A C-Type Lectin, RfCTL27, Activates the Immune Defense in the Red Palm Weevil Rhynchophorus ferrugineus (A.G. Olivier, 1791) (Coleoptera: Curculionidae: Dryophthorinae) by the Recognition of Gram-Negative Bacteria
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Red Palm Weevil Rearing and Breeding
2.2. Sequence Characterization of RfCTL27
2.3. Analysis of RfCTL27 Expression in Different Tissues of the RPW Larvae
2.4. Expression of RfCTL27 in the Gut and Fat Body of RPW Larvae Challenge with Pathogenic Microbes
2.5. The Impact of RfCTL27 Knockdown on RPW Larvae Immune Response and the Count of Gut eGFP-Labeled E. coli Colonies
2.6. Data Analysis and Processing
3. Results
3.1. Sequence Characteristics of RfCTL27
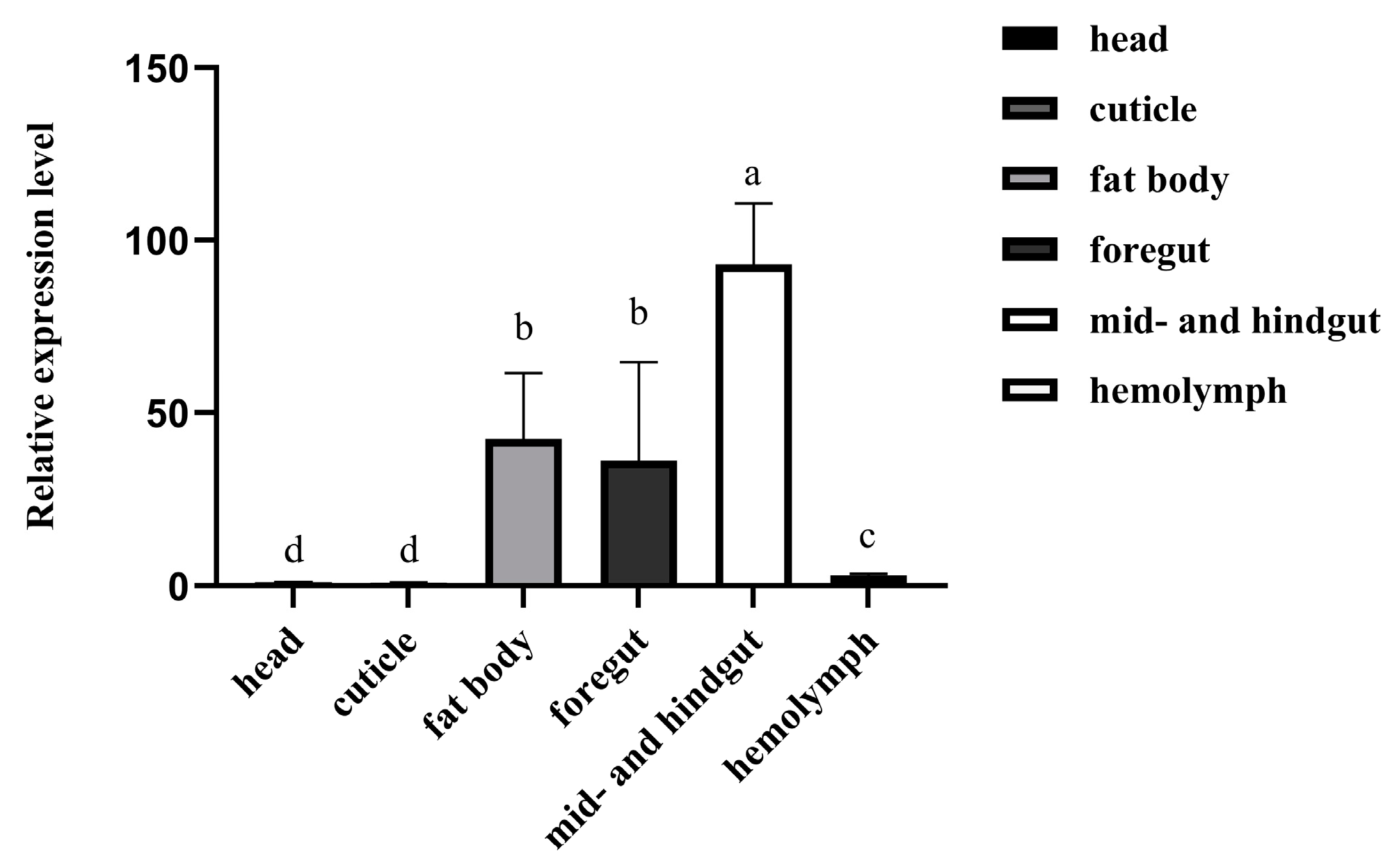
3.2. Expression Profiles of RfCTL27 in the Tissues of RPW Larvae
3.3. Expression Patterns of RfCTL27 upon Pathogen Challenge in RPW Larvae
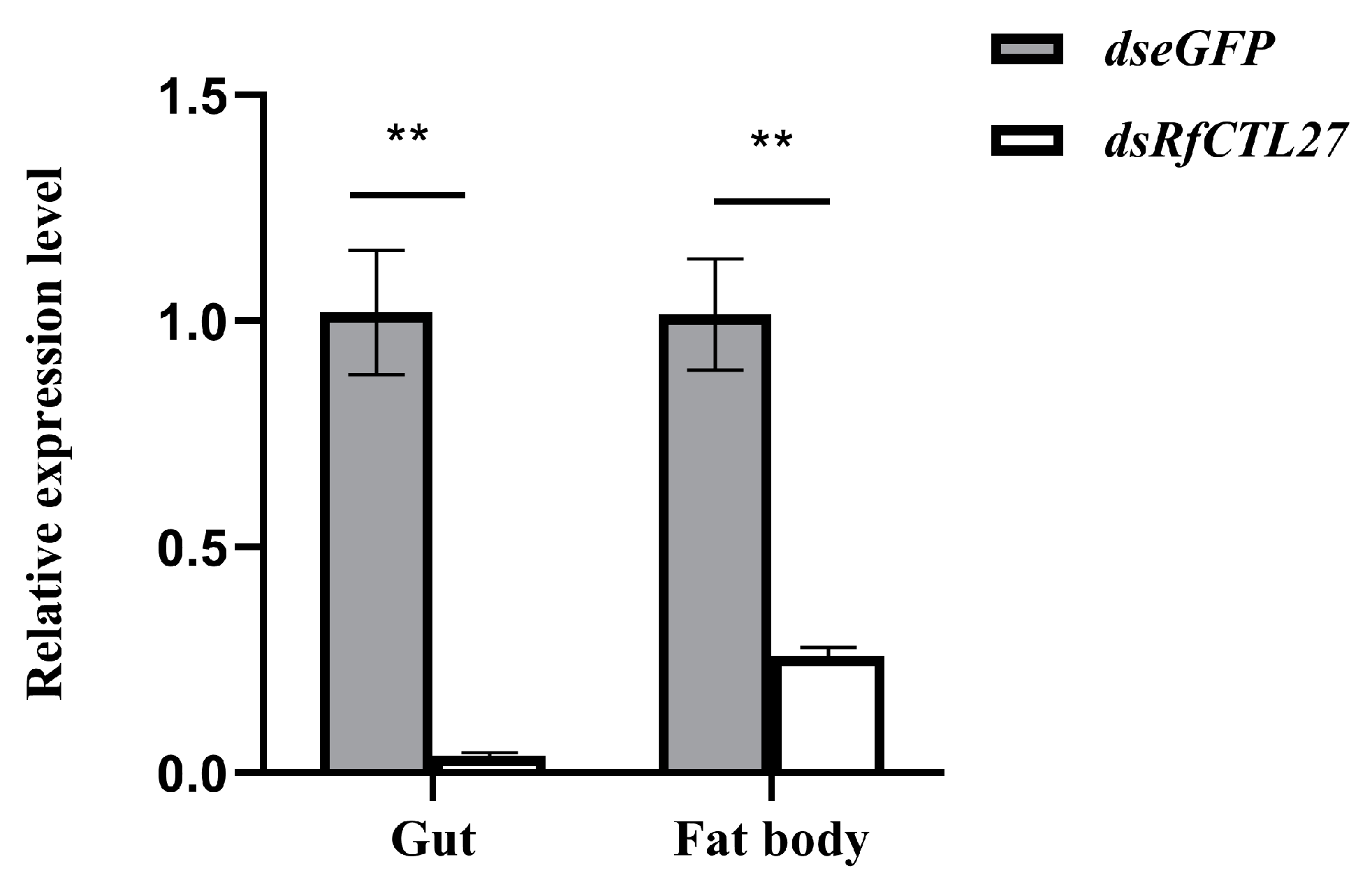
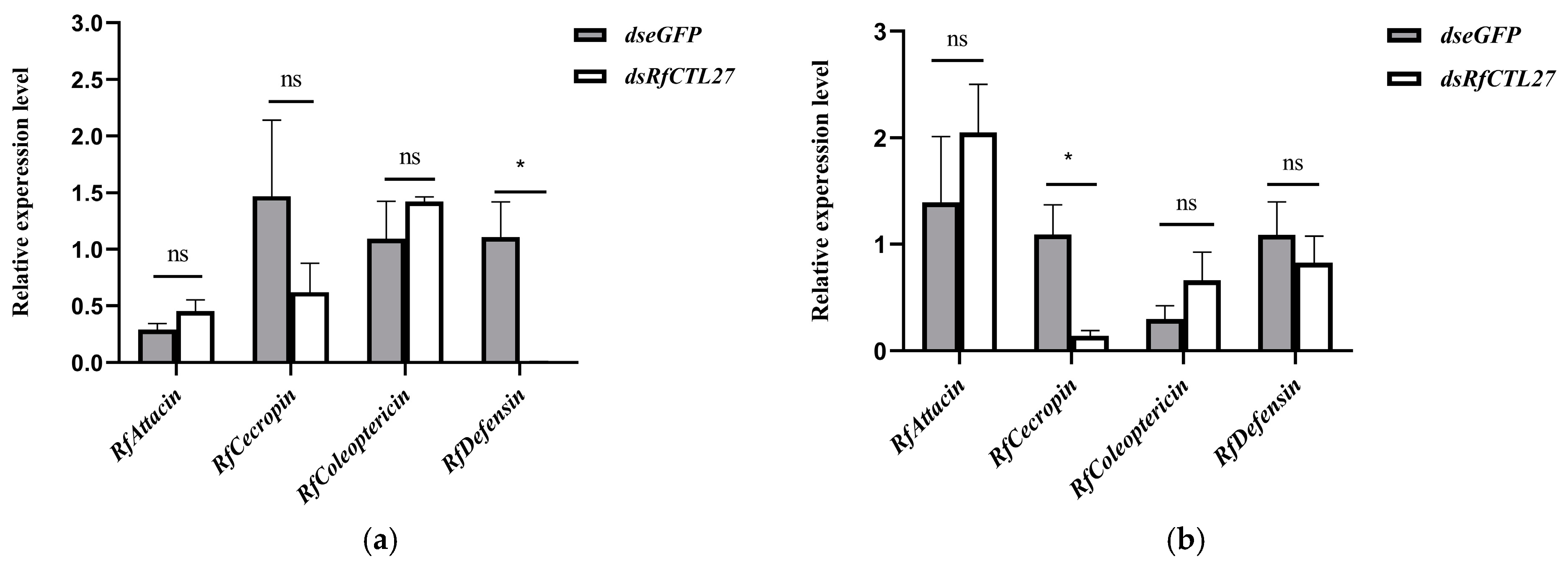
3.4. Effect of RfCTL27 Silencing on the Immunocompetence of RPW Larvae and Expression of Antimicrobial Peptide Genes in the Gut and Fat Body
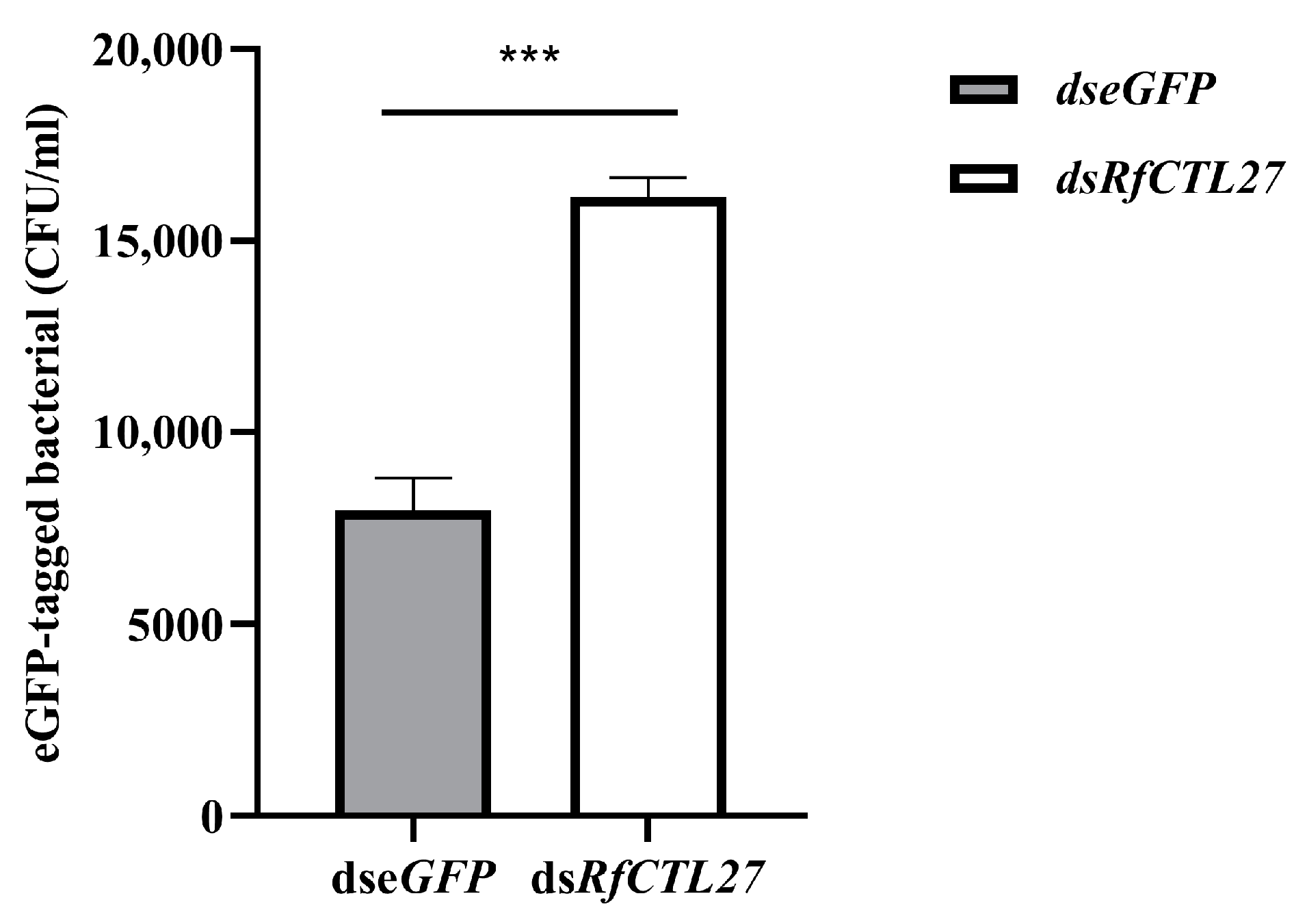
3.5. The Effect of RfCTL27 Knockdown on the Ability of RPW Larvae to Clear to the Invaded E. coli in Hemolymph
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Knutelski, S.; Awad, M.; Łukasz, N.; Bukowski, M.; Śmiałek, J.; Suder, P.; Dubin, G.; Mak, P. Isolation, identification, and bioinformatic analysis of antibacterial proteins and peptides from immunized hemolymph of red palm weevil Rhynchophorus ferrugineus. Biomolecules 2021, 11, 83. [Google Scholar] [CrossRef]
