Monoaminergic Systems in Flight-Induced Potentiation of Phonotactic Behavior in Female Crickets Gryllus bimaculatus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavioral Test
2.3. Pharmacological Treatments
2.4. Evoked Flight
2.5. Serotonin and Octopamine Measurement by High Performance Liquid Chromatography with Fluorescent Detection (HPLC-FLD)
2.6. Statistical Analysis
3. Results
3.1. Octopaminergic Ligands and Phonotactic Behavior in Female Crickets
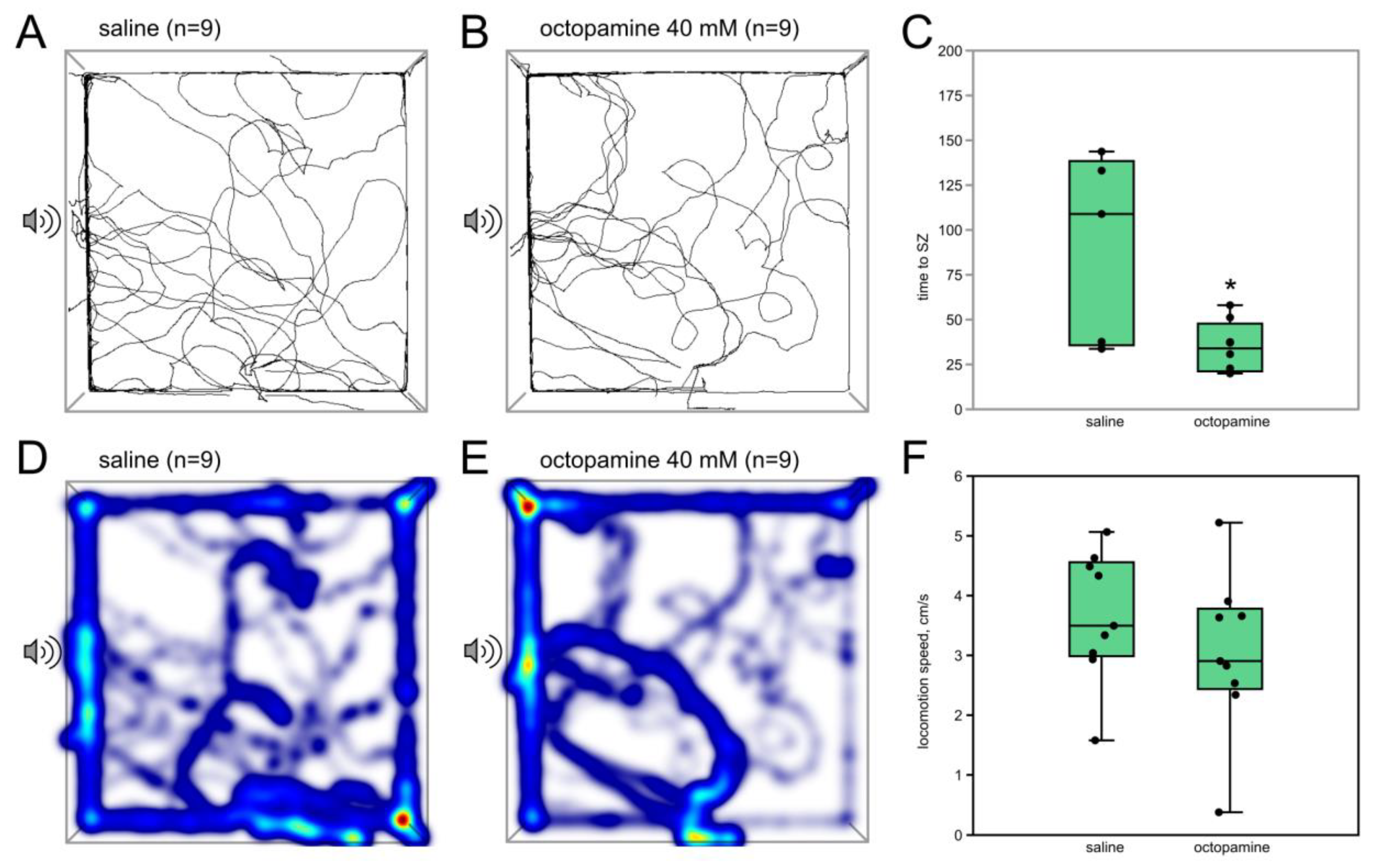
3.1.1. Octopamine Receptor Antagonist Epinastine Potentiates the Effect of Flight
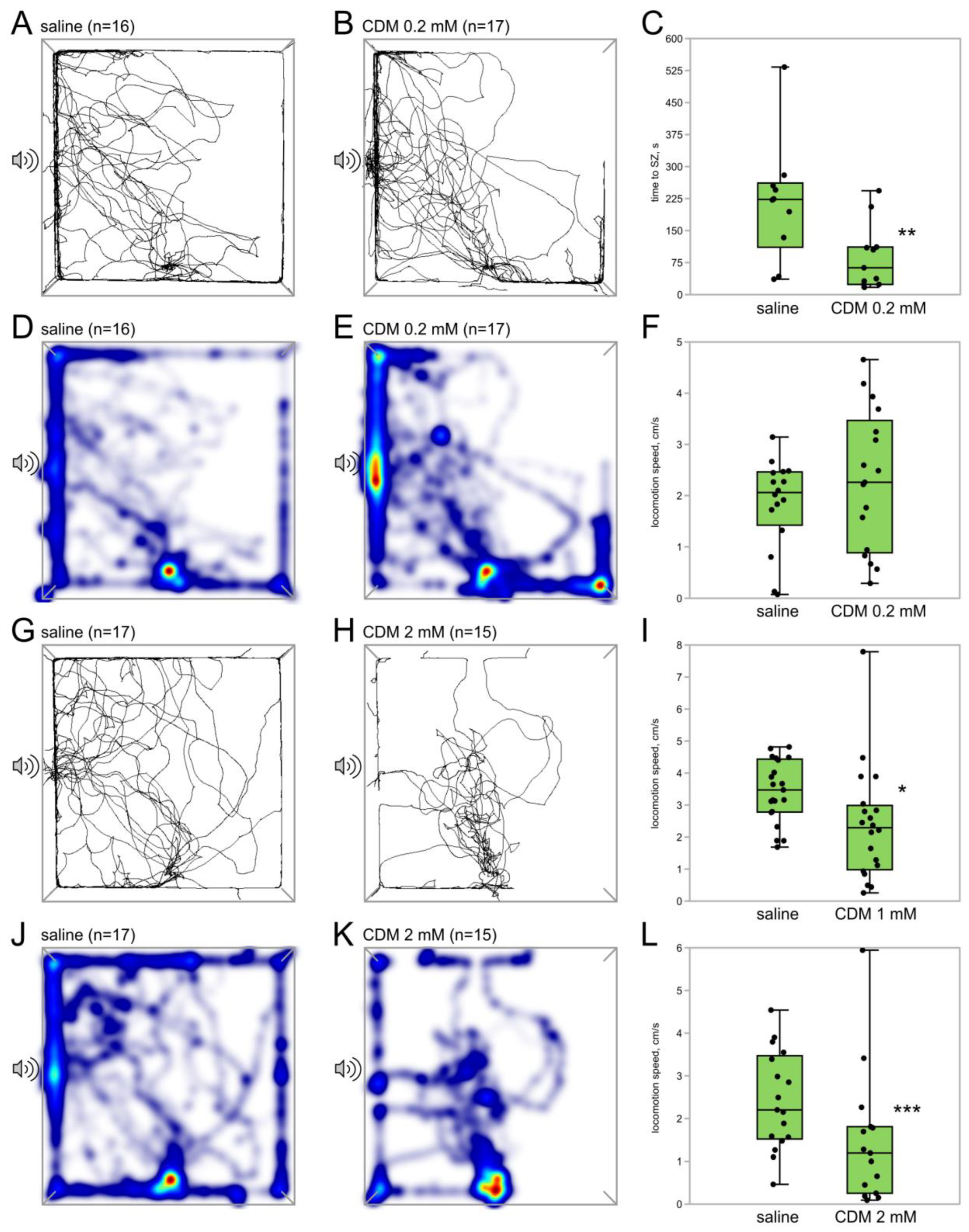
3.1.2. Octopamine Receptor Agonist Chlordimeform Exerts Dose-Dependent Effects on Phonotaxis
3.1.3. Octopamine (40 mM) Acts Similarly to the Low Dose of CDM
3.2. Serotonergic Ligands and Phonotactic Behavior in Female Crickets
