Global Potential Distribution of Invasive Species Pseudococcus viburni (Hemiptera: Pseudococcidae) under Climate Change
Abstract
:Simple Summary
Abstract
1. Introduction
- (1)
- to identify the mainly environmental factors affecting the potential distribution of P. viburni;
- (2)
- to observe the trends of suitable habitat range under climate change background;
- (3)
- to provide a theoretical reference for the relevant departments to formulate corresponding quarantine measures and ensure agriculture safety.
2. Materials and Methods
2.1. Data on Occurrence of the Species
2.2. Selection and Comparison of Environmental variables
2.3. Model Construction
2.4. Identification of Potential Habitat Threshold
2.5. Model Evaluation
3. Results
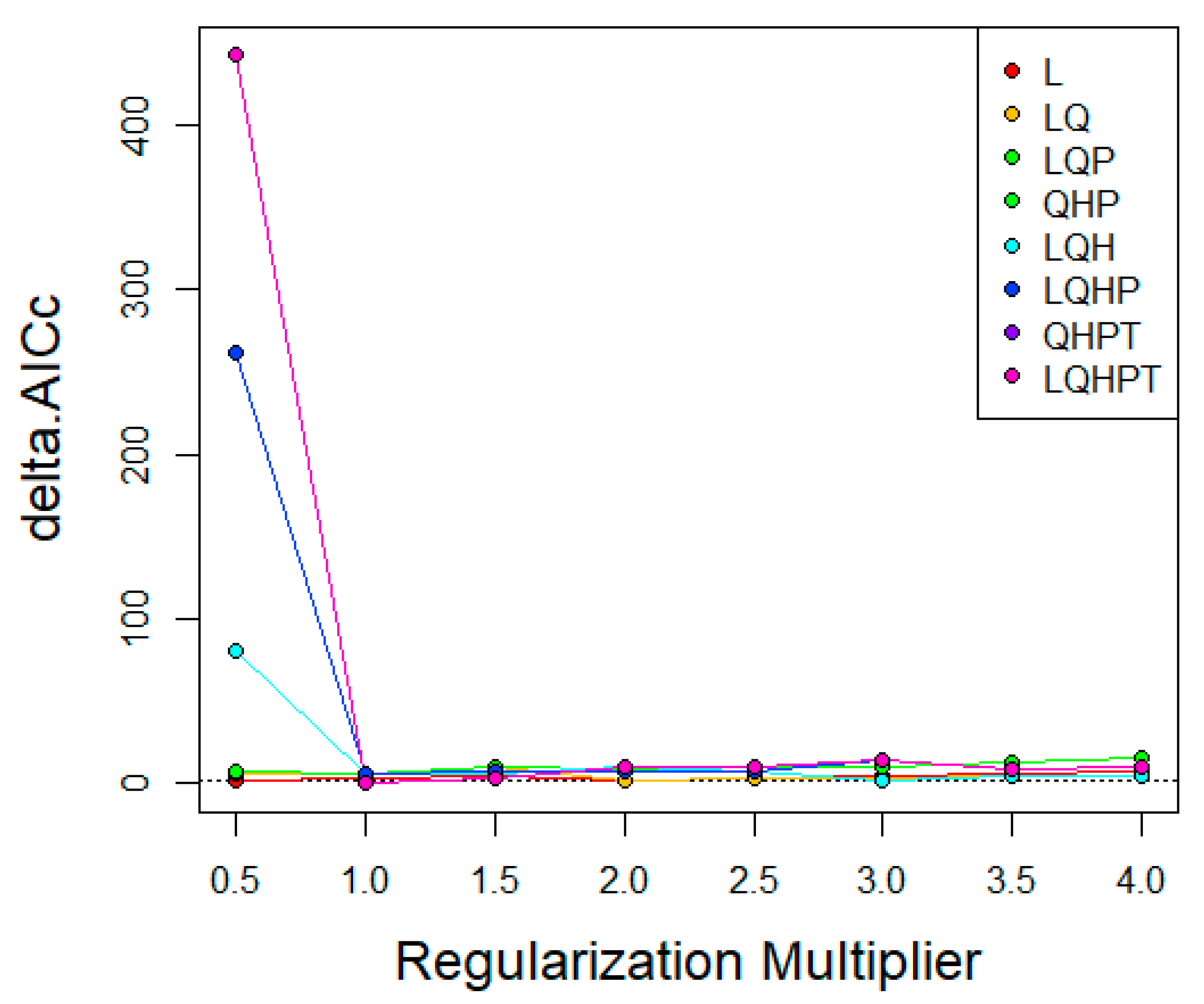
3.1. Model Performance Evaluation
3.2. Climate Variables
3.3. Response Curves
3.4. Current Suitable Area
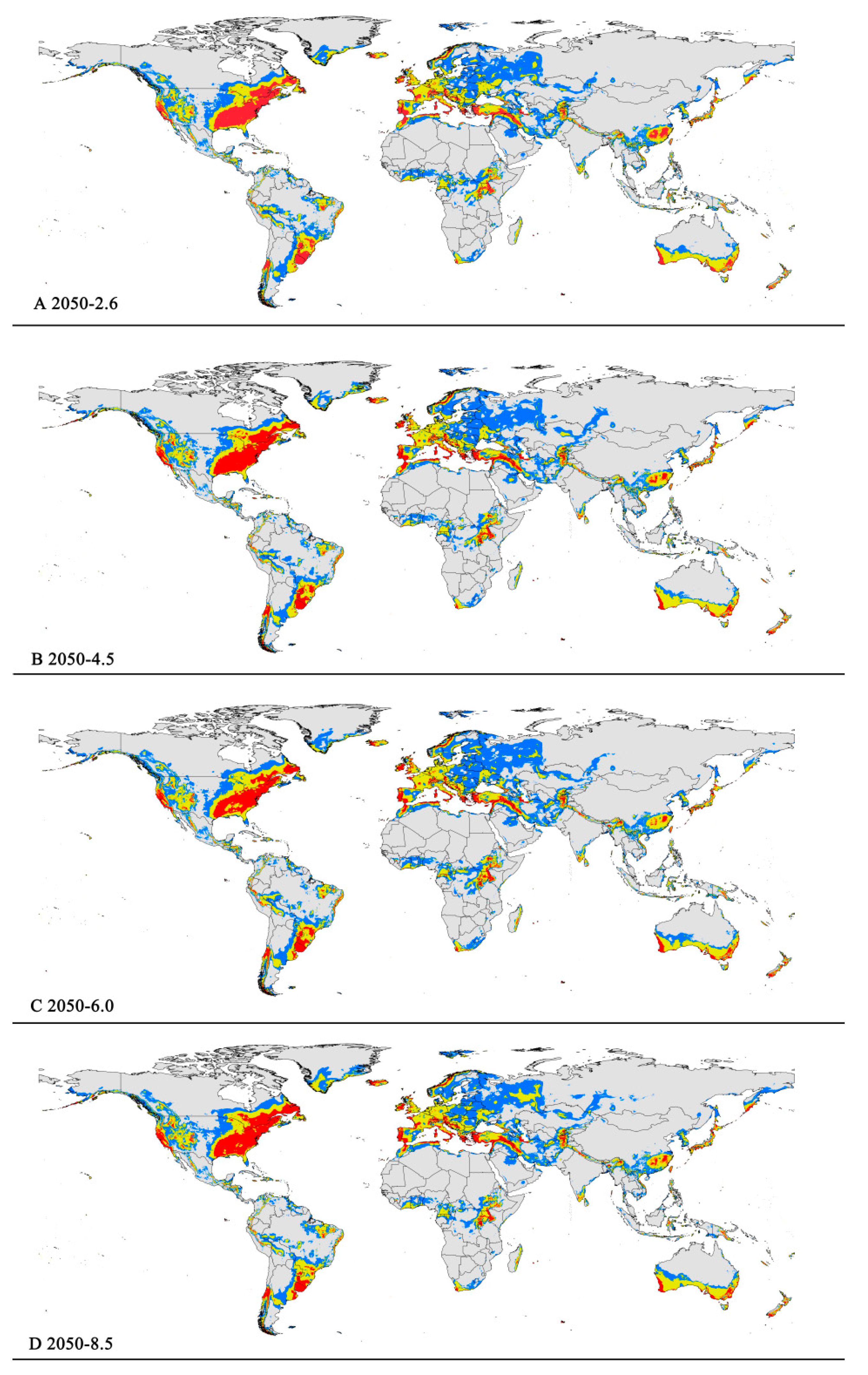
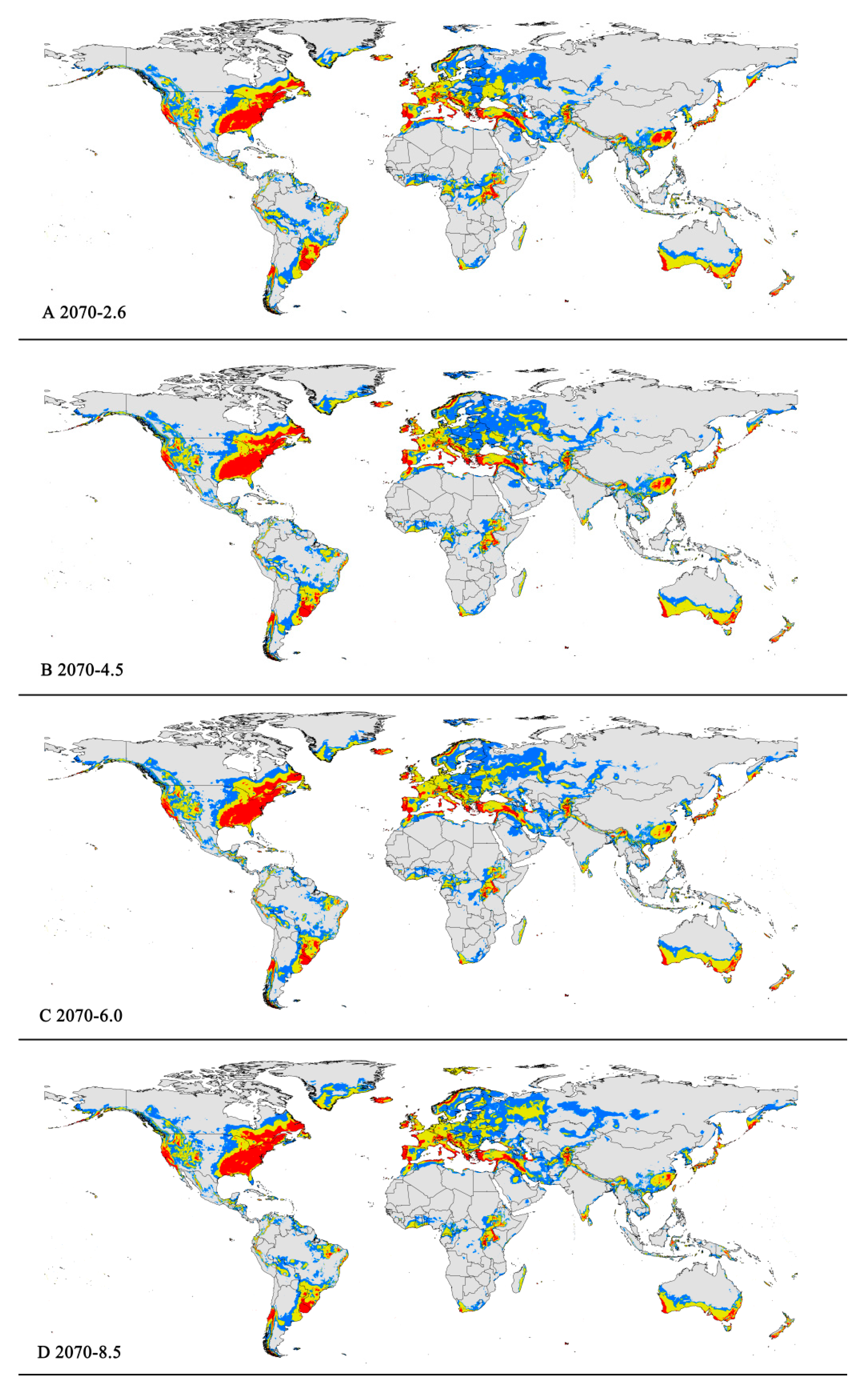
3.5. Future Potential Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Franco, J.C.; Zada, A.; Menel, Z. Novel approaches for the management of mealybug pests. In Biorational Control of Arthropod Pests; Ishaaya, I., Horowitz, A.R., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 233–278. [Google Scholar]
