Simple Summary
Bacterial symbionts, especially Wolbachia, are vital in many physiological processes of insects. However, the mean infection prevalence of Wolbachia in many species of lepidopteran insects is relatively low. Here, we investigated the infection, composition, abundance, and diversity of bacterial symbionts, especially Wolbachia, associated with Spodoptera frugiperda. Our results revealed that Wolbachia was found in the ovaries and salivary glands of S. frugiperda female adults. Although the infection and abundance of Wolbachia varied between geographical populations, they all belonged to the supergroup B and were named the wFru strain, which has been considered to potentially induce cytoplasmic incompatibility. These findings may provide a foundation for developing potential biocontrol techniques for S. frugiperda.
Abstract
Bacterial symbionts associated with insects can be crucial in insect nutrition, metabolism, immune responses, development, and reproduction. However, the bacterial symbionts of the fall armyworm Spodoptera frugiperda remain unclear. S. frugiperda is an invasive polyphagous pest that severely damages many crops, particularly maize and wheat. Here, we investigated the infection, composition, abundance, and diversity of bacterial symbionts, especially Wolbachia, in different tissues of S. frugiperda female adults. The infection prevalence frequencies of Wolbachia in five provinces of China, namely Pu’er, Yunnan; Nanning, Guangxi; Sanya, Hainan; Yunfu, Guangdong; and Nanping, Fujian, were assessed. The results indicated that Proteobacteria, Firmicutes, and Bacteroidetes were the three most dominant bacterial phyla in S. frugiperda adults. At the genus level, the abundant microbiota, which included Enterobacter and Enterococcus, varied in abundance between tissues of S. frugiperda. Wolbachia was found in the ovaries and salivary glands of S. frugiperda adults, and was present in 33.33% of the Pu’er, Yunnan, 23.33% of the Nanning, Guangxi, and 13.33% of the Sanya, Hainan populations, but Wolbachia was absent in the Yunfu, Guangdong and Nanping, Fujian populations. Further phylogenetic analyses revealed that all of the Wolbachia strains from the different S. frugiperda populations belonged to the supergroup B and were named the wFru strain. Since there were Wolbachia strains inducing cytoplasmic incompatibility in supergroup B, these findings may provide a foundation for developing potential biocontrol techniques against S. frugiperda.
1. Introduction
The fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), which is native to tropical and subtropical areas in America [1,2], is considered one of the most serious, polyphagous, invasive insect pests in the world [3]. S. frugiperda displays two subpopulations called the “Rice” and “Corn” strains, which have affected more than 350 species of plants from 76 families, including maize, wheat, rice, cotton, sugarcane, and sorghum [4]. In China, S. frugiperda was first found in western Yunnan Province in 2018 [5] and then rapidly expanded to the other 26 provinces within a year, except for Heilongjiang, Jilin, Liaoning, Qinghai, and Xinjiang [6,7,8]. S. frugiperda has evolved to acquire strong resistance against most insecticides (e.g., pyrethroids, organophosphates, and carbamates) [9] and overcome the resistance of transgenic maize [10,11]. Consequently, alternative control strategies for S. frugiperda management are urgently needed, and population manipulation with bacterial symbionts is one of the emerging strategies used in pest management [12,13,14].
Biological interactions between bacteria and insects, which can be parasitic, symbiotic, or neutral, are very common in nature [15]. Insects generally host a gut microbiota, which is extracellular and can vary depending on the environmental conditions and on the insect’s diet, and endosymbiotic bacteria, which are intracellular and can be obligate or facultative [16]. Previous studies have revealed that symbiotic bacteria play vital roles in many physiological processes of insects, including nutrition, metabolism, immune responses, development, and reproduction [17,18]. For instance, the IIa bacteriocin (mundticin KS) produced by the gut bacterium Enterococcus mundtii inhibited pathogen colonization in Spodoptera littoralis (Lepidoptera: Noctuidae) [19]. Xia et al. [20] revealed that the most abundant gut bacteria (Enterobacter spp.) contributed to plant cell wall degradation in Plutella xylostella (Lepidoptera: Plutellidae) and enhanced food utilization efficiency. Wang et al. [21] hypothesized that Wolbachia may be a crucial factor influencing the reproductive isolation of Ectropis obliqua (Lepidoptera: Geometridae) and Ectropis grisescens (Lepidoptera: Geometridae). In addition, Lei et al. [22] reported that Wolbachia reduced the susceptibility of the striped stem borer Chilo suppressalis (Lepidoptera: Crambidae) to two insecticides (fipronil and avermectin). Among the intracellular bacteria endosymbionts, Wolbachia (Alphaproteobacteria: Rickettsiales) is one of the most abundant in nematodes, insects, and other arthropods, and approximately 40–60% of all insect species in nature are estimated to be infected with Wolbachia [23,24]. Normally, these bacteria can manipulate the reproductive pattern of their hosts in diverse ways, including through cytoplasmic incompatibility (CI), feminization, male killing (MK), and parthenogenesis (PI), to increase the chance of infected individuals reproducing successfully and spreading the infection through their progeny [25,26]. Wolbachia, due to its inability to be cultured outside host cells, and the infection rate of Wolbachia in many species of lepidopteran insects is relatively low, which presents a serious challenge for studying its infection patterns [27,28,29]. Consequently, understanding the composition, diversity, and potential functions of bacterial symbionts in S. frugiperda, particularly regarding Wolbachia, is a gray area in research.