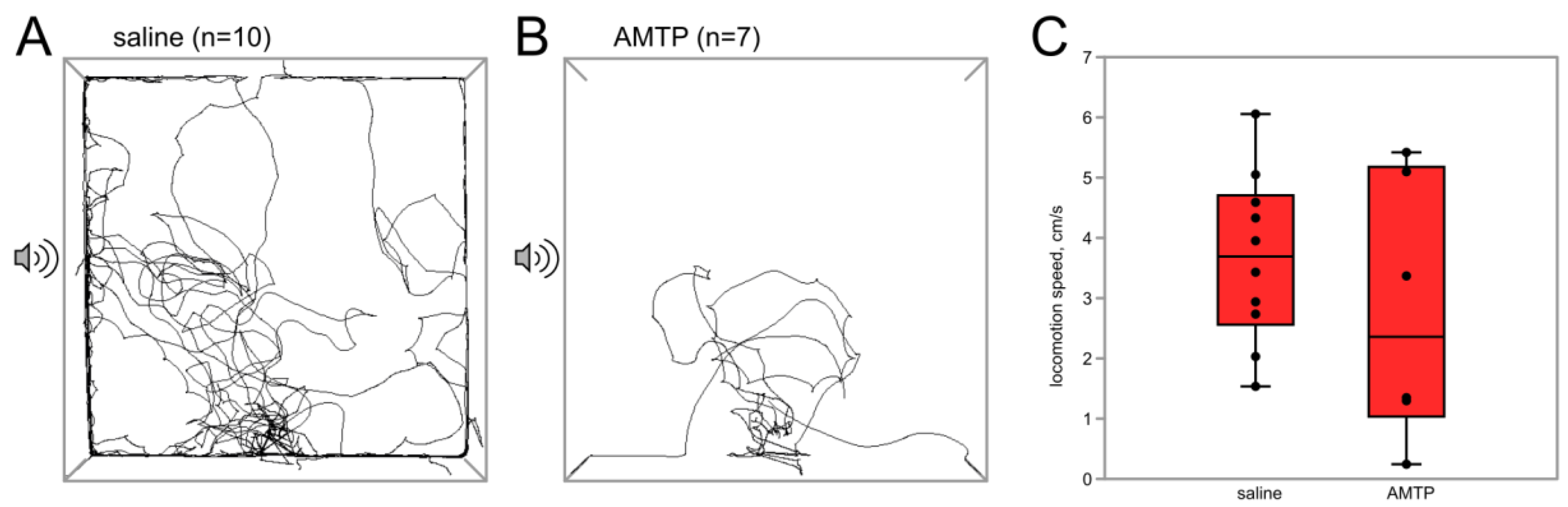
3.2.1. Serotonin Synthesis Inhibitor AMTP Abolishes the Effect of Flight on Phonotactic Behavior
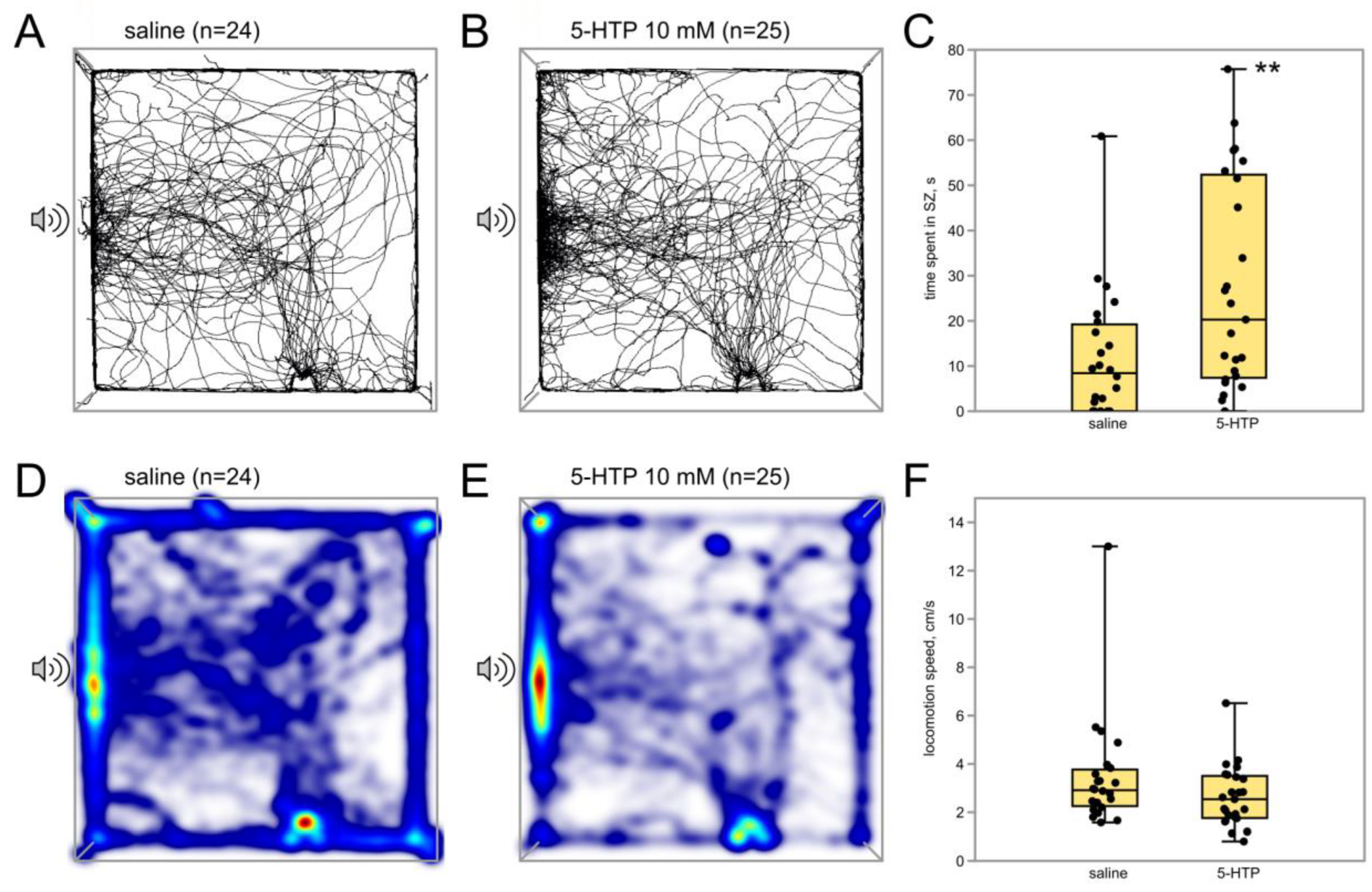
3.2.2. Immediate Serotonin Precursor 5-HTP Potentiates Phonotactic Behavior
3.3. Impact of Flight on Serotonin and Octopamine Content Measured by High Performance Liquid Chromatography in the Thoracic Ganglia of Female Crickets