- Shar, M.U.; Rustamani, M.A.; Nizamani, S.M.; Bhutto, L.A. Red palm weevil (Rhynchophorus ferrugineus Olivier) infestation and its chemical control in Sindh province of Pakistan. Afr. J. Agric. Res. 2012, 7, 1666–1673. [Google Scholar]
- Habib, D.M.; Mouna, N.C.I.B.; Wiem, H. Red palm weevil (Rhynchophorus ferrugineus) chemical treatments applied on ornamental palms in Tunisia: Results of extensive experiments. Int. J. Agr. Innov. Res. 2017, 5, 1062–1068. [Google Scholar]
- Pu, Y.C.; Ma, T.L.; Hou, Y.M.; Sun, M. An entomopathogenic bacterium strain, Bacillus thuringiensis, as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Pest. Manag. Sci. 2017, 73, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Habineza, P.; Ji, T.; Hou, Y.; Shi, Z. Intestinal microbiota confer protection by priming the immune system of red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae). Front. Physiol. 2019, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Dembilio, Ó.; Quesada-Moraga, E.; Santiago-Álvarez, C.; Jacas, J.A. Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the Red Palm Weevil, Rhynchophorus ferrugineus. J. Invertebr. Pathol. 2010, 104, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Hazzouri, K.M.; Sudalaimuthuasari, N.; Kundu, B.; Nelson, D.; Al-Deeb, M.A.; Le Mansour, A.; Amiri, K.M. The genome of pest Rhynchophorus ferrugineus reveals gene families important at the plant-beetle interface. Commun. Biol. 2020, 3, 323. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; AlJabr, A.M. Susceptibility and immune defence mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against entomopathogenic fungal infections. Int. J. Mol. Sci. 2016, 17, 1518. [Google Scholar] [CrossRef]