In the present study, 16S rRNA high-throughput sequencing and polymerase chain reaction (PCR) were used to investigate the infection, composition, abundance, and diversity of bacterial symbionts in S. frugiperda, with a particular focus on Wolbachia. Given the recognized potential of Wolbachia for biological control and the paucity of research on its interaction with S. frugiperda, our study of pest-associated symbiotic bacteria, especially Wolbachia, may lay a useful foundation for developing potential biocontrol techniques against S. frugiperda.
2. Materials and Methods
2.1. Insect Rearing and Plant Growth
Spodoptera frugiperda were initially collected from a corn field in July 2019 in Zhangzhou (24.52° N, 117.35° E), Fujian Province, Southeast China. S. frugiperda larvae were reared on fresh maize leaves in a lattice box (inner grid size: 4.1 cm, × 2.3 cm, × 3.3 cm, 3 × 8 grids) under a 14:10 h light–dark cycle in an incubator at 26 ± 1 °C and 70 ± 10% relative humidity (RH). Thereafter, the pupae were transferred to a new cage (35 cm, × 35 cm, × 35 cm), where emerging adults were fed an artificial diet with a 10% honey solution under the same conditions for reproduction. The experimental populations were reared for at least 10 generations before use.
To supply maize leaves as food for S. frugiperda, seeds (Zea mays L. var. Zhengdan no. 958) were sown in soil (10% sand, 5% clay, and 85% peat) in a greenhouse under ambient conditions. Plants without any pest or disease damage were used at the 6–12-expanded leaf stage.
2.2. PCR Amplification, Library Preparation, and Sequencing
Approximately 30 newly emerged, unmated S. frugiperda female adults were sterilized with 75% alcohol and dissected under a stereomicroscope on an ultraclean workbench, after which gut, ovary, salivary gland, and fat body tissues were isolated. These tissues were divided into three replicates for testing. Total genomic DNA for all samples was extracted using the TIANamp Genomic DNA Kit (Tiangen, Beijing, China) following the manufacturer’s instructions. DNA extraction was also carried out on an ultraclean workbench after ultraviolet sterilization to avoid contamination by environmental DNA. The specific primers 27F (5′-AGRGTTTGATYNTGGCTCAG-3′) and 1492R (5′-TASGGHTACCTTGTTASGACTT-3′) were used to amplify the V1–V9 regions of the 16S rRNA gene. PCR amplification was performed using the following thermocycling procedures: 95 °C for 5 min, 25 cycles of 95 °C for 30 s, 50 °C for 30 s, and 72 °C for 40 s, with a final elongation step at 72 °C for 7 min. Homogenized PCR products were visualized through 1.5% agarose gel electrophoresis, with gel purified using a TIANgel Midi Purification Kit (Tiangen, Beijing, China), and subsequently homogenized to generate a sequencing library (SMRT Bell). The quality of the established library was checked first, and single-molecule sequencing was conducted on the PacBio Sequel platform at Biomarker Bioinformatics Technology, Co., Ltd. (Beijing, China). Filtering and demultiplexing of the raw readings were performed to obtain circular consensus sequence (CCS) readings via SMRT Link software (version 8.0) (minPasses ≥ 5 and minPredictedAccuracy ≥ 0.9). Subsequently, the CCS readings were assigned to corresponding samples based on their barcodes using lima v1.7.0. The Cutadapt quality control program (version 2.7) was utilized to eliminate CCS readings lacking primers or falling outside the specified length range (1200–1650 bp). The chimeric sequences were identified and excised employing UCHIME v8.1 [30]. The CCS readings exhibiting a similarity level of 97% or greater were clustered as operational taxonomic units (OTUs) using USEARCH v10.0 [31]. OTUs with sequences < 0.005% of the total number of sequences were discarded [32]. The resulting representative sequences from each OTU cluster were screened for further annotation. Taxonomic classification of all OTUs was conducted using the RDP classifier v2.2, with a confidence threshold of 80% [33], based on the SILVA database (release 132) [34]. Finally, to facilitate downstream diversity analysis, selected OTUs were aligned to the core alignment template of the SILVA database using PyNAST v1.2.2.