- Thancharoen, A.; Lankawe, S.; Moonjuntha, P.; Wongphanuwat, T.; Sangtongpraow, B.; Ngoenklan, R.; Kittipadakul, P.; Wyckhuys, K.A.G. Effective biological control of an invasive mealybug pest enhances root yield in cassava. J. Pest. Sci. 2018, 91, 1199–1211. [Google Scholar] [CrossRef]
- Naidu, R.A.; Maree, H.J.; Burger, J.T. Grapevine leafroll disease and associated viruses: A unique pathosystem. Annu. Rev. Phytopathol. 2015, 53, 613–634. [Google Scholar] [CrossRef]
- Mathulwe, L.L.; Malan, A.P.; Stokwe, N.F. A review of the biology and control of the obscure mealybug, Pseudococcus viburni (Hemiptera: Pseudococcidae), with special reference to biological control using entomopathogenic fungi and nematodes. Afr. Entomol. 2021, 29, 1–16. [Google Scholar] [CrossRef]
- Puig, A.S.; Wurzel, S.; Suarez, S.; Marelli, J.P.; Niogret, J. Mealybug (Hemiptera: Pseudococcidae) species associated with cacao mild mosaic virus and evidence of virus acquisition. Insects 2021, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Miller, G.L.; Watson, G.W. Invasive species of mealybugs (Hemiptera: Pseudococcidae) and their threat to U.S. Agriculture. Proc. Entomol. Soc. Wash. 2002, 104, 825–836. [Google Scholar]
