First Morpho-Functional Assessment of Immature Stages of Pelecocera Species (Diptera: Syrphidae) Feeding on False Truffles
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
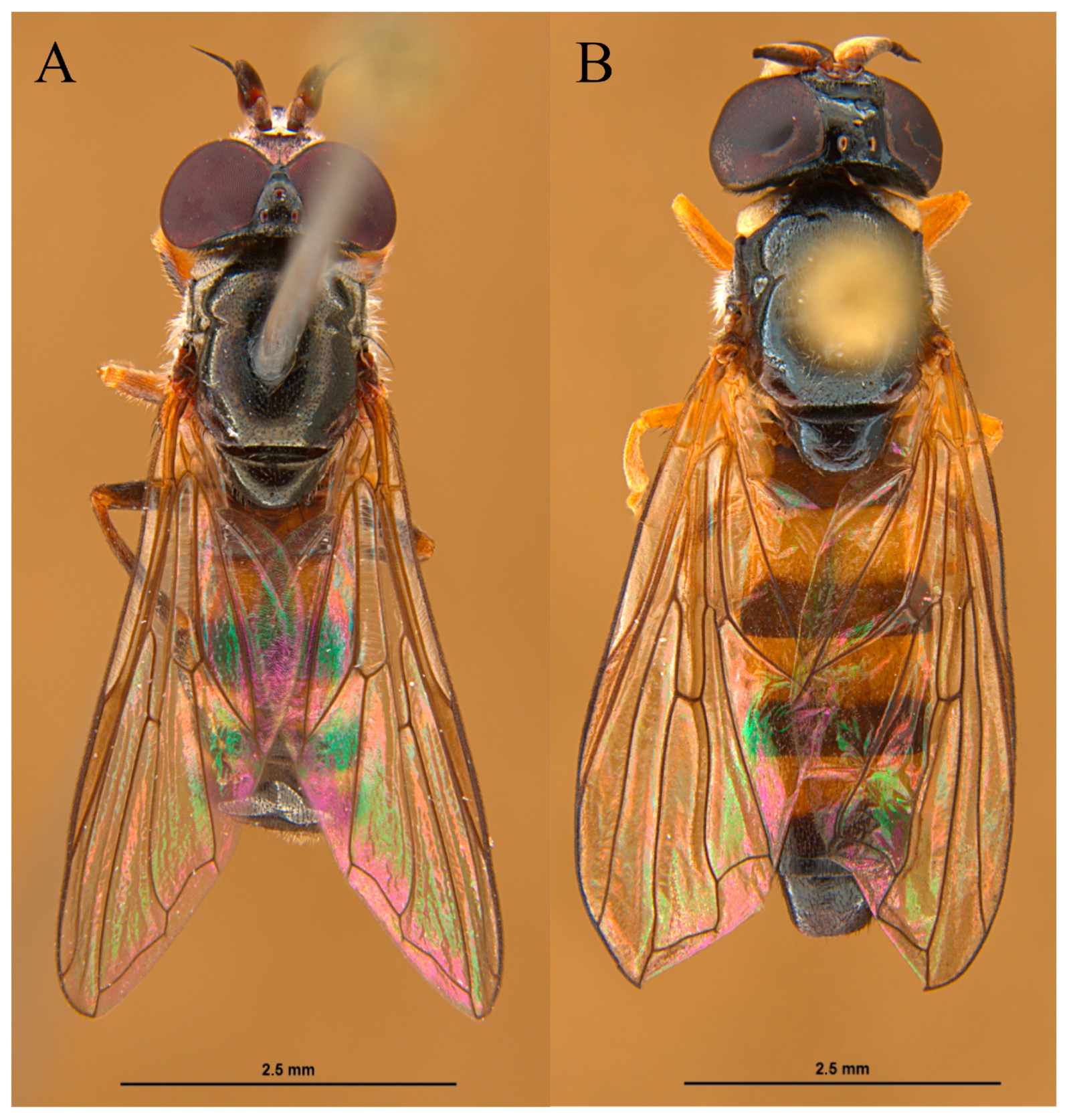
2.1. Examined Material and Adult/Larva Identification
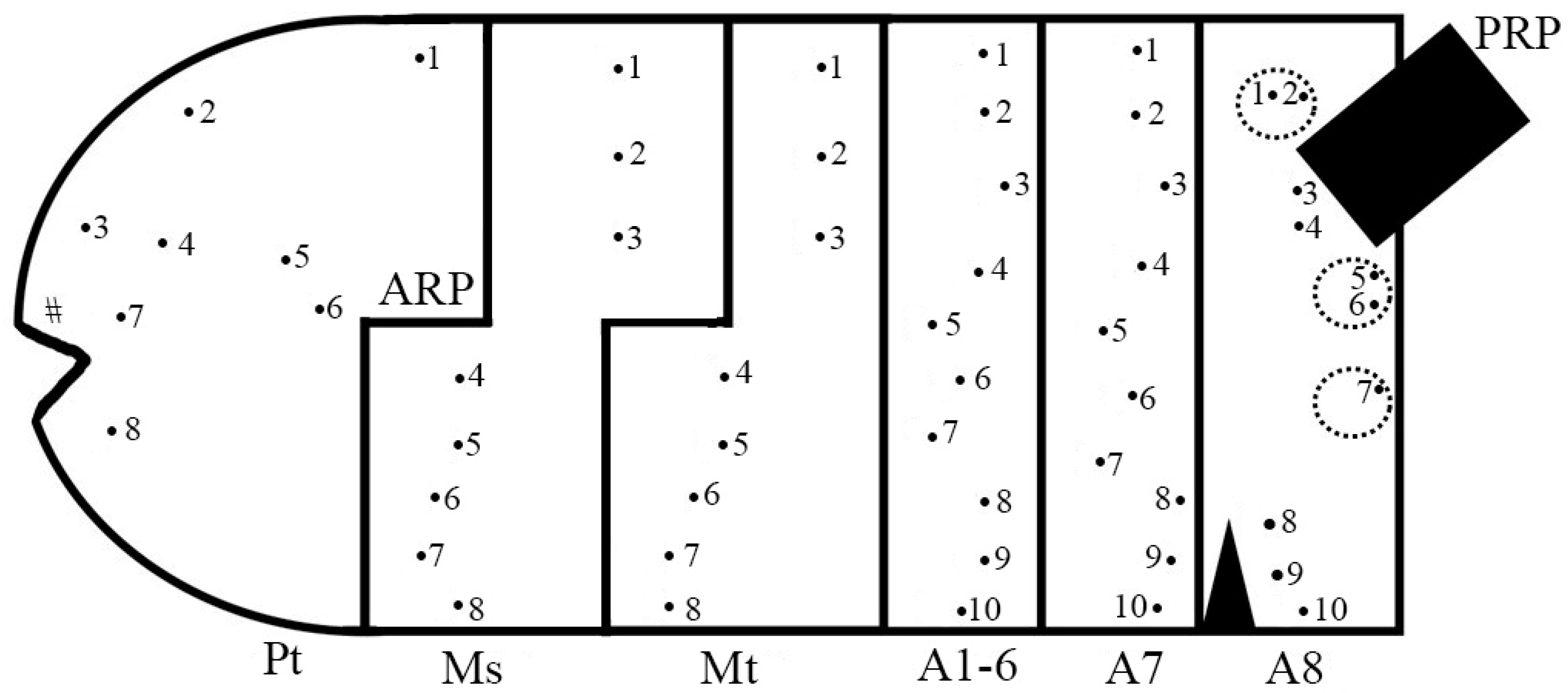
2.2. Sample Preparation and Study
2.3. Morphological Terminology
3. Results
3.1. Shared Descriptions of the Larvae/Puparia of Pelecocera (Pelecocera) tricincta and Pelecocera (Chamaesyrphus) lugubris
3.2. Immature Stages of Pelecocera (Pelecocera) tricincta
3.2.1. L1 Larva
3.2.2. L2 Larva (Figure 4)

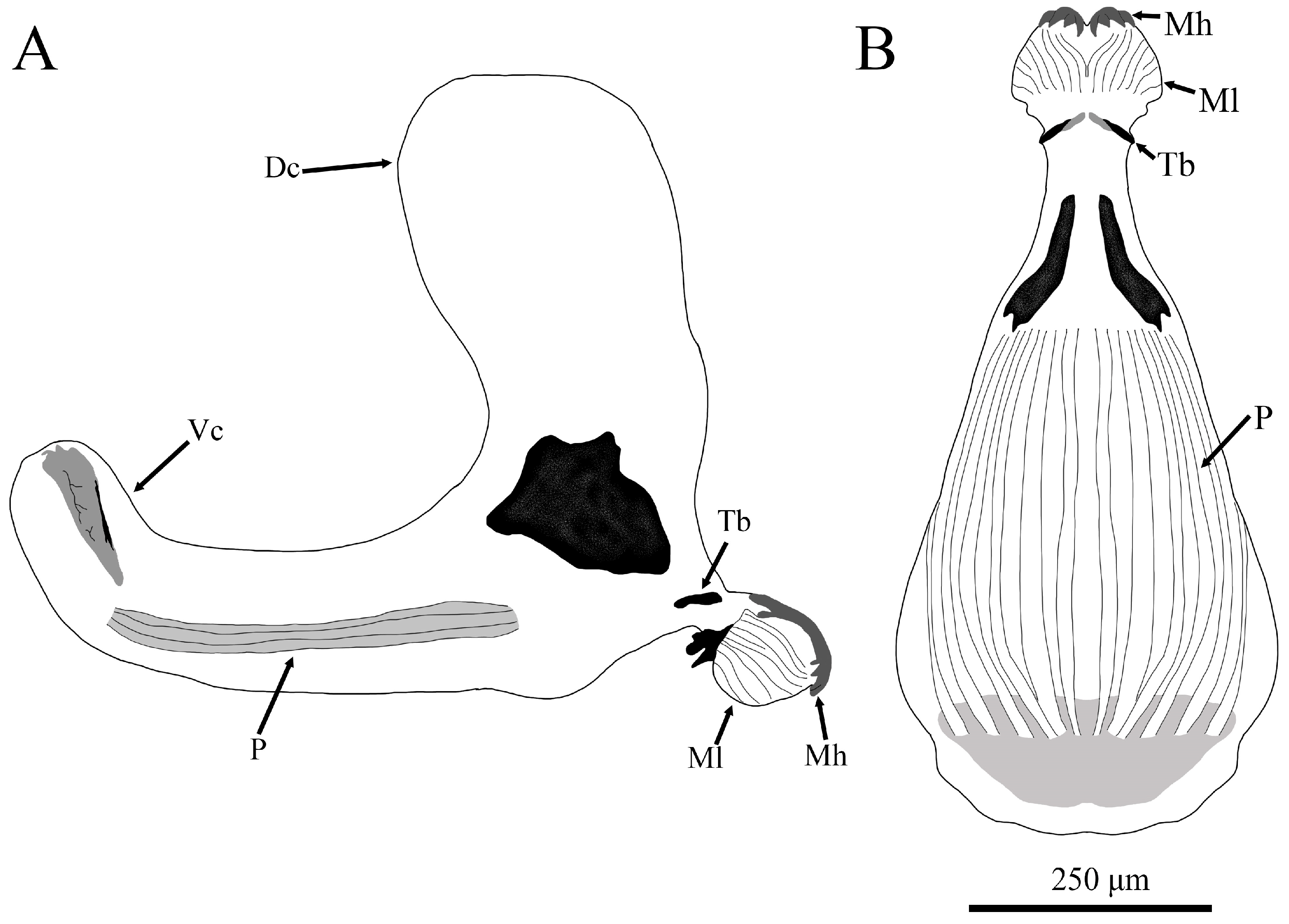
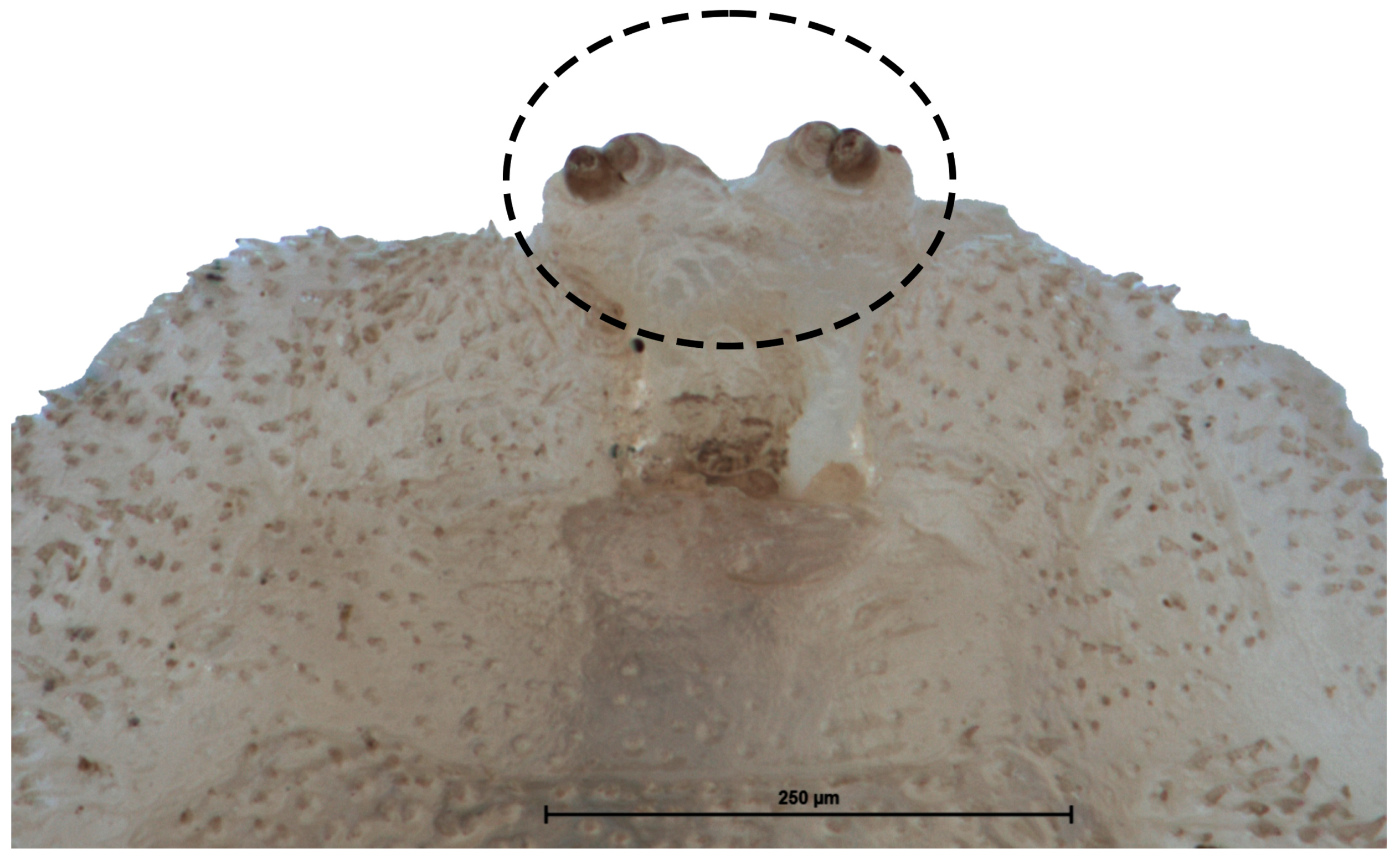



3.2.3. Puparium of Pelecocera (Pelecocera) tricincta (Figure 11)


3.3. Immature Stages of Pelecocera (Pelecocera) lugubris
3.3.1. L3 Larva (Figure 13)