2.3. Microbiome Diversity Analysis
For further bacterial community analysis, the microbial diversity of each sample was estimated using alpha diversity indices (including the ACE, Chao1, Simpson, and Shannon indices). The ACE and Chao 1 indices are primarily used to estimate species richness, while the Simpson index focuses on dominance, and the Shannon index combines species richness and evenness. These indices, along with the OTU numbers and coverage of the library, were calculated in Mothur v.1.30 [35]. Beta diversity was assessed using QIIME software (version 2), and the microbial community structures across different groups were compared using principal component analysis (PCA) based on the Bray–Curtis index and weighted UniFrac distance matrices [36]. To assess the statistical significance of the differences in the bacterial symbionts among the groups, analysis of similarities (ANOSIM) and permutational multivariate analysis of variance (PERMANOVA) were conducted using the anosim and adonis functions of the package vegan v2.3.0 [37] in the R v3.1.1 programming environment [38]. In ANOSIM, an R value closer to 1 implies greater dissimilarity between groups than within groups [39], while a larger R2 value in PERMANOVA indicates that the grouping factor significantly contributes to the overall variation [40]. p values less than 0.05 in both the ANOSIM and PERMANOVA were considered to indicate statistical significance.
2.4. PCR Detection of Endosymbionts from Different S. frugiperda Populations
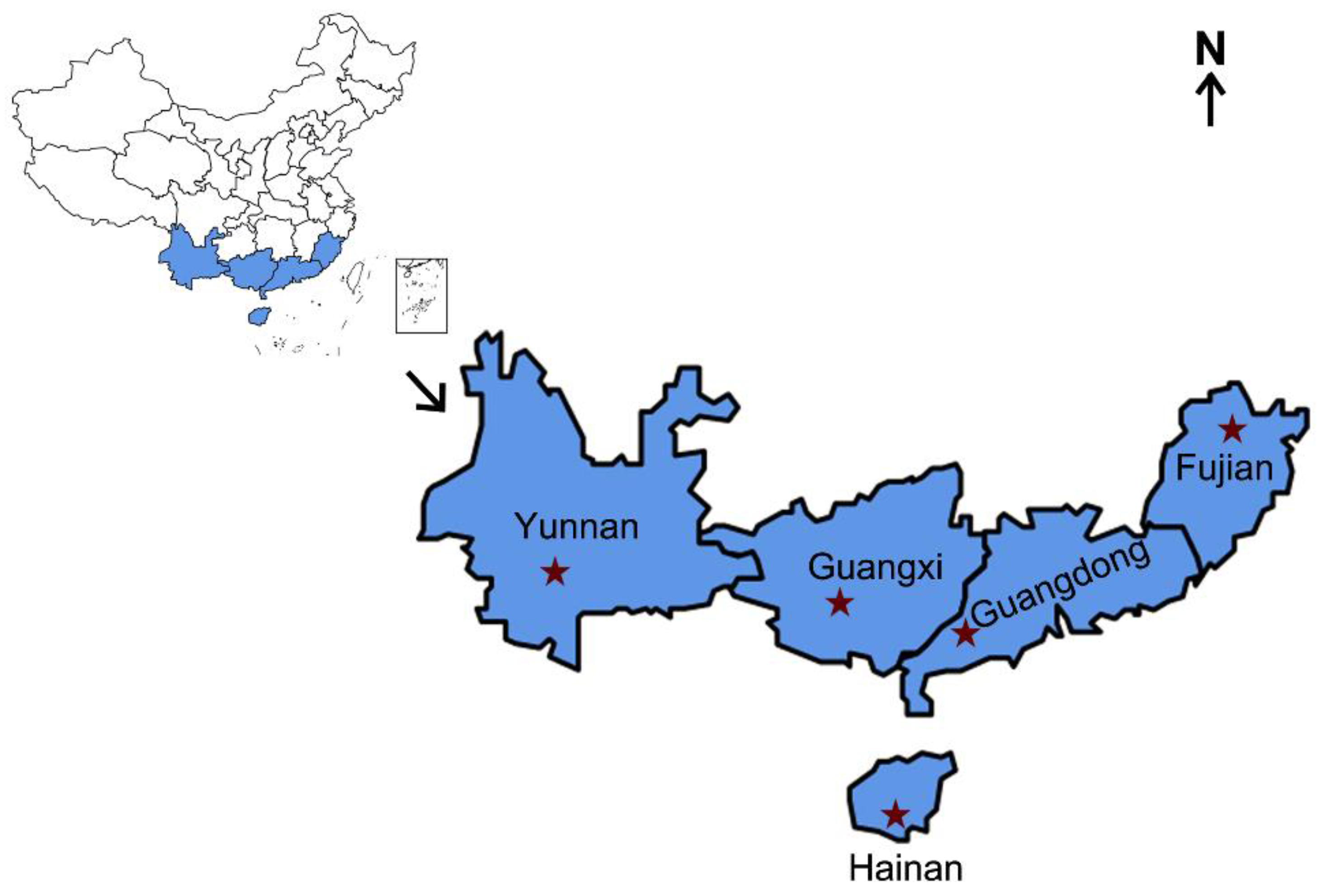
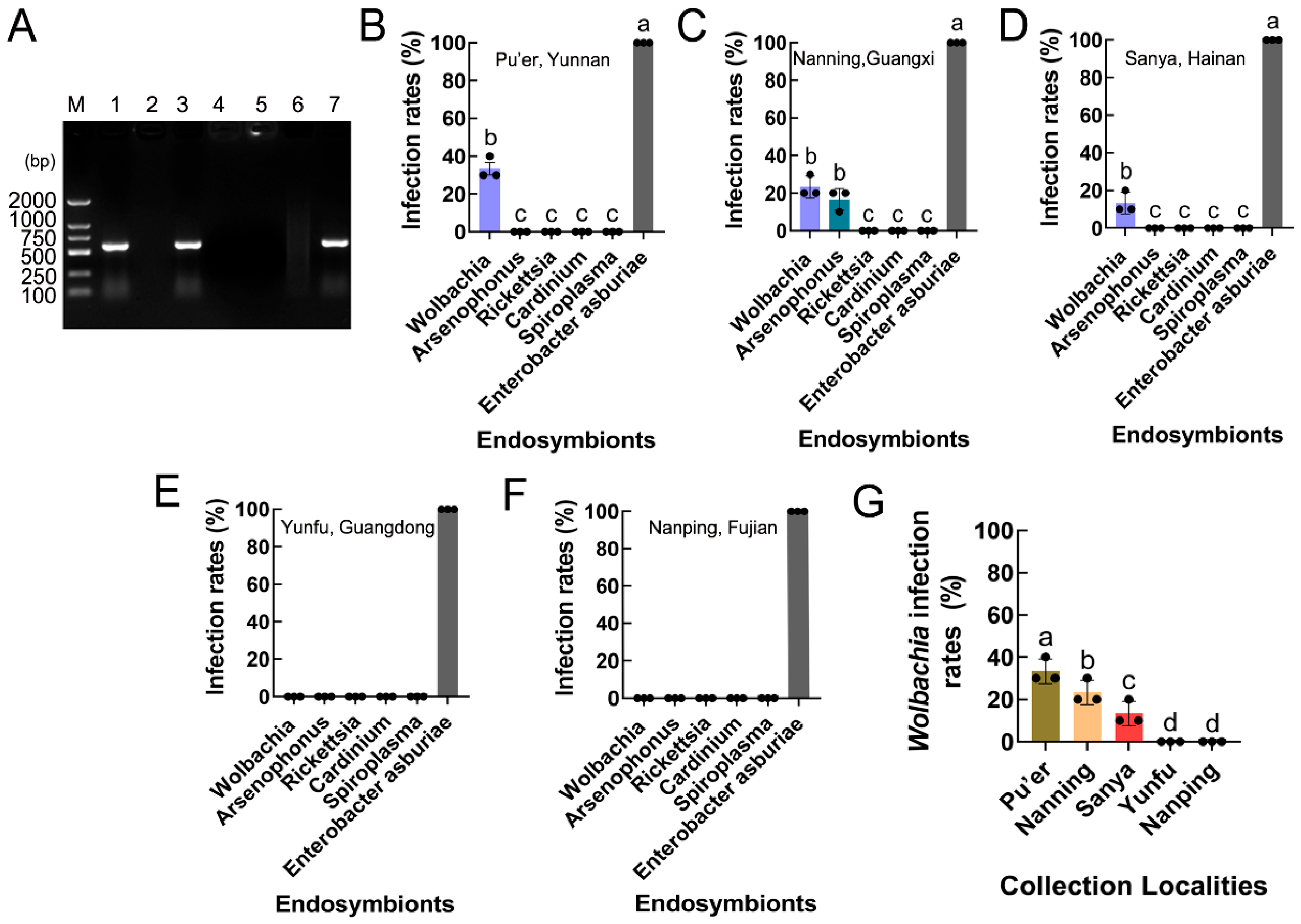
S. frugiperda female adults were collected from five collection localities in China, namely Pu’er, Yunnan; Nanning, Guangxi; Sanya, Hainan; Yunfu, Guangdong; and Nanping, Fujian (Figure 1). Approximately 30 adults from each province were immersed separately in 95% ethyl alcohol and frozen at −20 °C before being assessed for the infection prevalence frequencies of endosymbionts, including Wolbachia, Arsenophonus, Cardinium, Rickettsia, and Spiroplasma, which have been reported to be present in many species of studied lepidopteran insects [41]. Enterobacter asburiae, which exhibited 100% infection among the samples analyzed, was used as a positive control. The sample information is provided in Table 1.
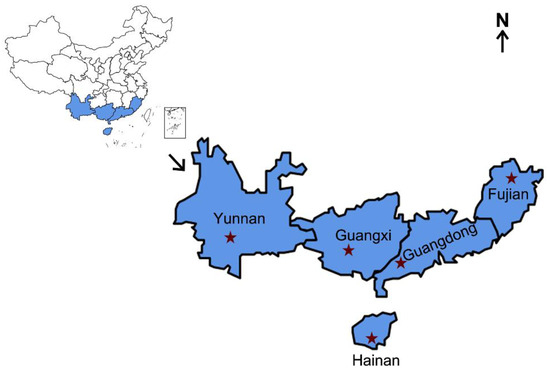
Figure 1.
Collection regions of S. frugiperda. The blue-shadowed parts represent the sampled provinces, and the red stars represent the exact locations. Information on the different S. frugiperda samples is provided in Table 1.

Table 1.
Information on the S. frugiperda samples used in this study.
Total DNA was extracted from a single S. frugiperda female adult following the method of Ahmed et al. [42], where 30 individuals were tested in each province, 10 individuals were treated as one experimental replicate to determine the infection prevalence frequencies of endosymbionts, and three replicates were performed. PCR amplification, which included assays for 16S rRNA (targeting conserved regions within the ribosomal RNA of Wolbachia), wsp (encoding the Wolbachia surface protein), and 16S rDNA (amplifying a fragment that includes the 16S rRNA coding sequence and other ribosomal DNA elements of Wolbachia) genes of endosymbionts, was performed in 25 μL of buffer containing 1 μL of the template DNA lysate, 1 μL of each primer, 2.5 mM MgCl2, each dNTP at 200 mM, and 1 unit of DNA Taq polymerase (Invitrogen, Guangzhou, China) [43]. The specific primers used in this study are shown in Table 2. The remainder of the PCR products of single colonies were then sent to the Beijing Institute (BGI) for sequencing after the expected bands were visible on a 1.5% agarose gel containing Gold-View colorant. The results were compared to known sequences using NCBI’s BLAST algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 15 March 2022). Finally, both the species and prevalence frequencies of endosymbionts in the five collection localities were determined.

Table 2.
Details of the primers used in this study.