- Ma, B.; Wang, X.; Liu, Q.; Zhao, Y.; Su, Z.; Chen, Y.; Shi, Z. A peptidoglycan recognition protein regulates the immune response of Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae) during exposure to pathogenic Gram-positive bacteria and fungi. Dev. Comp. Immunol. 2023, 144, 104705. [Google Scholar] [CrossRef]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef]
- Li, J.; Bi, J.; Zhang, P.; Wang, Z.; Zhong, Y.; Xu, S.; Wang, L.; Li, B. Functions of a C-type lectin with a single carbohydrate-recognition domain in the innate immunity and movement of the red flour beetle, Tribolium castaneum. Insect. Mol. Biol. 2019, 30, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Tanji, T.; Ohashi-Kobayashi, A.; Natori, S. Participation of a galactose-specific C-type lectin in Drosophila immunity. Biochem. J. 2006, 396, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Kanost, M.R. Immulectin-2, a lipopolysaccharide-specific lectin from an insect, Manduca sexta, is induced in response to gram-negative bacteria. J. Biol. Chem. 2000, 275, 37373–37381. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.Y.; Shahzad, T.; Yang, P.J.; Liu, S.; Yu, X.Q.; Rao, X.J. A single-CRD C-type lectin is important for bacterial clearance in the silkworm. Dev. Comp. Immunol. 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, F.; Zhu, K.; Zhu, M.; Li, Q.; Hu, Q.; Yu, X.Q. Comparative genomic analysis of C-type lectin-domain genes in seven holometabolous insect species. Insect. Biochem. Mol. Biol. 2020, 126, 103451. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Ma, C.; Li, H.; Zuo, H.; Weng, S.; Chen, X.; Zeng, D.; He, J.; Xu, X. Identification of a C-type lectin with antiviral and antibacterial activity from pacific white shrimp Litopenaeus vannamei. Dev. Comp. Immunol. 2014, 46, 231–240. [Google Scholar] [CrossRef]
