Age Determination of Chrysomya megacephala Pupae through Reflectance and Machine Learning Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
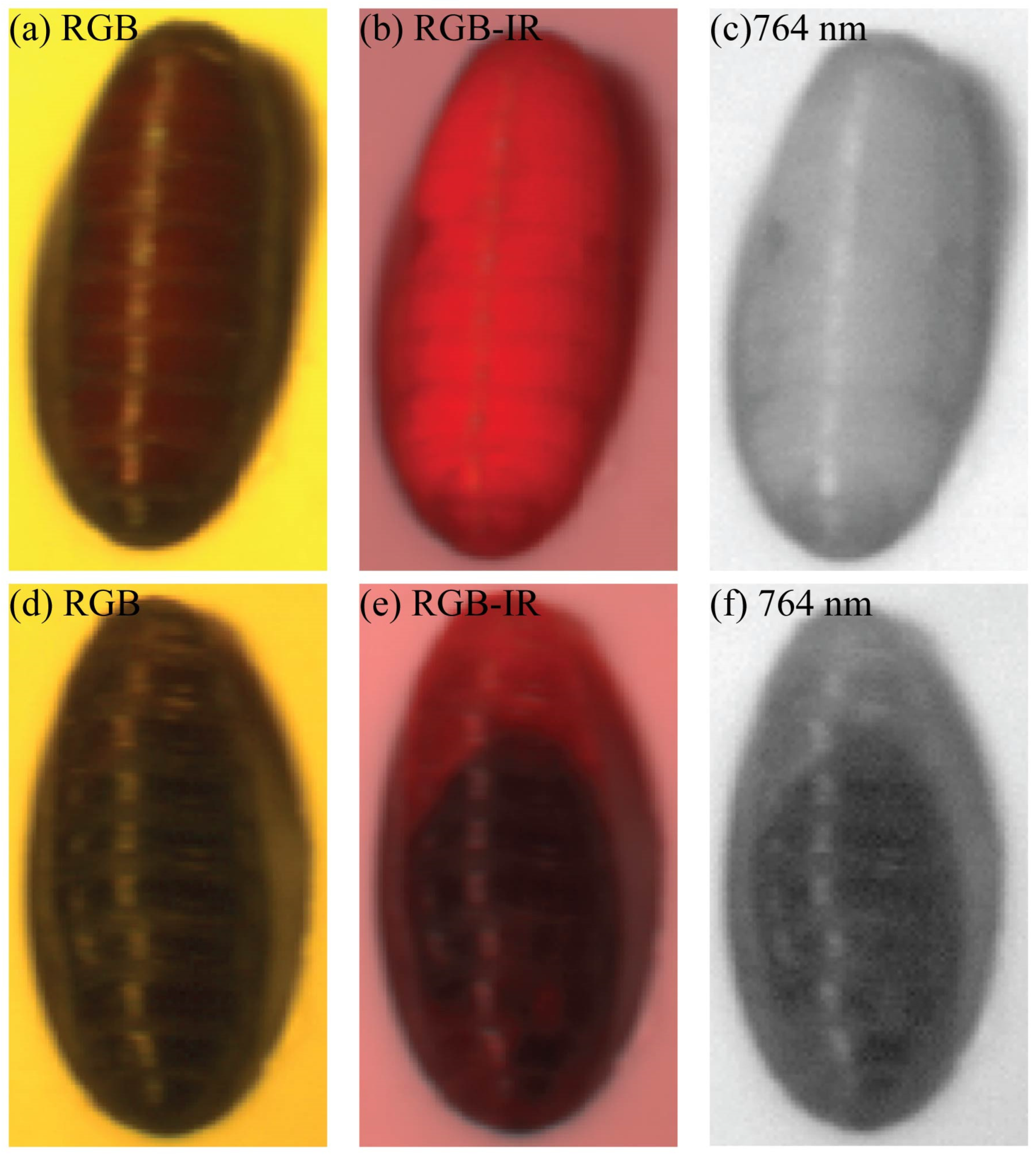
2.2. Hyperspectral Imaging
2.3. Hyperspectral Image Reflectance Transformation
2.4. Data Analysis
3. Results
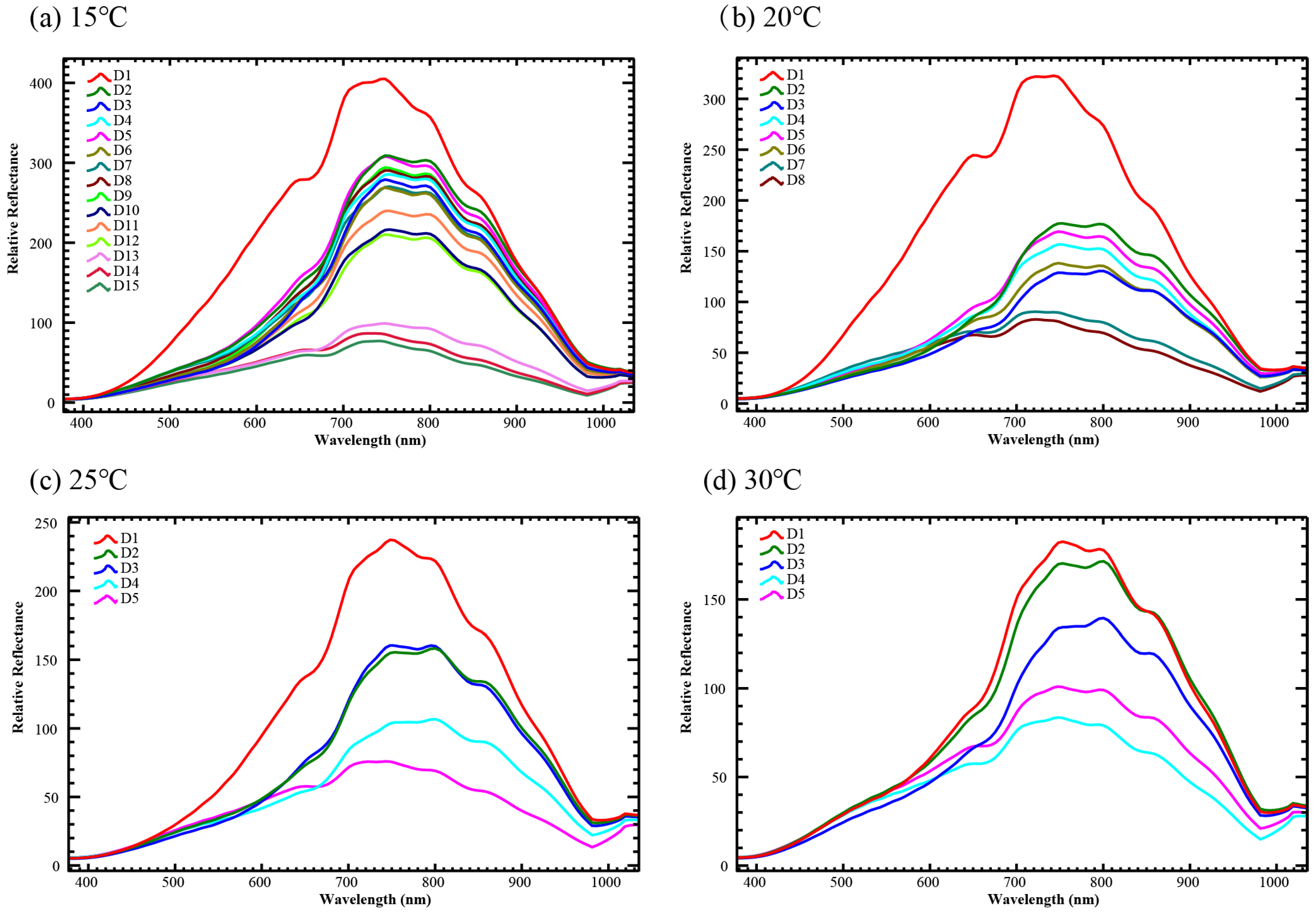
3.1. Hyperspectral Imaging Spectroscopy of Ch. megacephala Pupa Stage under Different Temperatures
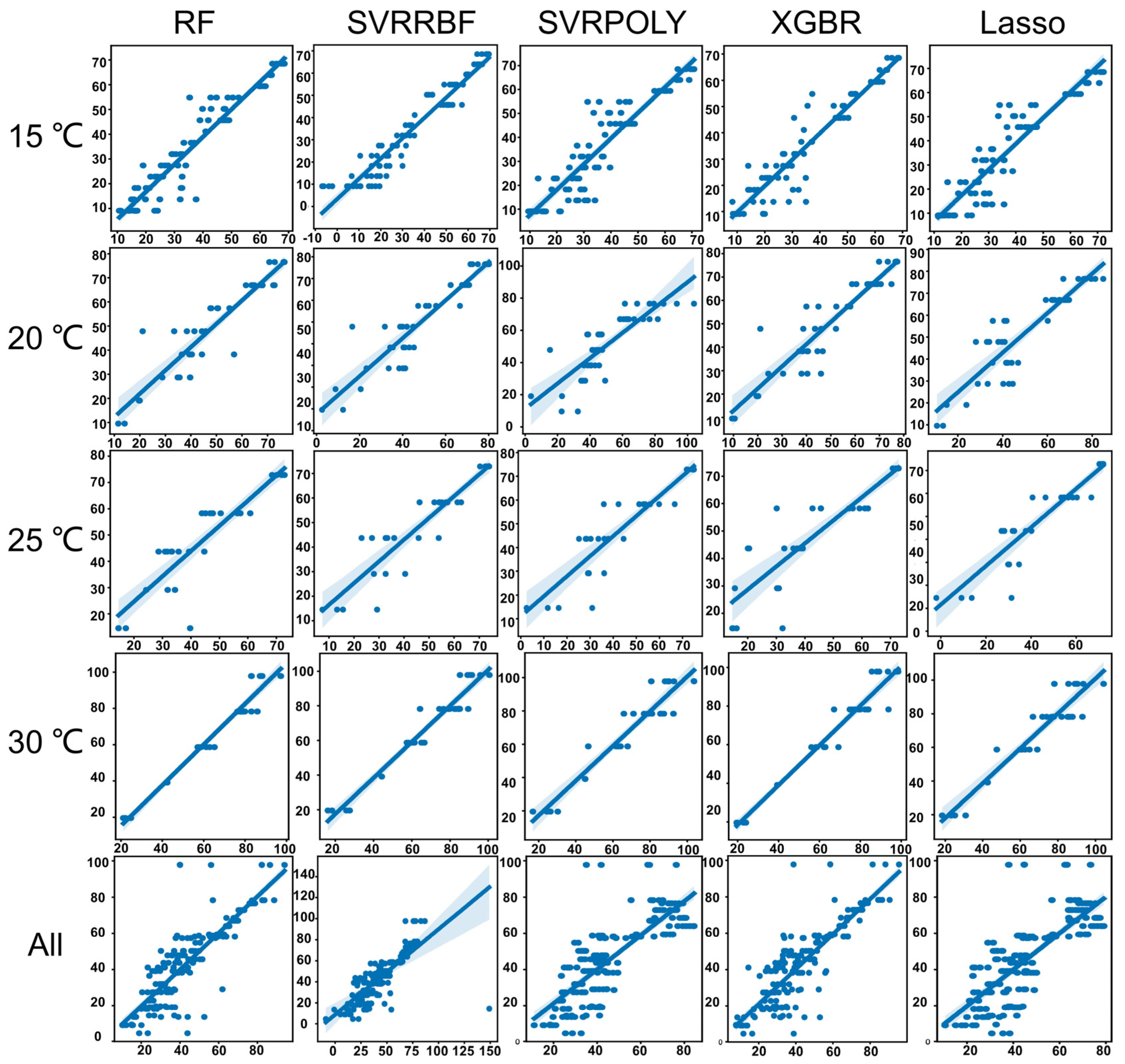
3.2. Machine Learning Models for Estimating the Development Time of Pupa
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Annu. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Benbow, M.E.; Tarone, A.M.; Mohr, R.M. Basic research in evolution and ecology enhances forensics. Trends Ecol. Evol. 2011, 26, 53–55. [Google Scholar] [CrossRef]
- Benecke, M. A brief history of forensic entomology. Forensic Sci. Int. 2001, 120, 2–14. [Google Scholar] [CrossRef]
- Muñoz, J.I.; Suárez-Peñaranda, J.M.; Otero, X.L.; Rodríguez-Calvo, M.S.; Costas, E.; Miguéns, X.; Concheiro, L. A new perspective in the estimation of postmortem interval (PMI) based on vitreous. J. Forensic Sci. 2001, 46, 209–214. [Google Scholar] [CrossRef]
- Madea, B. Estimation of the Time since Death, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- DuPre, D.P. Homicide Investigation Field Guide; Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Benecke, M.; Josephi, E.; Zweihoff, R. Neglect of the elderly: Forensic entomology cases and considerations. Forensic Sci. Int. 2004, 146, S195–S199. [Google Scholar] [CrossRef]
