North African Endemism: A New Species of Black Fly (Diptera: Simuliidae) from the Djurdjura Mountains of Algeria †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chromosome Preparation
2.2. Morphological Preparation
2.3. Type Depository
3. Results
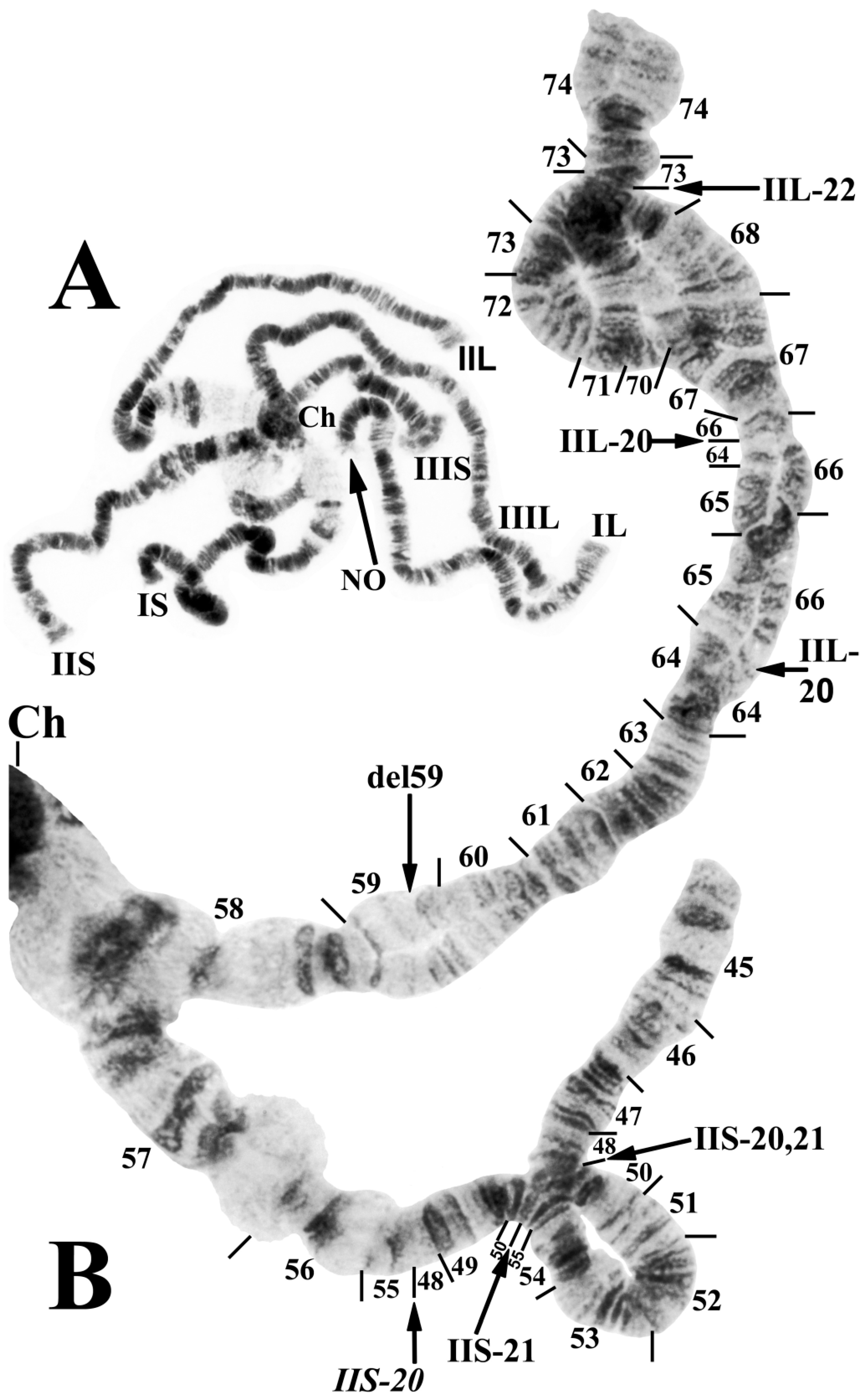
3.1. Chromosomal Description
3.2. Morphological Description
3.3. Diagnosis
3.4. Type Material
3.5. Bionomics
3.6. Associated Species
3.7. Natural Enemies
3.8. Etymology
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Carsten, R.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldså, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar]
- Hardy, N.B.; Williams, D.J. Doubling the known endemic species diversity of New Caledonian armored scale insects (Hemiptera, Diaspididae). ZooKeys 2018, 78, 11–47. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.P.; Fink, D.; Fletcher, W.J. Late Pleistocene glaciers and climate in the High Atlas, North Africa. In Untangling the Quaternary Period—A Legacy of Stephen C. Porter; Waitt, R.B., Thackray, G.D., Gillespie, A.R., Eds.; Geological Society of America: Boulder, CO, USA, 2021; Volume 548, pp. 155–174. [Google Scholar]
- Yasri-Cheboubi, N.; Vinçon, G.; Lounaci, A. The Nemouridae from Algeria (Insecta: Plecoptera). Zoosystema 2016, 38, 295–308. [Google Scholar] [CrossRef]
- Ayache, S.; Strangi, A.; Chakali, G.; Dahmani, L.; Chellali, M.; Pennacchio, F.; Roversi, P.F.; Binazzi, F. A new species, Cinara tellenica Binazzi F. et Strangi (Aphididae Lachninae) associated with Cedrus atlantica in the Tell Atlas of Algeria. Bull. Insectol. 2020, 73, 275–283. [Google Scholar]