4. Discussion
4.1. The Dual Role of OA in the Modulation of Phonotaxis
4.1.1. Behaviors Activated by Octopamine Can Interfere with Positive Phonotaxis
4.1.2. Is There Evidence for a Facilitating Effect of Octopamine on Insect Hearing?
4.2. Serotonin Potentiates Phonotaxis
4.2.1. Does Flight Change the State of the Serotonergic System?
4.2.2. Serotonin May Affect Phonotaxis via Changes in the Motivational State
4.2.3. Serotonin and Hearing in Insects
4.3. Conclusions. Possible Roles of 5-HT and OA in the Flight-Dependent Modulation of Female Phonotactic Behavior
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nolen, T.G.; Hoy, R.R. Phonotaxis in flying crickets: I. Attraction to the calling song and avoidance of bat-like ultrasound are discrete behaviors. J. Comp. Physiol. A 1986, 159, 423–439. [Google Scholar] [CrossRef]
- Moiseff, A.; Pollack, G.S.; Hoy, R.R. Steering responses of flying crickets to sound and ultrasound: Mate attraction and predator avoidance. Proc. Natl. Acad. Sci. USA 1978, 75, 4052–4056. [Google Scholar] [CrossRef]
- Popov, A.V.; Shuvalov, V.F. Phonotactic behavior of crickets. J. Comp. Physiol. 1977, 119, 111–126. [Google Scholar] [CrossRef]
- Ulagaraj, S.M.; Walker, T.J. Phonotaxis of crickets in flight: Attraction of male and female crickets to male calling songs. Science 1973, 182, 1278–1279. [Google Scholar] [CrossRef]
- Baker, C.A.; Clemens, J.; Murthy, M. Acoustic pattern recognition and courtship songs: Insights from insects. Annu. Rev. Neurosci. 2019, 42, 129–147. [Google Scholar] [CrossRef]
- Hedwig, B. Pulses, patterns and paths: Neurobiology of acoustic behaviour in crickets. J. Comp. Physiol. A 2006, 192, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Sergejeva, M.V.; Popov, A.V. Ontogeny of positive phonotaxis in female crickets, Gryllus bimaculatus De Geer: Dynamics of sensitivity, frequency-intensity domain, and selectivity to temporal pattern of the male calling song. J. Comp. Physiol. A 1994, 174, 381–389. [Google Scholar] [CrossRef]
- Mezheritskiy, M.; Vorontsov, D.; Lapshin, D.; Dyakonova, V. Previous flight facilitates partner finding in female crickets. Sci. Rep. 2020, 10, 22328. [Google Scholar] [CrossRef] [PubMed]
- Shiga, S.; Kogawauchi, S.; Yasuyama, K.; Yamaguchi, T. Flight behaviour and selective degeneration of flight muscles in the adult cricket (Gryllus bimaculatus). J. Exp. Biol. 1991, 155, 661–667. [Google Scholar] [CrossRef]
- Lorenz, M.W. Oogenesis-flight syndrome in crickets: Age-dependent egg production, flight performance, and biochemical composition of the flight muscles in adult female Gryllus bimaculatus. J. Insect Physiol. 2007, 8, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.A.; Stevenson, P.A. Flight restores fight in crickets. Nature 2000, 403, 613. [Google Scholar] [CrossRef]
- Stevenson, P.A.; Dyakonova, V.E.; Rillich, J.; Schildberger, K. Octopamine and experience-dependent modulation of aggression in crickets. J. Neurosci. 2005, 25, 1431–1441. [Google Scholar] [CrossRef]
- Dyakonova, V.E.; Krushinsky, A.L. Previous motor experience enhances courtship behavior in male cricket Gryllus bimaculatus. J. Insect Behav. 2008, 21, 172–180. [Google Scholar] [CrossRef]
- Adamo, S.A.; Linn, C.E.; Hoy, R.R. The role of neurohormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J. Exp. Biol. 1995, 198, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.A.; Hofmann, H.; Schoch, K.; Schildberger, K. The fight and flight responses of crickets depleted of biogenic amines. J. Neurobiol. 2000, 43, 107–120. [Google Scholar] [CrossRef]
- Magno, D. The Role of Octopamine in Syllable-Period Selective Phonotaxis in Female Cricket Acheta domesticus. Honors Thesis, Andrews University, Berrien County, MI, USA, 2017; p. 159. [Google Scholar]