- Niederegger, S.; Pastuschek, J.; Mall, G. Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic Sci. Int. 2010, 199, 72–78. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.L.; Wang, J.F.; Wang, M.; Yang, L.J.; Tao, L.Y.; Zhang, Y.N.; Hou, Y.D.; Chu, J.; Hou, Z.L. Development of the green bottle fly Lucilia illustris at constant temperatures. Forensic Sci. Int. 2016, 267, 136–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Sun, J.; Hu, G.; Wang, M.; Amendt, J.; Wang, J. Temperature-dependent development of the blow fly Chrysomya pinguis and its significance in estimating postmortem interval. R. Soc. Open Sci. 2019, 6, 190003. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.J.; Zhang, Y.N.; Tao, L.Y.; Wang, J.F. Development of Musca domestica at constant temperatures and the first case report of its application for estimating the minimum postmortem interval. Forensic Sci. Int. 2018, 285, 172–180. [Google Scholar] [CrossRef]
- Hu, G.L.; Wang, Y.; Sun, Y.; Zhang, Y.N.; Wang, M.; Wang, J.F. Development of Chrysomya rufifacies (Diptera: Calliphoridae) at Constant Temperatures Within its Colony Range in Yangtze River Delta Region of China. J. Med. Entomol. 2019, 56, 1215–1224. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Li, L.; Wang, J.; Wang, M.; Zhang, Y.; Chu, J.; Liu, K.; Hou, Y.; Tao, L. Temperature-dependent Development of Parasarcophaga similis (Meade 1876) and its Significance in Estimating Postmortem Interval. J. Forensic Sci. 2017, 62, 1234–1243. [Google Scholar] [CrossRef]
- Alotaibi, F.; Alkuriji, M.; AlReshaidan, S.; Alajmi, R.; Metwally, D.M.; Almutairi, B.; Alorf, M.; Haddadi, R.; Ahmed, A. Body Size and Cuticular Hydrocarbons as Larval Age Indicators in the Forensic Blow Fly, Chrysomya albiceps (Diptera: Calliphoridae). J. Med. Entomol. 2020, 58, 1048–1055. [Google Scholar] [CrossRef]
- Barros-Cordeiro, K.B.; Pujol-Luz, J.R.; Báo, S.N. A Study of the Pupal Development of Five Forensically Important Flies (Diptera: Brachycera). J. Med. Entomol. 2021, 58, 1643–1653. [Google Scholar] [CrossRef]