- Garcia Morales, M.; Denno, B.D.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy, N.B. ScaleNet: A literature-based model of scale insect biology and systematics. Database 2016. [Google Scholar] [CrossRef]
- Hambleton, E.J. [Notes on Pseudococcinae of economic importance in Brazil with a description of four new species.] Notas sobre Pseudococcinae de importancia economica no Brasil com a descripção de quatro especies novas. Arch. Inst. Biol. 1935; 6, 105–120. (In Portuguese) [Google Scholar]
- Lepage, H.S. Catalogo dos Cocci deos do Brasil. Rev. Do Mus. Paul. São Paulo 1938, 23, 327–491. (In Portuguese) [Google Scholar]
- Williams, D.J.; Granara de Willink, M.C. Mealybugs of Central and South America; CAB International: London, UK, 1992; p. 635. [Google Scholar]
- Charles, J.G. Using parasitoids to infer a native range for the obscure mealybug, Pseudococcus viburni, in South America. BioControl 2011, 56, 155–161. [Google Scholar] [CrossRef]
- Culik, M.P.; Gullan, P.J. A new pest of tomato and other records of mealybugs (Hemiptera: Pseudococcidae) from Espirito Santo, Brazil. Zootaxa 2005, 964, 1–8. [Google Scholar] [CrossRef]