3.3.2. Puparium of Pelecocera (Chamaesyrphus) lugubris (Figure 14)

3.4. Immature Stages of Pelecocera: Taxonomic Diagnosis
3.5. Taxonomic Key for the Immature Stages of the Rhingiini Tribe
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mengual, X.; Kazerani, F.; Talebi, A.S.; Gilasian, E. A revision of the genus Pelecocera Meigen with the description of the male Pelecocera persina Kuznetzov from Iran (Diptera: Syrphidae). Zootaxa 2015, 3947, 99–108. [Google Scholar] [CrossRef][Green Version]
- Ricarte, A.; Marcos-García, M.Á. A checklist of the Syrphidae (Diptera) of Spain, Andorra and Gibraltar. Zootaxa 2017, 4216, 401–440. [Google Scholar] [CrossRef] [PubMed]
- van Eck, A.; Mengual, X. Review of the genus Pelecocera Meigen, 1822 (Diptera, Syrphidae) in the Palearctic with the description of a new species from Cyprus. Contrib. Entomol. 2021, 71, 321–343. [Google Scholar] [CrossRef]
- Lair, X.; Ropars, L.; Skevington, J.H.; Kelso, S.; Geslin, B.; Minssieux, E.; Nève, G. Revision of the Pelecocera Meigen, 1822 (Diptera: Syrphidae) from France: Taxonomy, ecology and distribution. Zootaxa 2022, 5141, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Peck, L. Syrphidae. In Catalogue of Palearctic Diptera, Vol. 8, Syrphidae-Conopidae; Soos, A., Papp, L., Eds.; Elsevier: Budapest, Hungary, 1988; Volume 8, pp. 11–229. [Google Scholar]
- Ståhls, G.; Stuke, J.H.; Vujić, A.; Doczkal, D.; Muona, J. Phylogenetic relationships of the genus Cheilosia and the tribe Rhingiini (Diptera, Syrphidae) based on morphological and molecular characters. Cladistics 2004, 20, 105–122. [Google Scholar] [CrossRef]
- Vujić, A.; Ståhls, G.; Radenković, S. Hidden European diversity: A new monotypic hoverfly genus (Diptera: Syrphidae: Eristalinae: Rhingiini). Zool. J. Linn. Soc. 2019, 185, 1188–1211. [Google Scholar] [CrossRef]