- Archer, M.S.; Elgar, M.A.; Briggs, C.A.; Ranson, D.L. Fly pupae and puparia as potential contaminants of forensic entomology samples from sites of body discovery. Int. J. Leg. Med. 2006, 120, 364–368. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Shang, Y.; Ren, L.; Chen, W.; Wang, S.; Guo, Y. Development of Sarcophaga dux (diptera: Sarcophagidae) at constant temperatures and differential gene expression for age estimation of the pupae. J. Therm. Biol. 2020, 93, 102735. [Google Scholar] [CrossRef]
- Shang, Y.; Amendt, J.; Wang, Y.; Ren, L.; Yang, F.; Zhang, X.; Zhang, C.; Guo, Y. Multimethod combination for age estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) with implications for estimation of the postmortem interval. Int. J. Leg. Med. 2023, 137, 329–344. [Google Scholar] [CrossRef]
- Sukontason, K.L.; Kanchai, C.; Piangjai, S.; Boonsriwong, W.; Bunchu, N.; Sripakdee, D.; Chaiwong, T.; Kuntalue, B.; Siriwattanarungsee, S.; Sukontason, K. Morphological observation of puparia of Chrysomya nigripes (Diptera: Calliphoridae) from human corpse. Forensic Sci. Int. 2006, 161, 15–19. [Google Scholar] [CrossRef]
- Schoborg, T.A.; Smith, S.L.; Smith, L.N.; Morris, H.D.; Rusan, N.M. Micro-computed tomography as a platform for exploring Drosophila development. Development 2019, 146, dev176685. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, Y.; Ren, L.; Qu, H.; Zhu, G.; Guo, Y. A Study of Cuticular Hydrocarbons of All Life Stages in Sarcophaga peregrina (Diptera: Sarcophagidae). J. Med. Entomol. 2022, 59, 108–119. [Google Scholar] [CrossRef]
- Brown, K.; Harvey, M. Optical coherence tomography: Age estimation of Calliphora vicina pupae in vivo? Forensic Sci. Int. 2014, 242, 157–161. [Google Scholar] [CrossRef]
- Nansen, C.; Elliott, N. Remote Sensing and Reflectance Profiling in Entomology. Annu. Rev. Entomol. 2016, 61, 139–158. [Google Scholar] [CrossRef]