- Dey, L.-S.; Hochkirch, A.; Moussi, A.; Simões, M.V.P.; Husemann, M. Diversification in and around the Atlas Mountains: Insights into the systematics and biogeography of the genus Thalpomena (Orthoptera: Acrididae: Oedipodinae). Syst. Entomol. 2022, 47, 402–419. [Google Scholar] [CrossRef]
- Dambri, B.M.; Godunko, R.J.; Benhadji, N. Baetidae (Insecta: Ephemeroptera) of Aurès Mountains (Algeria): A new species of the Baetis alpinus species group, with notes on Baetis Laech [sic], 1815 biogeography within Maghreb. Insects 2023, 14, 899. [Google Scholar] [CrossRef]
- Lamine, S.; Lounaci, A.; Reding, J.-P.; Vinçon, G. Marthamea bayae, a new species of stonefly from Algeria (Plecoptera: Perlidae). Zootaxa 2019, 4603, 311–326. [Google Scholar] [CrossRef]
- Kechemir, L.H.; Sartori, M.; Lounaci, A. An unexpected new species of Habrophlebia from Algeria (Ephemeroptera, Leptophlebiidae). ZooKeys 2020, 953, 31–47. [Google Scholar] [CrossRef]
- Meddour, R.; Sahar, O.; Jury, S. New analysis of the endemic vascular plants of Algeria, their diversity, distribution pattern and conservation status. Willdenowia 2023, 53, 25–43. [Google Scholar] [CrossRef]
- Mohammed, T.; Al-Amin, A.Q. Climate change and water resources in Algeria: Vulnerability, impact and adaptation strategy. Econ. Environ. Stud. 2018, 18, 411–429. [Google Scholar] [CrossRef]
- Sayre, R.; Comer, P.; Hak, J.; Josse, C.; Bow, J.; Warner, H.; Larwanou, M.; Kelbessa, E.; Bekele, T.; Kehl, H.; et al. A New Map of Standardized Terrestrial Ecosystems of Africa; Association of American Geographers: Washington, DC, USA, 2013; p. 24. [Google Scholar]
- Adler, P.H. World Blackflies (Diptera: Simuliidae): A Comprehensive Revision of the Taxonomic and Geographical Inventory. 2022. Available online: http://biomia.sites.clemson.edu/pdfs/blackflyinventory.pdf (accessed on 12 September 2023).