- Ling, E.; Ao, J.; Yu, X.Q. Nuclear translocation of immulectin-3 stimulates hemocyte proliferation. Mol. Immunol. 2008, 45, 2598–2606. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Muhammad, A.; Habineza, P.; Wang, X.; Xiao, R.; Ji, T.; Hou, Y.; Shi, Z. Spätzle Homolog-Mediated Toll-Like Pathway Regulates Innate Immune Responses to Maintain the Homeostasis of Gut Microbiota in the Red Palm Weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae). Front. Microbiol. 2020, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Zelensky, A.N.; Gready, J.E. The C-type lectin-like domain superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Kahn, R.; Fourme, R.; Drickamer, K.; Hendrickson, W.A. Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science 1991, 254, 1608–1615. [Google Scholar] [CrossRef]
- Yu, X.Q.; Ma, Y. Calcium is not required for immulectin-2 binding, but protects the protein from proteinase digestion. Insect. Biochem. Mol. Biol. 2006, 36, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Wang, L.; Song, X.; Zhao, J.; Qiu, L.; Li, L.; Song, L. A novel C-type lectin (Cflec-3) from Chlamys farreri with three carbohydrate-recognition domains. Fish. Shellfish. Immunol. 2009, 26, 707–715. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Y.; Shi, Y.R.; Ren, Q.; Wang, W. Function of a novel C-type lectin with two CRD domains from Macrobrachium rosenbergii in innate immunity. Dev. Comp. Immunol. 2015, 49, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Jacopo, V.; Adam, M.; Richma, B.; Sandrine, U.; Claudia, B.; Philippe, B. The defensin peptide of the malaria vector mosquito Anopheles gambiae: Antimicrobial activities and expression in adult mosquitoes. Insect. Biochem. Mol. 2001, 31, 241–248. [Google Scholar]