- Hren, R.; Sersa, G.; Simoncic, U.; Milanic, M. Imaging perfusion changes in oncological clinical applications by hyperspectral imaging: A literature review. Radiol. Oncol. 2022, 56, 420–429. [Google Scholar] [CrossRef]
- Sarić, R.; Nguyen, V.D.; Burge, T.; Berkowitz, O.; Trtílek, M.; Whelan, J.; Lewsey, M.G.; Čustović, E. Applications of hyperspectral imaging in plant phenotyping. Trends Plant Sci. 2022, 27, 301–315. [Google Scholar] [CrossRef]
- Pu, H.; Wei, Q.; Sun, D.-W. Recent advances in muscle food safety evaluation: Hyperspectral imaging analyses and applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Nansen, C.; Ribeiro, L.P.; Dadour, I.; Roberts, J.D. Detection of temporal changes in insect body reflectance in response to killing agents. PLoS ONE 2015, 10, e0124866. [Google Scholar] [CrossRef]
- Voss, S.C.; Magni, P.; Dadour, I.; Nansen, C. Reflectance-based determination of age and species of blowfly puparia. Int. J. Leg. Med. 2017, 131, 263–274. [Google Scholar] [CrossRef]
- Shang, Y.; Feng, Y.; Ren, L.; Zhang, X.; Yang, F.; Zhang, C.; Guo, Y. Pupal Age Estimation of Sarcophaga peregrina (Diptera: Sarcophagidae) at Different Constant Temperatures Utilizing ATR-FTIR Spectroscopy and Cuticular Hydrocarbons. Insects 2023, 14, 143. [Google Scholar] [CrossRef]
- Frere, B.; Suchaud, F.; Bernier, G.; Cottin, F.; Vincent, B.; Dourel, L.; Lelong, A.; Arpino, P. GC-MS analysis of cuticular lipids in recent and older scavenger insect puparia. An approach to estimate the postmortem interval (PMI). Anal. Bioanal. Chem. 2014, 406, 1081–1088. [Google Scholar] [CrossRef]
- Martín-Vega, D.; Simonsen, T.J.; Wicklein, M.; Hall, M.J.R. Age estimation during the blow fly intra-puparial period: A qualitative and quantitative approach using micro-computed tomography. Int. J. Leg. Med. 2017, 131, 1429–1448. [Google Scholar] [CrossRef]