- Adler, P.H.; Cherairia, M.; Arigue, S.F.; Samraoui, B.; Belqat, B. Cryptic biodiversity in the cytogenome of bird-biting black flies in North Africa. Med. Vet. Entomol. 2015, 29, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Lounaci, A.; Brosse, S.; Mouloud, S.A.; Lounaci-Daoudi, D.; Mebarki, N.; Thomas, A. Current knowledge of benthic invertebrate diversity in an Algerian stream: A species check-list of the Sébaou River basin (Tizi Ouzou). Bull. Soc. Hist. Nat. Toulouse 2000, 136, 43–55. [Google Scholar]
- Lounaci, A.; Brosse, S.; Thomas, A.; Lek, S. Abundance, diversity and community structure of macroinvertebrates in an Algerian stream: The Sébaou wadi. Ann. Limnol.-Int. J. Limnol. 2000, 36, 123–133. [Google Scholar] [CrossRef]
- Haouchine, S.; Lounaci, A. Les macroinvertébrés benthiques des cours d’eau de Kabylie (Algérie): Faunistique, écologie et répartition géographique. Bull. Soc. Zool. France 2012, 137, 133–156. [Google Scholar]
- Adler, P.H.; Huang, S. Chromosomes as barcodes: Discovery of a new species of black fly (Diptera: Simuliidae) from California, USA. Insects 2022, 13, 903. [Google Scholar] [CrossRef] [PubMed]
- Adler, P.H.; Crosskey, R.W. Cytotaxonomy of the Simuliidae (Diptera): A systematic and bibliographic conspectus. Zootaxa 2015, 3975, 1–139. [Google Scholar] [CrossRef]
- Adler, P.H.; Reeves, W.K. North–south differentiation of black flies in the Western Cordillera of North America: A new species of Prosimulium (Diptera: Simuliidae). Diversity 2023, 15, 212. [Google Scholar] [CrossRef]
- Adler, P.H.; Şirin, Ü. Cytotaxonomy of the Prosimulium (Diptera: Simuliidae) of Western Asia. Zool. J. Linn. Soc. 2014, 171, 753–768. [Google Scholar] [CrossRef]
- Basrur, P.K. The salivary gland chromosomes of seven segregates of Prosimulium (Diptera: Simuliidae) with a transformed centromere. Can. J. Zool. 1959, 37, 527–570. [Google Scholar] [CrossRef]
- Adler, P.H.; Currie, D.C.; Wood, D.M. The Black Flies (Simuliidae) of North America; Cornell University Press: Ithaca, NY, USA, 2004; p. 941. [Google Scholar]
- Basrur, P.K. The salivary gland chromosomes of seven species of Prosimulium (Diptera: Simuliidae) from Alaska and British Columbia. Can. J. Zool. 1962, 40, 1019–1033. [Google Scholar] [CrossRef]
- Craig, D.A. Mouthparts and feeding behaviour of Tahitian larval Simuliidae (Diptera: Nematocera). Quaest. Entomol. 1977, 13, 195–218. [Google Scholar]
- Rothfels, K.H. Cytotaxonomy of black flies (Simuliidae). Annu. Rev. Entomol. 1979, 24, 507–539. [Google Scholar] [CrossRef]
- Henderson, C.A.P. A cytological study of the Prosimulium onychodactylum complex (Diptera, Simuliidae). Can. J. Zool. 1986, 64, 32–44. [Google Scholar] [CrossRef]
- Nelder, M.P.; Beard, C.E.; Adler, P.H.; Kim, S.-K.; McCreadie, J.W. Harpellales (Zygomycota: Trichomycetes) associated with black flies (Diptera: Simuliidae): World review and synthesis of their ecology and taxonomy. Fungal Divers. 2006, 22, 121–169. [Google Scholar]
- Kúdela, M.; Adler, P.H.; Kúdelová, T. Taxonomic status of the black fly Prosimulium italicum Rivosecchi (Diptera: Simuliidae) based on genetic evidence. Zootaxa 2018, 4377, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Bedo, D.G. Cytogenetics and evolution of Simulium ornatipes Skuse (Diptera: Simuliidae) I. Sibling speciation. Chromosoma 1977, 64, 37–65. [Google Scholar] [CrossRef]