- Poortinga, W.; Whitmarsh, L.; Steg, L.; Böhm, G.; Fisher, S. Climate change perceptions and their individual-level determinants: A cross-European analysis. Glob. Environ. Chang. 2019, 55, 25–35. [Google Scholar] [CrossRef]
- Hossain, M.S.; Arshad, M.; Qian, L.; Kächele, H.; Khan, I.; Islam, M.D.I.; Mahboob, M.G. Climate change impacts on farmland value in Bangladesh. Ecol. Indic. 2020, 112, 106181. [Google Scholar] [CrossRef]
- Cao, C.Y.; Tao, J. Predicting the Areas of Suitable Distribution for Zelkova serrata in China under Climate Change. Sustainability 2021, 13, 1493. [Google Scholar] [CrossRef]
- Broennimann, O.; Treier, U.A.; Müller-Schärer, H.; Thuiller, W.; Peterson, A.T.; Guisan, A. Evidence of climatic niche shift during biological invasion. Ecol. Lett. 2007, 10, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, G.; Blanchon, D.; Foote, H.; Pollonais, C.; Mosee, A. Queensland fruit fly invasion of New Zealand: Predicting area suitability under future climate change scenarios. In Perspectives in Biosecurity Research Series; Blanchon, D., Galbraith, M., Eds.; Unitec ePress: Auckland, New Zealand, 2015; Volume 2, pp. 1–8. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.yp7ene (accessed on 5 December 2023).