- Ngando, F.J.; Zhang, X.; Qu, H.; Xiao, J.; Ren, L.; Yang, F.; Feng, Y.; Shang, Y.; Chen, S.; Zhang, C.; et al. Age determination of Chrysomya megacephala (Diptera: Calliphoridae) using lifespan patterns, gene expression, and pteridine concentration under constant and variable temperatures. Forensic Sci. Int. 2024, 354, 111916. [Google Scholar] [CrossRef]
- Babatunde, H.A.; Collins, J.; Lukman, R.; Saxton, R.; Andersen, T.; McDougal, O.M. SVR Chemometrics to Quantify β-Lactoglobulin and α-Lactalbumin in Milk Using MIR. Foods 2024, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Wang, Z.; Li, G.; Xie, L. Sex determination of silkworm pupae using VIS-NIR hyperspectral imaging combined with chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 208, 7–12. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Liu, B.; Wu, Y.; Ma, J.; Zhang, W.; Wang, B.; Chen, Z. Estimation of Winter Wheat Yield Using Multiple Temporal Vegetation Indices Derived from UAV-Based Multispectral and Hyperspectral Imagery. Remote Sens. 2023, 15, 4800. [Google Scholar] [CrossRef]
- Fei, S.; Yu, X.; Lan, M.; Li, L.; Xia, X.; He, Z.; Xiao, Y. Research on Winter Wheat Yield Estimation Based on Hyperspectral Remote Sensing and Ensemble Learning Method. Sci. Agric. Sin. 2021, 54, 3417–3427. [Google Scholar] [CrossRef]
- Bai, X.; Song, Y.-Q.; Yu, R.; Xiong, J.; Peng, Y.; Jiang, Y.M.; Yang, G.; Li, Z.; Zhu, X. Hyperspectral Estimation of Apple Canopy Chlorophyll Content Using an Ensemble Learning Approach. Appl. Eng. Agric. 2021, 37, 505–511. [Google Scholar] [CrossRef]
| Temperature | Data Set | Revised Data Set * | Training Set | Testing Set |
|---|---|---|---|---|
| 15 °C | 449 | 423 | 338 | 85 |
| 20 °C | 241 | 220 | 176 | 44 |
| 25 °C | 150 | 143 | 114 | 29 |
| 30 °C | 150 | 149 | 119 | 30 |
| All | 990 | 935 | 748 | 187 |