- Moran, K.M.; Skevington, J.H.; Kelson, S.; Mengual, X.; Jordaens, K.; Young, A.D.; Ståhls, G.; Mutin, V.; Bot, S.; Van Zuijen, M.; et al. A multigene phylogeny of the eristalinae flower flies (Diptera: Syrphidae), with emphasis on the subtribe Criorhinina. Zool. J. Linn. Soc. 2021, 194, 120–135. [Google Scholar] [CrossRef]
- Wong, D.; Norman, H.; Creedy, T.J.; Jordaens, K.; Moran, K.M.; Young, A.; Mengual, X.; Skevington, J.H.; Vogler, A.P. The phylogeny and evolutionary ecology of hoverflies (Diptera: Syrphidae) inferred from mitochondrial genomes. Mol. Phylogenetics Evol. 2023, 184, 107759. [Google Scholar] [CrossRef] [PubMed]
- Kehlmaier, C. Hoverflies (Diptera, Syrphidae) from northern Spain, with notes on Pelecocera tricincta Meigen, 1822. Volucella 2002, 6, 139–153. [Google Scholar]
- Okada, H.; Sueyoshi, M.; Suetsugu, K. Consumption of the ectomycorrhizal fungi Rhizopogon roseolus and R. luteolus by Chamaesyrphus japonicus (Diptera: Syrphidae). Entomol. Sci. 2021, 24, 123–126. [Google Scholar] [CrossRef]
- Speight, M.C.D. Species accounts of European Syrphidae. In Syrph the Net, the Database of European Syrphidae (Diptera); Syrph the Net publications: Dublin, Ireland, 2020; Volume 104, pp. 1–314. [Google Scholar]
- Bot, S.; van de Meutter, F. Veldgids Zweefvliegen; KNNV Uitgeverij: Zeist, The Netherlands, 2019; pp. 1–388. [Google Scholar]
- Kuznetzov, S.Y. Morphology of the eggs of hoverflies (Diptera, Syrphidae). Rev. Ent. URSS 1988, 67, 741–753. [Google Scholar]
- QGis Development Team. QGis. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2024. Available online: https://qgis.osgeo.org (accessed on 30 September 2023).