- Madahar, D.P. The salivary gland chromosomes of seven taxa in the subgenus Stegopterna (Diptera, Simuliidae, Cnephia). Can. J. Zool. 1969, 47, 115–119. [Google Scholar] [CrossRef]
- Rubzow, I.A. Simuliidae (Melusinidae) [part]. In Die Fliegen der Palaearktichen Region (III) 14; Lindner, E., Ed.; E. Schweizerbart: Stuttgart, Germany, 1961; pp. 161–208. [Google Scholar]
- Zwick, H. Faunistisch-ökologische und taxonomische Untersuchungen an Simuliidae (Diptera), unter besonderer Berücksichtigung der Arten des Fulda-Gebietes. Abh. Senckenb. Naturforsch. Ges. 1974, 533, 1–116. [Google Scholar]
- Bentinck, W.C. The Black Flies of Japan and Korea (Diptera: Simuliidae); 406th Medical General Laboratory: Tokyo, Japan, 1955; pp. 1–23. [Google Scholar]
- Knoz, J. To identification of Czechoslovakian black-flies (Diptera, Simuliidae). Folia Facult. Scient. Nat. Univ. Purkynianae Brun. Biol. 1965, 6, 54. [Google Scholar]
- Belqat, B. Etude Systématique, Écologique et Caryologique des Simulies (Diptera: Simuliidae) du Maroc: Cas Particulier du Rif. Ph.D. Thesis, Université Abdelmalek Essaâdi, Tetouan, Morocco, 2002. [Google Scholar]
- Rivosecchi, L. Simuliidae: Diptera Nematocera. Fauna d’Italia 1978, 13, 1–533. [Google Scholar]
- Clergue-Gazeau, M.; Boumaiza, M. Les Simuliidae (Diptera, Nématocera) de la Tunisie. II. Clés pour la reconnaissance des espèces actuellement recensées. Arch. L’institut Pasteur Tunis 1986, 63, 601–637. [Google Scholar]






| Site | Location | Longitude and Latitude | Elevation (m above Sea Level) | Date |
|---|---|---|---|---|
| 1 | spring brook 0.5 km upstream of village Tirurda (TR2) | 36°29′26′′ N 04°21′32′′ E | 1045 | 7 April 2015 |
| 2 | stream 1 km upstream from a drinking water fountain called “l3insar [sic] n’biya” (TR3) | 36°29′36′′ N 04°21′18′′ E | 1255 | 16 April 2015 |
| 3 | stream 1 km downstream of Tirurda Pass (TR1) | 36°29′26′′ N 04°21′42′′ E | 1120 | 10 May 2015 |
| 4 | stream near town of Illithen | 36°30′25′′ N 04°24′17′′ E | 1150 | 14 April 2016 |
| 5 | stream near village of Ath Atsou | 36°29’43′′N 04°22′23′′ E | 1080 | 14 April 2016 |
| Site 1 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Females:Males | 6:0 | 10:4 | 2:1 | 9:3 | 3:3 |
| IS-40 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| IS-41 | 0.92 | 0.86 | 1.00 | 0.88 | 1.00 |
| IL-17 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| IIS-20 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| IIS-21 2 | * | * | * | * | * |
| IIL-20 2 | * | * | * | * | * |
| IIL-21 2 | * | * | * | * | * |
| IIL-22 3 | ** | ** | ** | ** | ** |
| IIL del59 4 | *** | ||||
| IIIL-39 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| IIIL-40 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adler, P.H.; Haouchine, S.; Belqat, B.; Lounaci, A. North African Endemism: A New Species of Black Fly (Diptera: Simuliidae) from the Djurdjura Mountains of Algeria. Insects 2024, 15, 150. https://doi.org/10.3390/insects15030150
Adler PH, Haouchine S, Belqat B, Lounaci A. North African Endemism: A New Species of Black Fly (Diptera: Simuliidae) from the Djurdjura Mountains of Algeria. Insects. 2024; 15(3):150. https://doi.org/10.3390/insects15030150
Chicago/Turabian StyleAdler, Peter H., Sabrina Haouchine, Boutaïna Belqat, and Abdelkader Lounaci. 2024. "North African Endemism: A New Species of Black Fly (Diptera: Simuliidae) from the Djurdjura Mountains of Algeria" Insects 15, no. 3: 150. https://doi.org/10.3390/insects15030150