2.5. Phylogenetic Analysis of Wolbachia in S. frugiperda
A phylogenetic tree of Wolbachia was constructed based on the wsp gene sequence. The wsp gene sequences of Wolbachia from different insect hosts were selected as references for homology analysis in the GenBank database using basic local alignment search tools (BLASTs). These wsp gene sequences of Wolbachia are listed in Table S1, and were edited and aligned using Lasergene v7.1 (DNASTAR, Inc., Madison, WI, USA). The Bayesian information criterion was used to select the best model and partitioning scheme in PartitionFinder v.1.0.1 [51]. In addition, the phylogenetic tree of Wolbachia was generated with IQ-TREE v1.6.8 using the TUM+F+R3 model for the wsp gene. The phylogenetic tree of Wolbachia was constructed based on the maximum likelihood (ML) method with 1000 nonparametric bootstrap replications in RAxML [52].
2.6. Statistical Analyses
All of the alpha diversity indices (ACE, Chao1, Simpson, and Shannon indices), circular consensus sequencing (CCS) readings of Wolbachia in different tissues, and infection and prevalence of endosymbionts in different S. frugiperda populations were analyzed using one-way analysis of variance (ANOVA), and the means were compared using Tukey’s HSD test (SPSS 21.0) at p < 0.05. All graphs were drawn with SigmaPlot 10.0.
3. Results
3.1. Bacterial Abundance in Different Tissues of S. frugiperda
A total of 80,521 CCS readings were obtained after 12 samples were sequenced and identified by barcode; each sample generated at least 4853 CCS readings with an average of 6710 CCS readings. The raw sequence data obtained from this study were deposited in the National Center for Biotechnology Information (NCBI) under accession number PRJNA995535. The clean CCS readings (gut: 6892.67 ± 473.32; ovary: 5588.67 ± 398.40; salivary gland: 6981.00 ± 249.03; fat body: 7375.00 ± 83.74) were retained after primer removal and length