M.; Tan, J.S. Histopathological changes of Ceroplastes japonicus infected by Lecanicillium lecanii. J. Invertebr. Pathol. 2009, 101, 96–105. [Google Scholar] [CrossRef]
- Benoit, J.B.; Lopez-Martinez, G.; Michaud, M.R.; Elnitsky, M.A.; Lee, R.E.; Denlinger, D.L. Mechanisms to reduce dehydration stress in larvae of the Antarctic midge, Belgica antarctica. J. Insect Physiol. 2007, 53, 656–667. [Google Scholar] [CrossRef]
- Zhao, K.F.; Shi, Z.P.; Wu, J.C. Insecticide-induced enhancement of flight capacity of the brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae). Crop Prot. 2011, 30, 476–482. [Google Scholar] [CrossRef]
- Zou, Q.; Wei, P.; Xu, Q.; Zheng, H.Z.; Tang, B.; Wang, S.G. cDNA cloning and characterization of two trehalases from Spodoptera litura (Lepidoptera; Noctuidade). Genet Mol. Res. 2013, 12, 901–915. [Google Scholar] [CrossRef]
- Tang, B.; Chen, X.F.; Liu, Y.; Tian, H.G.; Liu, J.; Hu, J.; Xu, W.H.; Zhang, W.Q. Characterization and expression patterns of a membrane-bound trehalase from Spodoptera exigua. BMC Mol. Biol. 2008, 9, 51. [Google Scholar] [CrossRef]
- Ma, L.; Dai, W.; Li, X.C.; Zhang, Y.L.; Zhang, C.N. Molecular cloning and expression analysis of soluble and membrane-bound trehalase genes in the cotton bollworm, Helicoverpa armigera. J. Asia Pac. Entomol. 2015, 18, 187–195. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Guan, R.B.; Li, H.C.; Miao, X.X. RNAi pest control and enhanced BT insecticidal efficiency achieved by dsRNA of chymotrypsin-like genes in Ostrinia furnacalis. J. Pest Sci. 2017, 90, 745–757. [Google Scholar] [CrossRef]
- Tang, B.; Wei, P.; Zhao, L.; Shi, Z.K.; Shen, Q.D.; Yang, M.M.; Xie, G.Q.; Wang, S.G. Knockdown of five trehalase genes using RNA interference regulates the gene expression of the chitin biosynthesis pathway in Tribolium castaneum. BMC Biotechnol. 2016, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Zhang, S.; Kong, X.; Liu, F.; Zhang, Z. RNAi-Mediated Silencing of the Chitinase 5 Gene for Fall Webworm (Hyphantria cunea) Can Inhibit Larval Molting Depending on the Timing of dsRNA Injection. Insects 2021, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kakumani, P.K.; Kumar, A.; Malhotra, P.; Mukherjee, S.K.; Bhatnagar, R.K. Genome wide screening of RNAi factors of Sf21 cells reveal several novel pathway associated proteins. BMC Genom. 2014, 15, 775. [Google Scholar] [CrossRef] [PubMed]
- Arakane, Y.; Specht, C.A.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Chitin synthases are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect Biochem Mol. Biol. 2008, 38, 959–962. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Ge, L.Q.; Jiang, Y.P.; Lu, X.L.; Li, X.; Stanley, D.; Song, Q.S.; Wu, J.C. RNAi knockdown of acetyl-CoA carboxylase gene eliminates jinggangmycin-enhanced reproduction and population growth in the brown planthopper, Nilaparvata lugens. Sci. Rep. 2015, 5, 15360. [Google Scholar] [CrossRef]
- Tang, B.; Chen, J.; Yao, Q.; Pan, Z.Q.; Xu, W.H.; Wang, S.G.; Zhang, W.Q. Characterization of a trehalose-6-phosphate synthase gene from Spodoptera exigua and its function identification through RNA interference. J. Insect Physiol. 2010, 56, 813–821. [Google Scholar] [CrossRef]
- Cha, W.H.; Lee, D.W. RNA interference of trehalose phosphate synthase inhibits metamorphosis and decreases cold tolerance in the diamondback moth, Plutella xylostella (L.). J. Asia Pac. Entomol. 2018, 21, 1034–1039. [Google Scholar] [CrossRef]
- Shao, Z.M.; Ding, J.H.; Jiang, D.L.; Liu, Z.X.; Li, Y.J.C.; Wang, J.; Wang, J.; Sheng, S.; Wu, F.A. Characterization and functional analysis of trehalase related to chitin metabolism in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Insects 2021, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.C.; Wang, J.; Li, J.H.; Deng, Y.Q.; Pu, P.; Fan, H.; Liu, Y.H. RNA interference of a trehalose-6-phosphate synthase gene reveals its roles during larval-pupal metamorphosis in Bactrocera minax (Diptera: Tephritidae). J. Insect Physiol. 2016, 91–92, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.M.; Zhao, L.N.; Shen, Q.D.; Xie, G.Q.; Wang, S.G.; Tang, B. Knockdown of two trehalose-6-phosphate synthases severely affects chitin metabolism gene expression in the brown planthopper Nilaparvata lugens. Pest Manag. Sci. 2017, 73, 206–216. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wang, Y.; Zhang, W.; Zhang, X.; Wang, S.; Cui, M.; Zhao, X.; Fan, D.; Dai, C. Knockdown of the Expression of Two Trehalase Genes with RNAi Disrupts the Trehalose and Chitin Metabolism Pathways in the Oriental Armyworm, Mythimna separata. Insects 2024, 15, 142. https://doi.org/10.3390/insects15030142
Yang H, Wang Y, Zhang W, Zhang X, Wang S, Cui M, Zhao X, Fan D, Dai C. Knockdown of the Expression of Two Trehalase Genes with RNAi Disrupts the Trehalose and Chitin Metabolism Pathways in the Oriental Armyworm, Mythimna separata. Insects. 2024; 15(3):142. https://doi.org/10.3390/insects15030142
Chicago/Turabian StyleYang, Hongjia, Yixiao Wang, Weijia Zhang, Xinxin Zhang, Sibo Wang, Mengyao Cui, Xiaohui Zhao, Dong Fan, and Changchun Dai. 2024. "Knockdown of the Expression of Two Trehalase Genes with RNAi Disrupts the Trehalose and Chitin Metabolism Pathways in the Oriental Armyworm, Mythimna separata" Insects 15, no. 3: 142. https://doi.org/10.3390/insects15030142
APA StyleYang, H., Wang, Y., Zhang, W., Zhang, X., Wang, S., Cui, M., Zhao, X., Fan, D., & Dai, C. (2024). Knockdown of the Expression of Two Trehalase Genes with RNAi Disrupts the Trehalose and Chitin Metabolism Pathways in the Oriental Armyworm, Mythimna separata. Insects, 15(3), 142. https://doi.org/10.3390/insects15030142