- Rotheray, G.E. Forms, Functions and Names. In Ecomorphology of Cyclorrhaphan larvae (Diptera); Feldhaar, H., Schmidt-Rhaesa, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 4, pp. 1–286. [Google Scholar] [CrossRef]
- Rotheray, G.E.; Gilbert, F. Phylogeny of Palearctic Syrphidae (Diptera): Evidence from larval stages. Zool. J. Linn. Soc. 1999, 127, 1–112. [Google Scholar] [CrossRef]
- Hartley, J.C. The cephalopharyngeal apparatus of syrphid larvae and its relationship to other Diptera. Proc. Zool. Soc. Lond. 1963, 141, 261–280. [Google Scholar] [CrossRef]
- Dušek, J.; Láska, P. Saprophage larven von Ferdinandea cuprea und Brachypalpus valgus (Diptera, Syrphdiae). Acta Entomol. Bohemoslov. 1988, 85, 307–312. [Google Scholar]
- Rotheray, G.E. Larval stages of 17 rare and poorly known British hoverflies (Diptera: Syrphidae). J. Nat. Hist. 1991, 25, 945–969. [Google Scholar] [CrossRef]
- Speight, M.C.D. Portevinia maculata: Last instar larva and puparium, with notes on the relationship between this hoverfly and its larval hostplant, Allium ursinum (Dipt., Syrphidae). Nouv. Rev. Entomol. 1986, 3, 37–43. [Google Scholar]
- Harley, J.C. A taxonomic account of the larvae of some British Syrphidae. Proc. Zool. Soc. Lond. 1961, 136, 505–573. [Google Scholar] [CrossRef]
- Lundbeck, W. Diptera Danica: Genera and Species of Flies Hitherto Found in Denmark; Part V: Lonchopteridae, Syrphidae; G.E.C. Gad: Copenhagen, Denmark, 1916; pp. 541–553. [Google Scholar]
- Rotheray, G.E. Larval and puparial records of some hoverflies associated with dead wood (Diptera, Syrphidae). Dipter. Dig. 1990, 7, 2–7. [Google Scholar]
- Ricarte, A.; Marcos-García, M.Á.; Pérez-Bañón, C.; Rotheray, G.E. The early stages and breeding sites of four rare saproxylic hoverflies (Diptera: Syrphidae) from Spain. J. Nat. Hist. 2007, 41, 1717–1730. [Google Scholar] [CrossRef]
- Thompson, F.C.; Rotheray, G.E. Family Syrphidae. In Contributions to a Manual of Palaearctic Diptera (with Species References to Flies of Economic Importance); Papp, L., Darvas, B., Eds.; Science Heral: Budapest, Hungary, 1998; pp. 81–139. [Google Scholar]
- Rotheray, G.E. Colour Guide to Hoverfly Larvae (Diptera, Syrphidae) in Britain and Europe; Dipterists Digest No. 9; Derek Whitely: Sheffield, UK, 1993; pp. 1–156. [Google Scholar]
- Molina, R.; Trappe, J.M. Biology of the ectomycorrhizal genus Rhizopogon. I. Host associations, host-specificity and pure culture syntheses. New Phytol. 1994, 125, 653–675. [Google Scholar] [CrossRef]
- Sulzbacher, M.A.; Grebenc, T.; García, M.Á.; Silva, B.D.; Silveria, A.; Antoniolli, Z.I.; Marinho, P.; Münzenberger, B.; Telleria, M.T.; Baseia, I.G.; et al. Molecular and morphological analyses confirm Rhizopogon verii as a widely distributed ectomycorrhizal false truffle in Europe, and its presence in South America. Mycorrhiza 2016, 26, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Trappe, J.M.; Grubisha, L.C.; Spatafora, J.W. Rhizopogon. In Ectomycorrhizal Fungi Key Genera in Profile; Cairney, J.W.G., Chambers, S.M., Eds.; Springer: Berling/Heidelberg, Germany, 1999; pp. 129–161. [Google Scholar] [CrossRef]
- Beiler, K.J.; Durall, D.M.; Simard, S.W.; Maxwell, S.A.; Kretzer, A.M. Architecture of the wood-wide web: Rhizopogon spp. genets link multiple Douglas-fir cohort. New Phytol. 2010, 185, 543–553. [Google Scholar] [CrossRef]