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. R Package Version 1.3-14. 2023. Available online: https://CRAN.R-project.org/package=dismo (accessed on 10 November 2023).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Clim. 2005, 25, 195–204. [Google Scholar] [CrossRef]
- Thapa, A.; Wu, B.; Hu, Y.B.; Nie, Y.G.; Singh, P.B.; Khatiwada, R.R.; Yan, L.; Gu, X.D.; Wei, F.W. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10544. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better MaxEnt models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 47, 629–643. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zhao, Q.; Cheng, L.F.; Zhao, L.; Zhang, H.F.; Wei, J.F. The Potential Global Distribution of the White Peach Scale Pseudaulacaspis pentagona (Targioni Tozzetti) under Climate Change. Forests 2020, 11, 192. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudik, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, J.E.; Ditommaso, A.; Wang, R.L.; Wu, R.S. Predicting invasions of Wedelia trilobata (L.) Hitchc. with Maxent and GARP models. J. Plant Res. 2015, 128, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.F.; Peng, L.F.; He, Z.Q.; Lu, Y.Y.; Wang, F. Potential distribution of two invasive pineapple pests under climate change. Pest. Manag. Sci. 2020, 76, 1652–1663. [Google Scholar] [CrossRef]
- Bosso, L.; Febbraro, M.D.; Cristinzio, G.; Zoina, A.; Russo, D. Shedding light on the effects of climate change on the potential distribution of Xylela fastidiousa in the Mediterranean basin. Biol. Invasions 2016, 18, 1759–1768. [Google Scholar] [CrossRef]
- Wang, F.; Wang, D.; Guo, G.; Hu, Y.H.; Wei, J.F.; Liu, J.Z. Species delimitation of the Dermacentor ticks based on phylogenetic clustering and niche modeling. PeerJ. 2019, 7, e69112019. [Google Scholar] [CrossRef]
- Morato, T.; González-Irusta, J.M.; Dominguez-Carrió, C.; Wei, C.L.; Davies, A.; Sweetman, A.K.; Taranto, G.H.; Beazley, L.; García-Alegre, A.; Grehan, A.; et al. Climate-induced changes in the suitable habitat of cold-water corals and commercially important deep-sea fishes in the North Atlantic. Glob. Chang. Biol. 2020, 26, 2181–2202. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. Assessing Accuracy Methods of Species Distribution Models: AUC, Specificity, Sensitivity and the True Skill Statistic. Glob. J. Hum. Soc. Sci. 2018, 18, 6–18. [Google Scholar]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Osorio-Olvera, L.; Lira-Noriega, A.; Soberón, J.; Townsend Peterson, A.; Falconi, M.; Contreras-Díaz, R.G.; Martínez-Meyer, E.; Barve, V.; Barve, N. ntbox: An R package with graphical user interface for modeling and evaluating multidimensional ecological niches. Methods Ecol. Evol. 2020, 11, 1199–1206. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Weltzin, J.F. Ecological niche models and the geography of biological invasions: A review and a novel application. In Invasive Plants: Ecological and Agricultural Aspects; Springer: Berlin/Heidelberg, Germany, 2005; pp. 45–46. [Google Scholar]
- Byeon, D.; Jung, S.; Lee, W.H. Review of CLIMEX and MaxEnt for studying species distribution in South Korea. J. Asia Pac. Biodivers. 2018, 11, 325–333. [Google Scholar] [CrossRef]
- Abbasipour, H.; Taghavi, A. Description and seasonal abundance of the tea mealybug, Pseudococcus viburni (Affinis) (Signoret) (Homoptera: Pseudococcidae) found on tea in Iran. J. Entomol. 2007, 4, 474–478. [Google Scholar] [CrossRef]
- Wang, Y.S.; Zhou, P.; Tian, H.; Wan, F.H.; Zhang, G.F. First Record of the Invasive Pest Pseudococcus jackbeardsleyi (Hemiptera: Pseudococcidae) on the Chinese Mainland and Its Rapid Identification Based on Species-Specific Polymerase Chain Reaction. J. Econ. Entomol. 2018, 111, 2120–2128. [Google Scholar] [CrossRef]