| Temperature | PCs * | Evaluation Metrics | Hyperparameter | ||||
|---|---|---|---|---|---|---|---|
| ML Model | R2_Score | MSE | RMSE | MAE | |||
| 15 °C | 6 | RF | 0.8777 | 46.5585 | 6.8234 | 4.7646 | max_features = 4, n_estimators = 21 |
| SVR-RBF | 0.9154 | 32.1844 | 5.6731 | 4.2668 | C = 10000, gamma = 0.01, kernal = ‘rbf’ | ||
| SVR-POLY | 0.8304 | 64.5724 | 8.0357 | 5.9882 | C = 1000, degree = 1, kernal = ‘poly’ | ||
| XGBR | 0.9069 | 35.4416 | 5.9533 | 3.9156 | max_depth = 7, n_estimators = 1000 | ||
| Lasso | 0.8265 | 66.0429 | 8.1267 | 6.2645 | alpha = 0.5995, fit_intercept = Ture, max_iter = 1000 | ||
| 20 °C | 7 | RF | 0.8694 | 49.1035 | 7.0074 | 4.7850 | max_features = 6, n_estimators = 41 |
| SVR-RBF | 0.8459 | 57.9510 | 7.6126 | 5.4913 | C = 100, gamma = 0.01, kernal = ‘rbf’ | ||
| SVR-POLY | 0.6432 | 134.1670 | 11.5830 | 8.9330 | C = 1000, degree = 2, kernal = ‘poly’ | ||
| XGBR | 0.8629 | 51.5522 | 7.1800 | 4.5486 | max_depth = 7, n_estimators = 1000 | ||
| Lasso | 0.7793 | 82.9672 | 9.1086 | 6.8378 | alpha = 0.0215, fit_intercept = Ture, max_iter = 1000 | ||
| 25 °C | 6 | RF | 0.7855 | 71.9127 | 8.4801 | 6.4624 | max_features = 4, n_estimators = 51 |
| SVR-RBF | 0.8046 | 65.5165 | 8.0942 | 6.0240 | C = 10000, gamma = 0.001, kernal = ‘rbf’ | ||
| SVR-POLY | 0.7302 | 90.4554 | 9.5108 | 7.1135 | C = 100, degree = 1, kernal = ‘poly’ | ||
| XGBR | 0.6607 | 113.7281 | 10.6643 | 7.1470 | max_depth = 8, n_estimators = 100 | ||
| Lasso | 0.7347 | 88.9378 | 9.4307 | 7.1464 | alpha = 0.7743, fit_intercept = Ture, max_iter = 1000 | ||
| 30 °C | 8 | RF | 0.9376 | 37.7242 | 6.1420 | 4.7838 | max_features = 6, n_estimators = 51 |
| SVR-RBF | 0.9359 | 38.7600 | 6.2258 | 5.1263 | C = 1000, gamma = 0.001, kernal = ‘rbf’ | ||
| SVR-POLY | 0.8988 | 61.1887 | 7.8223 | 6.7235 | C = 100, degree = 1, kernal = ‘poly’ | ||