- Yamada, A.; Furukawa, H.; Yamanaka, T. Cultivation of edible ectomycorrhizal mushrooms in Japan. Rev. Fitotec. Mex. 2017, 40, 379–389. [Google Scholar]
- Dowding, V.M. The function and ecological significance of the pharyngeal ridges occurring in the larvae of some cyclorrhaphous Diptera. Parasitology 1967, 57, 371–388. [Google Scholar] [CrossRef]
- Souba-Dols, G.J.; Ricarte, A.; Hauser, M.; Speight, M.; Marcos-García, M.Á. What do Eumerus Meigen larvae feed on? New immature stages of three species (Diptera: Syrphidae) breeding in different plants. Org. Divers. Evol. 2020, 20, 267–284. [Google Scholar] [CrossRef]
- Orengo-Green, J.J.; Quinto, J.; Ricarte, A.; Marcos-García, M.Á. Combined stereomicroscope and SEM disentangle the fine morphology of the undescribed larva and puparium of the hoverfly Milesia crabroniformis (Fabricius, 1775) (Diptera: Syrphidae). Micron 2023, 165, 103397. [Google Scholar] [CrossRef] [PubMed]

| ARP | Anterior respiratory process | Mh | Mouth hook |
| Dc | Dorsal cornu | Ml | Mandible lobe |
| ES | Ecdysial scar | P | Pharyngeal ridge |
| IS | Interespiracular setae | Pg | Perispiracular gland |
| L1 | First larval stage | PRP | Posterior respiratory process |
| L2 | Second larval stage | Tb | Tentorial bar |
| L3 | Third larval stage | Vc | Ventral cornu |
| M | Mandible |
| Characters | Pelecocera Meigen, 1822 | Ferdinandea Rondani, 1844 | Portevinia Goffe, 1944 |
|---|---|---|---|
| PRP outline in dorsal view | M-shaped | Short and slightly constricted in the middle (see Figure 1 in Dušek and Láska [19]) | Barrel-shaped (see Figure 6 in Rotheray [20]) |
| PRP color | Yellowish/dark brown | Ochre | Shining black [21] |
| PRP: pairs of spiracular openings | 3 | 3 (see Figure 44 in Hartley [22]) | 4 [21] |
| Eighth abdominal segment in lateral view | Small and abruptly truncated | Particularly truncated [22] | Flat disc form (see Figure 1 in Speight [21]) |
| Pairs of lappets | 3 | 3 [19] | Without lappets [21] |
| Breeding site | Fruiting bodies of Rhizopogon spp. fungi | Sap run from Acer, Aesculus, Malus, Populus, Quercus, and Salix trees [19,23,24,25] | Allium bulbs [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orengo-Green, J.J.; Marcos-García, M.Á.; Carstensen, L.B.; Ricarte, A. First Morpho-Functional Assessment of Immature Stages of Pelecocera Species (Diptera: Syrphidae) Feeding on False Truffles. Insects 2024, 15, 191. https://doi.org/10.3390/insects15030191
Orengo-Green JJ, Marcos-García MÁ, Carstensen LB, Ricarte A. First Morpho-Functional Assessment of Immature Stages of Pelecocera Species (Diptera: Syrphidae) Feeding on False Truffles. Insects. 2024; 15(3):191. https://doi.org/10.3390/insects15030191
Chicago/Turabian StyleOrengo-Green, José J., M. Ángeles Marcos-García, Leif Bloss Carstensen, and Antonio Ricarte. 2024. "First Morpho-Functional Assessment of Immature Stages of Pelecocera Species (Diptera: Syrphidae) Feeding on False Truffles" Insects 15, no. 3: 191. https://doi.org/10.3390/insects15030191
APA StyleOrengo-Green, J. J., Marcos-García, M. Á., Carstensen, L. B., & Ricarte, A. (2024). First Morpho-Functional Assessment of Immature Stages of Pelecocera Species (Diptera: Syrphidae) Feeding on False Truffles. Insects, 15(3), 191. https://doi.org/10.3390/insects15030191