- Atkins, G.; Yoon, J.; Lee, K.; Koo, R.; Chung, K.; Zdor, J.; Magno, D.; Cho, E.B.; Kim, C.; Gonzalez-Socoloske, D.; et al. Nanoinjection of neurotransmitters into the prothoracic ganglion of female cricket Acheta domesticus changes phonotactic selectivity. Physiol. Entomol. 2020, 45, 131–139. [Google Scholar] [CrossRef]
- Dyakonova, V.E.; Schürmann, F.; Sakharov, D.A. Effects of serotonergic and opioidergic drugs on escape behaviors and social status of male crickets. Naturwissenschaften 1999, 86, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Ureshi, M.; Dainobu, M.; Sakai, M. Serotonin precursor (5-hydroxytryptophan) has a profound effect on the post-copulatory time-fixed sexually refractory stage in the male cricket, Gryllus bimaculatus DeGeer. J. Comp. Physiol. A 2002, 188, 767–779. [Google Scholar]
- Anstey, M.L.; Rogers, S.M.; Ott, S.R.; Burrows, M.; Simpson, S.J. Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 2009, 323, 627–630. [Google Scholar] [CrossRef]
- Alexander, R.D. Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae). Behaviour 1961, 17, 130–223. [Google Scholar] [CrossRef]
- Witney, A.; Hedwig, B. Kinematics of phonotactic steering in the walking cricket Gryllus bimaculatus (de Geer). J. Exp. Biol. 2011, 214, 69–79. [Google Scholar] [CrossRef]
- Sarmiento-Ponce, E.J.; Sutcliffe, M.P.F.; Hedwig, B. Substrate texture affects female cricket walking response to male calling song. R. Soc. Open Sci. 2018, 5, 172334. [Google Scholar] [CrossRef]
- Dyakonova, V.E.; Krushinsky, A.L. Serotonin precursor (5-hydroxytryptophan) causes substantial changes in the fighting behavior of male crickets, Gryllus bimaculatus. J. Comp. Physiol. A 2013, 199, 601–609. [Google Scholar] [CrossRef]
- Rillich, J.; Stevenson, P.A. Serotonin Mediates Depression of Aggression After Acute and Chronic Social Defeat Stress in a Model Insect. Front. Behav. Neurosci. 2018, 12, 233. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Sloley, B.D.; Orikasa, S. Selective Depletion of Dopamine, Octopamine, and 5-Hydroxytryptamine in the Nervous Tissue of the Cockroach (Periplaneta americana). J. Neurochem. 1988, 51, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Missala, K.; Sourkes, T.L. Functional cerebral activity of an analogue of serotonin formed in situ. Neurochem. Int. 1988, 12, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Dierick, H.A.; Greenspan, R.J. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 2007, 39, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Roeder, T.; Degen, J.; Gewecke, M. Epinastine, a highly specific antagonist of insect neuronal octopamine receptors. Eur. J. Pharmacol. 1998, 349, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Schofield, P.K.; Swales, L.S.; Treherne, J.E. Quantitative analysis of cellular and paracellular effects involved in the disruption of the blood brain barrier of an insect by hypertonic urea. J. Exp. Biol. 1984, 109, 333–340. [Google Scholar] [CrossRef]
- Pflüger, H.J.; Duch, C.; Heidel, E. Neuromodulatory octopaminergic neurons and their functions during insect motor behaviour. The Ernst Florey memory lecture. Acta Biol. Hung. 2004, 55, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pflüger, H.J.; Duch, C. Dynamic neural control of insect muscle metabolism related to motor behavior. Physiology 2011, 4, 293–303. [Google Scholar] [CrossRef]