- Ji, W.; Gao, G.; Wei, J.F. Potential Global Distribution of Daktulosphaira vitifoliae under Climate Change Based on MaxEnt. Insects 2021, 12, 347. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Mothes, C.C.; Stroud, J.T.; Clements, S.L.; Searcy, C.A. Evaluating ecological niche model accuracy in predicting biotic invasions using South Florida’s exotic lizard community. J. Biogeogr. 2019, 46, 432–441. [Google Scholar] [CrossRef]
- Yang, X.Q.; Kushwaha, S.P.S.; Saran, S.; Xu, J.; Roy, P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, M.Y.; Li, C.; Liu, Z.Z. Optimized Maxent Model Predictions of Climate Change Impacts on the Suitable Distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]



| Variable Abbreviation | Variables |
|---|---|
| Bio1 | Annual mean temperature (°C) |
| Bio2 | Mean diurnal range (°C) |
| Bio3 | Diurnal temperature difference to annual temperature difference ratio |
| Bio4 | Temperature seasonality (standard deviation × 100) |
| Bio5 | Max temperature of the warmest month (°C) |
| Bio6 | Min temperature of the coldest month (°C) |
| Bio7 | Temperature annual range |
| Bio8 | Mean temperature of the wettest quarter (°C) |
| Bio9 | Mean temperature of the driest quarter (°C) |
| Bio10 | Mean temperature of the warmest quarter (°C) |
| Bio11 | Mean temperature of the coldest quarter (°C) |
| Bio12 | Annual precipitation (mm) |
| Bio13 | Precipitation of the wettest month (mm) |
| Bio14 | Precipitation of the driest month (mm) |
| Bio15 | Precipitation seasonality |
| Bio16 | Precipitation of the wettest quarter (mm) |
| Bio17 | Precipitation of the driest quarter (mm) |
| Bio18 | Precipitation of the warmest quarter (mm) |
| Bio19 | Precipitation of the coldest quarter (mm) |
| Variables | Percentage Contribution (%) |
|---|---|
| Precipitation of the coldest quarter (Bio19) | 70.7 |
| Precipitation seasonality (Bio15) | 12.6 |
| Mean temperature of wettest quarter (Bio8) | 10.8 |
| Mean diurnal range (Bio2) | 3 |
| Precipitation of the warmest quarter (Bio18) | 2.9 |
| Pest Species | Habitat Level | Current Climate Conditions | Future Climate Conditions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rcp26-2050 | rcp26-2070 | rcp45-2050 | rcp45-2070 | rcp60-2050 | rcp60-2070 | rcp85-2050 | rcp85-2070 | |||
| obscure mealybug | LSA | −2.63 × 107 | −3.04 × 107 | −2.95 × 107 | −3.04 × 107 | −3.16 × 107 | −3.29 × 107 | −3.20 × 107 | −3.14 × 107 | −3.22 × 107 |
| (15.24%) | (12.04%) | (15.59%) | (19.78%) | (24.75%) | (21.55%) | (19.5%) | (22.21%) | |||
| MSA | −2.52 × 107 | −2.55 × 107 | −2.61 × 107 | −2.43 × 107 | −2.64 × 107 | −2.55 × 107 | −2.65 × 107 | −2.58 × 107 | −2.83 × 107 | |
| (1.17%) | (3.43%) | (−3.50%) | (4.69%) | (1.26%) | (5.19%) | (2.53%) | (12.27%) | |||
| HSA | −1.25 × 107 | −1.09 × 107 | −1.07 × 107 | −1.09 × 107 | −1.05 × 107 | −0.98 × 107 | −1.00 × 107 | −1.11 × 107 | −1.11 × 107 | |
| (−12.34%) | (−14.38%) | (−12.58%) | (−15.53%) | (−20.68%) | (−19.39%) | (−10.70%) | (−10.98%) | |||
| TSA | −6.40 × 107 | −6.68 × 107 | −6.62 × 107 | −6.57 × 107 | −6.85 × 107 | −6.83 × 107 | −6.86 × 107 | −6.83 × 107 | −7.16 × 107 | |
| (4.33%) | (3.51%) | (2.59%) | (6.97%) | (6.66%) | (7.14%) | (6.75%) | (11.84%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Niu, M.; Zhang, H.; Cai, B.; Ji, W. Global Potential Distribution of Invasive Species Pseudococcus viburni (Hemiptera: Pseudococcidae) under Climate Change. Insects 2024, 15, 195. https://doi.org/10.3390/insects15030195
Wei J, Niu M, Zhang H, Cai B, Ji W. Global Potential Distribution of Invasive Species Pseudococcus viburni (Hemiptera: Pseudococcidae) under Climate Change. Insects. 2024; 15(3):195. https://doi.org/10.3390/insects15030195
Chicago/Turabian StyleWei, Jiufeng, Minmin Niu, Hanxi Zhang, Bo Cai, and Wei Ji. 2024. "Global Potential Distribution of Invasive Species Pseudococcus viburni (Hemiptera: Pseudococcidae) under Climate Change" Insects 15, no. 3: 195. https://doi.org/10.3390/insects15030195