filtration. The effective CCS readings (gut: 6881.00 ± 472.02; ovary: 5528.00 ± 427.79; salivary gland: 6828.33 ± 270.84; fat body: 7274.33 ± 71.42) were retained after chimeric read removal. All of the effective tags were clustered into OTUs (gut: 110.00 ± 35.38; ovary: 194.33 ± 33.11; salivary gland: 209.33 ± 18.80; fat body: 44.33 ± 6.69). The Good’s coverage rates were >0.99, indicating that a high degree of sequencing coverage, with all microbiota in each group, was represented by the number of OTUs identified (Table 3).

Table 3.
Samples and their processed sequence data.
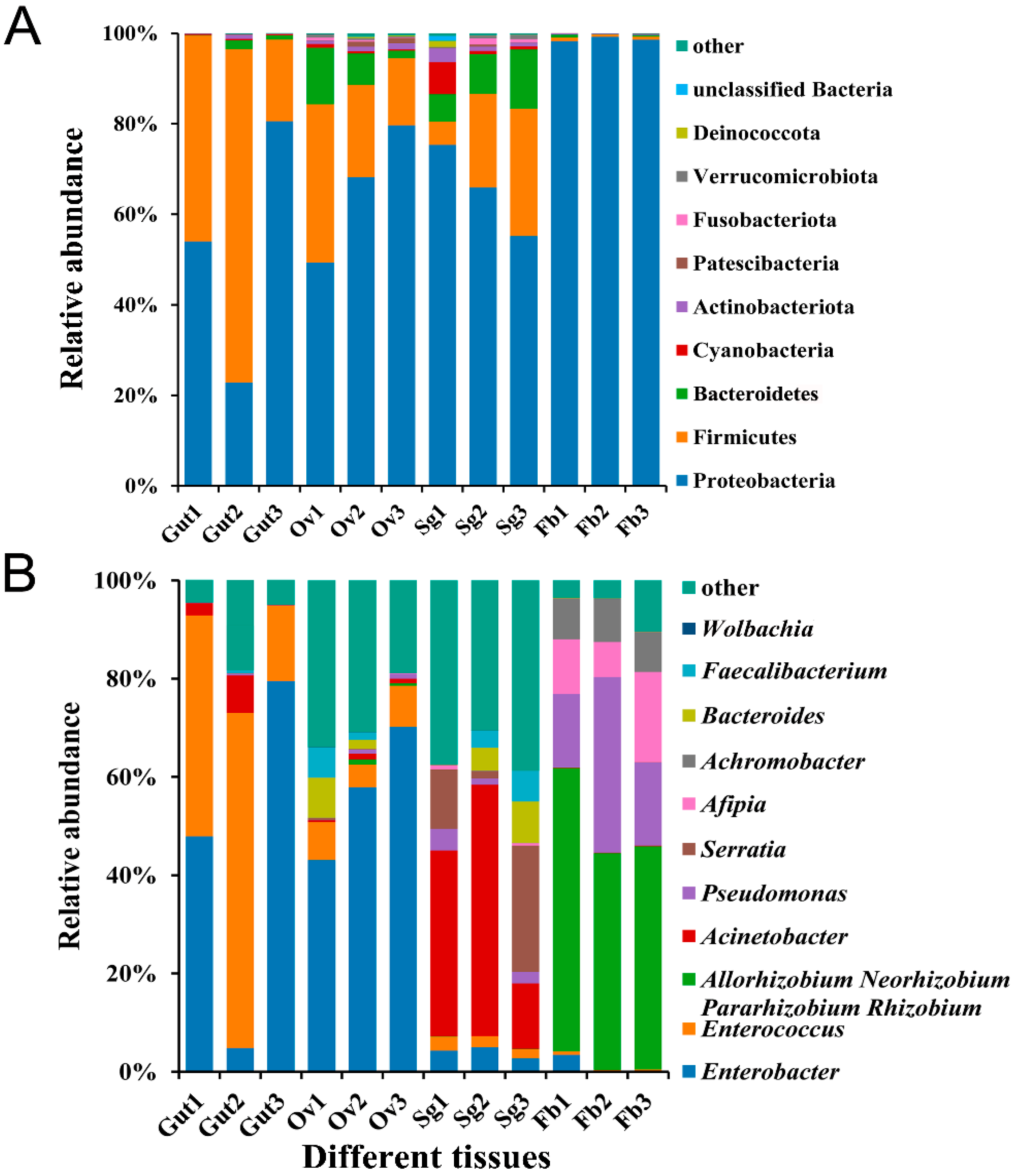
Based on their abundance and annotation information, 481 bacterial OTUs were assigned to 21 phyla, 33 classes, 81 orders, 148 families, and 290 genera. At the phylum level, the most abundant bacterial phyla identified were Proteobacteria, Firmicutes, and Bacteroidetes, among which Proteobacteria was the most abundant in the fat body tissues of S. frugiperda female adults (98.70%). The phyla detected in the ovaries were evenly represented, with Proteobacteria accounting for 65.76%, Firmicutes accounting for 23.40%, and Bacteroidetes accounting for 7.03% (Figure 2A). At the genus level, the abundances of microbiota members Enterobacter and Enterococcus varied between the tissues of S. frugiperda. The abundance of Enterobacter in the ovaries (57.07%) was much greater than that in the other three tissues (gut: 44.10%; salivary gland: 3.99%; fat body: 1.21%), while Enterococcus was the most abundant genus in the gut tissues (42.83%), representing 6.88% of the ovary, 2.32% of the salivary gland, and 0.42% of the fat body tissues (Figure 2B).
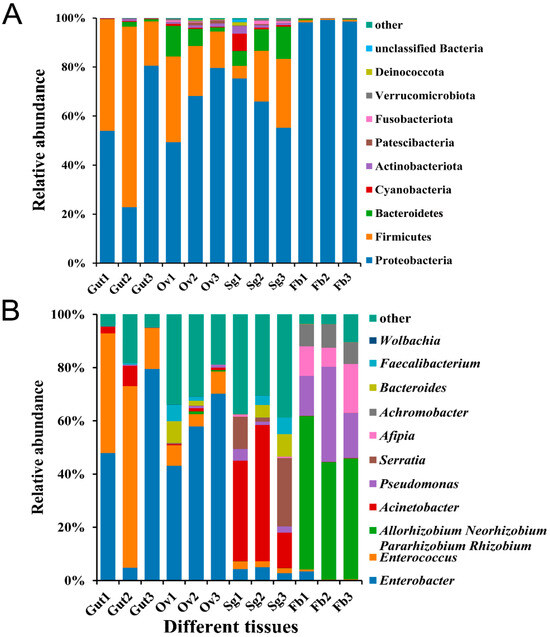
Figure 2.
Relative bacterial abundance in different tissues of S. frugiperda female adults. (A) Phylum-level relative abundances of bacteria in S. frugiperda; (B) genus-level relative abundances of bacteria in S. frugiperda.