- Verlinden, H.; Vleugels, R.; Marchal, E.; Badisco, L.; Pflüger, H.J.; Blenau, W.; Broeck, J.V. The role of octopamine in locusts and other arthropods. J. Insect Physiol. 2010, 56, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Rillich, J.; Rillich, B.; Stevenson, P.A. Differential modulation of courtship behavior and subsequent aggression by octopamine, dopamine and serotonin in male crickets. Horm. Behav. 2019, 114, 104542. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.; Daniel, H.; Bechtel, W.D.; Brandt, K. New tetracyclic guanidine derivatives with H1-antihistaminic properties. Chemistry of epinastine. Arzneim.-Forsch. 1990, 40, 440–446. [Google Scholar]
- Yanai, K.; Ryu, J.H.; Watanabe, T.; Iwata, R.; Ido, T.; Asakura, M.; Matsumura, R.; Itoh, M. Positron emission tomographic study of central histamine H1-receptor occupancy in human subjects treated with epinastine, a second-generation antihistamine. Methods Find. Exp. Clin. Pharmacol. 1995, 17, 64–69. [Google Scholar] [PubMed]
- Degen, J.; Gewecke, M.; Roeder, T. Octopamine receptors in the honeybee and locust nervous system: Pharmacological similarities between homologous receptors of distantly related species. Br. J. Pharmacol. 2000, 130, 587–594. [Google Scholar] [CrossRef]
- Hollingworth, R.M.; Murdock, L.L. Formamidine pesticides: Octopamine-like actions in a firefly. Science 1980, 28, 74–76. [Google Scholar] [CrossRef]
- Nathanson, J.A. Phenyliminoimidazolidines. Characterization of a class of potent agonists of octopamine-sensitive adenylate cyclase and their use in understanding the pharmacology of octopamine receptors. Mol. Pharmacol. 1985, 28, 254–268. [Google Scholar]
- Roeder, T. Pharmacology of the octopamine receptor from locust central nervous tissue (OAR3). Br. J. Pharmacol. 1995, 114, 210–216. [Google Scholar] [CrossRef]
- Roeder, T. Octopamine in invertebrates. Prog. Neurobiol. 1999, 59, 533–561. [Google Scholar] [CrossRef]
- Rezával, C.; Nojima, T.; Neville, M.C.; Lin, A.C.; Goodwin, S.F. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr. Biol. 2014, 24, 725–730. [Google Scholar] [CrossRef]
- White, M.A.; Chen, D.S.; Wolfner, M.F. She’s got nerve: Roles of octopamine in insect female reproduction. J. Neurogenet. 2021, 35, 132–153. [Google Scholar] [CrossRef]
- Ueda, A.; Kidokoro, Y. Aggressive behaviours of female Drosophila melanogaster are influenced by their social experience and food resources. Physiol. Entomol. 2002, 27, 21–28. [Google Scholar] [CrossRef]
- Nilsen, S.P.; Chan, Y.-B.; Huber, R.; Kravitz, E.A. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2004, 101, 12342–12347. [Google Scholar] [CrossRef] [PubMed]
- Bath, E.; Bowden, S.; Peters, C.; Reddy, A.; Tobias, J.A.; Easton-Calabria, E.; Seddon, N.; Goodwin, S.F.; Wigby, S. Sperm and sex peptide stimulate aggression in female Drosophila. Nat. Ecol. Evol. 2017, 1, 154. [Google Scholar] [CrossRef]
- Vedenina, V.Y.; Shestakov, L.S. Loser in fight but winner in love: How does inter-male competition determine the pattern and outcome of courtship in cricket Gryllus bimaculatus? Front. Ecol. Evol. 2018, 6, 197. [Google Scholar] [CrossRef]
- Tinsley, E.K.; Bailey, N.W. Intrasexual aggression reduces mating success in field crickets. Ecol. Evol. 2023, 13, e10557. [Google Scholar] [CrossRef] [PubMed]
- Kostarakos, K.; Hedwig, B. Calling song recognition in female crickets: Temporal tuning of identified brain neurons matches behavior. J. Neurosci. 2012, 32, 9601–9612. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.N.; Borst, A.; Haag, J. Flight activity alters velocity tuning of fly motion-sensitive neurons. J. Neurosci. 2011, 31, 9231–9237. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T. Octopamine-Mediated Neuromodulation of Insect Senses. Neurochem. Res. 2007, 32, 1511–1529. [Google Scholar] [CrossRef]