- Vizioli, J.; Bulet, P.; Charlet, M.; Lowenberger, C.; Blass, C.; Müller, H.M.; Dimopoulos, G.; Hoffmann, J.; Kafatos, F.C.; Richman, A. Cloning and analysis of a cecropin gene from the malaria vector mosquito, Anopheles gambiae. Insect. Mol. Biol. 2000, 9, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Richman, A.M.; Bulet, P.; Hetru, C.; Barillas, M.C.; Hoffmann, J.A.; Kafatos, F.C. Inducible immune factors of the vector mosquito Anopheles gambiae: Biochemical purification of a defensin antibacterial peptide and molecular cloning of preprodefensin cDNA. Insect. Mol. Biol. 1996, 5, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Samakovlis, C.; Kimbrell, D.A.; Kylsten, P.; Engström, A.; Hultmark, D. The immune response in Drosophila: Pattern of cecropin expression and biological activity. EMBO J. 1990, 9, 2969–2976. [Google Scholar] [CrossRef]
- Pang, X.; Xiao, X.; Liu, Y.; Zhang, R.; Liu, J.; Liu, Q.; Wang, P.; Cheng, G. Mosquito C-type lectins maintain gut microbiome homeostasis. Nat. Microbiol. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Liu, P.P.; Wei, Z.; Cheng, Z.H.; Wang, X.W. Small immune effectors coordinate peptidoglycan-derived immunity to regulate intestinal bacteria in shrimp. PLoS Pathog. 2022, 18, e1010967. [Google Scholar] [CrossRef]



| Reagent | Dosage |
|---|---|
| SYBR mix | 10 μL |
| Forward primer | 0.4 μL |
| Reverse primer | 0.4 μL |
| cDNA | 1 μL |
| ddH2O | 8.2 μL |
| Total | 20 μL |
| Primers | Sequences (5′-3′) |
|---|---|
| RT-qPCR | |
| Rfβ-Actin F | CCAAGGGAGCCAAGCAATT |
| Rfβ-Actin R | CGCTGATGCCCCTATGTATGT |
| RfCTL27 F | ATCAACGGATGGTTCTGGTC |
| RfCTL27 R | ACGAAGGGTTTCAGATGGT |