| XGBR | 0.9460 | 32.6807 | 5.7167 | 3.9733 | max_depth = 7, n_estimators = 1000 | ||
| Lasso | 0.8918 | 65.4251 | 8.0886 | 6.7544 | alpha = 0.2783, fit_intercept = Ture, max_iter = 1000 | ||
| ALL | 5 | RF | 0.7583 | 118.1160 | 10.8681 | 7.0315 | max_features = 3, n_estimators = 91 |
| SVR-RBF | 0.6041 | 193.4511 | 13.9087 | 7.6207 | C = 10000, gamma = 0.01, kernal = ‘rbf’ | ||
| SVR-POLY | 0.6111 | 190.0203 | 13.7848 | 10.6602 | C = 100, degree = 1, kernal = ‘poly’ | ||
| XGBR | 0.7449 | 124.6266 | 11.1636 | 7.3951 | max_depth = 7, n_estimators = 100 | ||
| Lasso | 0.6016 | 194.6762 | 13.9526 | 10.7460 | alpha = 1, fit_intercept = Ture, max_iter = 1000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Qu, H.; Zhou, Z.; Chen, S.; Ngando, F.J.; Yang, F.; Xiao, J.; Guo, Y.; Cai, J.; Zhang, C. Age Determination of Chrysomya megacephala Pupae through Reflectance and Machine Learning Analysis. Insects 2024, 15, 184. https://doi.org/10.3390/insects15030184
Zhang X, Qu H, Zhou Z, Chen S, Ngando FJ, Yang F, Xiao J, Guo Y, Cai J, Zhang C. Age Determination of Chrysomya megacephala Pupae through Reflectance and Machine Learning Analysis. Insects. 2024; 15(3):184. https://doi.org/10.3390/insects15030184
Chicago/Turabian StyleZhang, Xiangyan, Hongke Qu, Ziqi Zhou, Sile Chen, Fernand Jocelin Ngando, Fengqin Yang, Jiao Xiao, Yadong Guo, Jifeng Cai, and Changquan Zhang. 2024. "Age Determination of Chrysomya megacephala Pupae through Reflectance and Machine Learning Analysis" Insects 15, no. 3: 184. https://doi.org/10.3390/insects15030184
APA StyleZhang, X., Qu, H., Zhou, Z., Chen, S., Ngando, F. J., Yang, F., Xiao, J., Guo, Y., Cai, J., & Zhang, C. (2024). Age Determination of Chrysomya megacephala Pupae through Reflectance and Machine Learning Analysis. Insects, 15(3), 184. https://doi.org/10.3390/insects15030184