- Georgiades, M.; Alampounti, A.; Somers, J.; Su, M.P.; Ellis, D.A.; Bagi, J.; Terrazas-Duque, D.; Tytheridge, S.; Ntabaliba, W.; Moore, S.; et al. Hearing of malaria mosquitoes is modulated by a beta-adrenergic-like octopamine receptor which serves as an insecticide target. Nat. Commun. 2023, 14, 4338. [Google Scholar] [CrossRef]
- Vorontsov, D.D.; Lapshin, D.N. Effect of octopamine on the frequency tuning of the auditory system in Culex pipiens pipiens mosquito (Diptera, Culicidae). Sensornye Sist. 2023, 37, 244–257. [Google Scholar]
- Gras, H.; Hörner, M.; Runge, L.; Schürmann, F.W. Prothoracic DUM neurons of the cricket Gryllus bimaculatus—Responses to natural stimuli and activity in walking behavior. J. Comp. Physiol. A 1990, 166, 901–914. [Google Scholar] [CrossRef]
- Nikitin, S.O.; Lapitskii, V.P. Participation of segmentary octopaminergic neurons in modulation of processes of sensomotor integration in the cricket Gryllus bimaculatus. J. Evol. Biochem. Physiol. 2000, 36, 406–411. [Google Scholar] [CrossRef]
- D’iakonova, V.E. Behavioral functions of serotonin and octopamine: Some paradoxes of comparative physiology. Uspekhi Fiziol. Nauk 2007, 38, 3–20. [Google Scholar]
- Xu, Y.Y.J.; Loh, Y.M.; Lee, T.T.; Ohashi, T.S.; Su, M.P.; Kamikouchi, A. Serotonin modulation in the male Aedes aegypti ear influences hearing. Front. Physiol. 2022, 13, 931567. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.M.; Matheson, T.; Sasaki, K.; Kendrick, K.; Simpson, S.J.; Burrows, M. Substantial changes in central nervous system neurotransmitters and neuromodulators accompany phase change in the locust. J. Exp. Biol. 2004, 207, 3603–3617. [Google Scholar] [CrossRef]
- Sasaki, K.; Goto, K.; Harano, K. Timing of male territorial flight and foraging of the large carpenter bee Xylocopa appendiculata related to serotonin in the brain. Sci. Nat. 2020, 107, 25. [Google Scholar] [CrossRef]
- Banerjee, S.; Lee, J.; Venkatesh, K.; Wu, C.F.; Hasan, G. Loss of flight and associated neuronal rhythmicity in inositol 1,4,5-trisphosphate receptor mutants of Drosophila. J. Neurosci. 2004, 24, 7869–7878. [Google Scholar] [CrossRef]
- Majeed, Z.R.; Abdeljaber, E.; Soveland, R.; Cornwell, K.; Bankemper, A.; Koch, F.; Cooper, R.L. Modulatory Action by the Serotonergic System: Behavior and Neurophysiology in Drosophila melanogaster. Neural Plast. 2016, 2016, 7291438. [Google Scholar] [CrossRef]
- Dasari, S.; Viele, K.; Turner, A.C.; Cooper, R.L. Influence of PCPA and MDMA (ecstasy) on physiology, development and behavior in Drosophila melanogaster. Eur. J. Neurosci. 2007, 26, 424–438. [Google Scholar] [CrossRef]
- Claassen, D.E.; Kammer, A.E. Effects of octopamine, dopamine, and serotonin on production of flight motor output by thoracic ganglia of Manduca sexta. J. Neurobiol. 1986, 17, 1–14. [Google Scholar] [CrossRef]
- Silva, B.; Goles, N.I.; Varas, R.; Campusano, J.M. Serotonin receptors expressed in Drosophila mushroom bodies differentially modulate larval locomotion. PLoS ONE 2014, 9, e89641. [Google Scholar] [CrossRef]
- Monachon, M.A.; Burkard, W.P.; Jalfre, M.; Haefely, W. Blockade of central 5-hydroxytryptamine receptors by methiothepin. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1972, 274, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Vleugels, R.; Verlinden, H.; Vanden Broeck, J. Serotonin, serotonin receptors and their actions in insects. Neurotransmitter 2015, 2, e314. [Google Scholar]
- Ngai, M.; Shoue, D.A.; Loh, Z.; McDowell, M.A. The pharmacological and functional characterization of the serotonergic system in Anopheles gambiae and Aedes aegypti: Influences on flight and blood-feeding behavior. Sci. Rep. 2019, 9, 4421. [Google Scholar] [CrossRef]