| RfAttacin F | TGGTTCTGGTGCCCAAGTGA |
| RfAttacin R | GCCATAACGATTCTTGTTGGAGTA |
| RfCecropin F | CAGAAGCTGGTTGGTTGAAGA |
| RfCecropin R | GCAACACCGACATAACCCTGA |
| RfColeopericin F | TCGTGGTTTCTACCATGTTCACT |
| RfColeopericin R | TCAGCTAAAACCTGATCTTGGA |
| RfDefensin F | TTCGCCAAACTTATCCTCGTG |
| RfDefensin R | GGGTGCTTCGTTATCAACTTCC |
| RNAi | |
| RfdsCTL27 F | taatacgactcactatagggACCTGGAAGTCGACTGGTTG |
| RfdsCTL27 R | taatacgactcactatagggAGTTCCTCGCTATCTTCGCA |
| RfdseGFP F | taatacgactcactatagggCAGTGCTTCAGCCGCTAC |
| RfdseGFP R | taatacgactcactatagggGTTCACCTGCCGTTCTTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Xia, Y.; Su, Z.; Wang, X.; Kou, Y.; Ma, B.; Hou, Y.; Shi, Z. A C-Type Lectin, RfCTL27, Activates the Immune Defense in the Red Palm Weevil Rhynchophorus ferrugineus (A.G. Olivier, 1791) (Coleoptera: Curculionidae: Dryophthorinae) by the Recognition of Gram-Negative Bacteria. Insects 2024, 15, 212. https://doi.org/10.3390/insects15030212
Gong Y, Xia Y, Su Z, Wang X, Kou Y, Ma B, Hou Y, Shi Z. A C-Type Lectin, RfCTL27, Activates the Immune Defense in the Red Palm Weevil Rhynchophorus ferrugineus (A.G. Olivier, 1791) (Coleoptera: Curculionidae: Dryophthorinae) by the Recognition of Gram-Negative Bacteria. Insects. 2024; 15(3):212. https://doi.org/10.3390/insects15030212
Chicago/Turabian StyleGong, Yanru, Yongjian Xia, Zhiping Su, Xinghong Wang, Yishuo Kou, Bing Ma, Youming Hou, and Zhanghong Shi. 2024. "A C-Type Lectin, RfCTL27, Activates the Immune Defense in the Red Palm Weevil Rhynchophorus ferrugineus (A.G. Olivier, 1791) (Coleoptera: Curculionidae: Dryophthorinae) by the Recognition of Gram-Negative Bacteria" Insects 15, no. 3: 212. https://doi.org/10.3390/insects15030212