- Fuchs, S.; Ermelinda, R.; Andrea, C.; Tony, N. Disruption of aminergic signalling reveals novel compounds with distinct inhibitory effects on mosquito reproduction, locomotor function and survival. Sci. Rep. 2014, 4, 5526. [Google Scholar] [CrossRef] [PubMed]
- Dayan, P.; Huys, Q.J. Serotonin in affective control. Annu. Rev. Neurosci. 2009, 32, 95–126. [Google Scholar] [CrossRef]
- Curran, K.P.; Chalasani, S.H. Serotonin circuits and anxiety: What can invertebrates teach us? Invert. Neurosci. 2012, 12, 81–92. [Google Scholar] [CrossRef]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.J. Feeding, hunger, satiety and serotonin in invertebrates. Proc. Biol. Sci. 2020, 287, 20201386. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, Z.; Kang, L. Serotonin enhances solitariness in phase transition of the migratory locust. Front. Behav. Neurosci. 2013, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Andrés, M.; Seifert, M.; Spalthoff, C.; Warren, B.; Weiss, L.; Giraldo, D.; Winkler, M.; Pauls, S.; Göpfert, M.C. Auditory Efferent System Modulates Mosquito Hearing. Curr. Biol. 2016, 26, 2028–2036. [Google Scholar] [CrossRef]
- Loh, Y.M.M.; Su, M.P.; Ellis, D.A.; Andrés, M. The auditory efferent system in mosquitoes. Front. Cell Dev. Biol. 2023, 11, 1123738. [Google Scholar] [CrossRef]
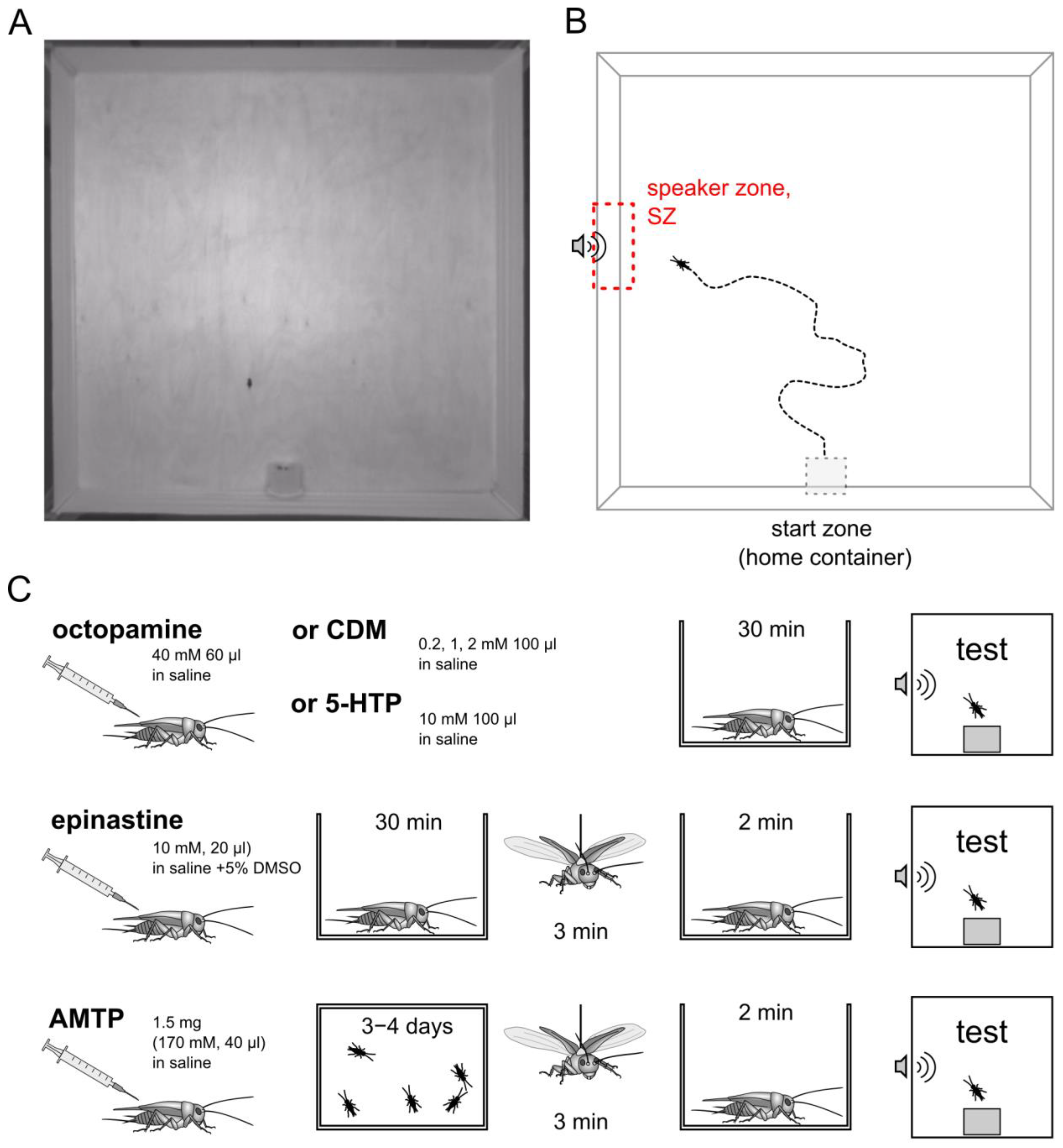


| Agent | Concentration | Volume of Injection | Vehicle | Remarks |
|---|---|---|---|---|
| Octopamine hydrochloride (Sigma) | 40 mM | 60 µL | saline | Crickets were injected 30 min prior to testing. Octopamine acted for at least 1.5–2 h after injection. |
| Chlordimeform (CDM, the octopamine receptors agonist) (Sigma) | 0.2 mM, 1 mM 2 mM | 100 μL | saline | Crickets were injected 30 min prior to testing. Chlordimeform acted for at least 1.5–2 h after injection. |
| Epinastine, a specific antagonist of insect octopamine receptors (Sigma) | 10 mM | 20 μL | 5% DMSO | Crickets were injected 30 min prior to the flight procedure. After the flight and before being placed in the experimental arena, the crickets were placed in individual containers for two minutes to rest. After a short rest, the crickets were placed in the experimental arena. Epinastine acted for at least 1.5–2 h after injection. |
| 5-hydroxytryptophan (5-HTP), immediate metabolic precursor of serotonin | 10 mM | 100 μL | saline | Crickets were injected 30 min prior to testing. 5-HTP acted for at least 1.5–2 h after injection. |
| Alpha-methyltryptophan (AMTP), serotonin synthesis inhibitor, the «false serotonin precursor» | 171 mM | 40 µL * | saline | Crickets were injected 3–4 days prior to the flight procedure. After the flight and before being placed in the experimental arena, the crickets were placed in individual containers for two minutes to rest. After a short rest, the crickets were placed in the experimental arena. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezheritskiy, M.; Melnikova, V.; Dyakonova, V.; Vorontsov, D. Monoaminergic Systems in Flight-Induced Potentiation of Phonotactic Behavior in Female Crickets Gryllus bimaculatus. Insects 2024, 15, 183. https://doi.org/10.3390/insects15030183
Mezheritskiy M, Melnikova V, Dyakonova V, Vorontsov D. Monoaminergic Systems in Flight-Induced Potentiation of Phonotactic Behavior in Female Crickets Gryllus bimaculatus. Insects. 2024; 15(3):183. https://doi.org/10.3390/insects15030183
Chicago/Turabian StyleMezheritskiy, Maxim, Victoria Melnikova, Varvara Dyakonova, and Dmitry Vorontsov. 2024. "Monoaminergic Systems in Flight-Induced Potentiation of Phonotactic Behavior in Female Crickets Gryllus bimaculatus" Insects 15, no. 3: 183. https://doi.org/10.3390/insects15030183