3.2. Bacterial Diversity and Community Structure in Different Tissues of S. frugiperda
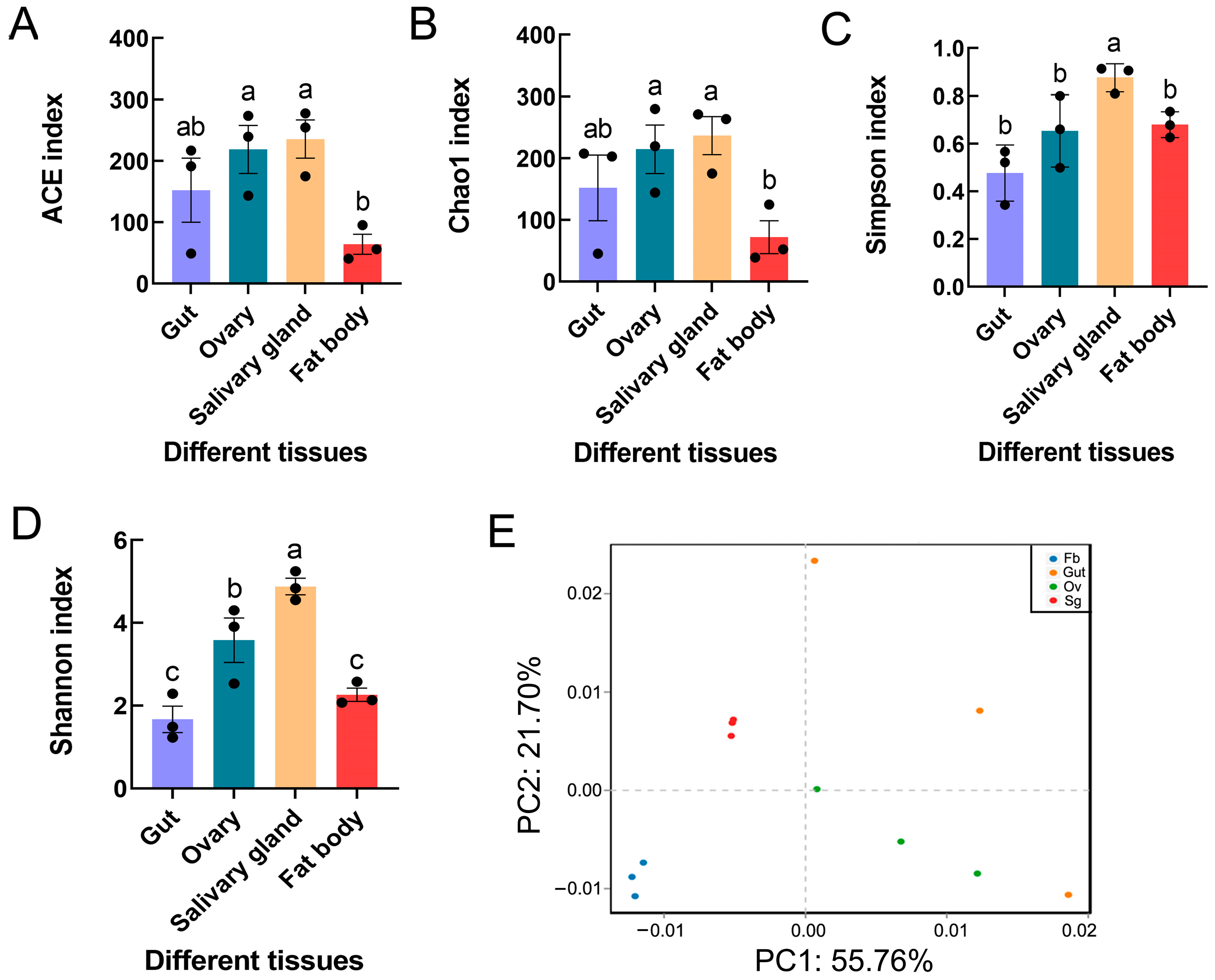
Microbiome diversity was estimated in relation to the alpha diversity, which was measured as the species richness and diversity in a sample. The Shannon and Simpson diversity metrics demonstrated that the salivary glands had significantly greater microbial diversity than the other tissues (Shannon F3,8 = 17.977, p = 0.001; Simpson F3,8 = 7.443, p < 0.05). Similar significant differences were also found for other alpha diversity indices (i.e., ACE F3,8 = 4.429, p < 0.05; Chao1 F3,8 = 3.610, p > 0.05) (Figure 3A–D). PCA revealed distinct clustering of microbes from different tissues of S. frugiperda, with obvious differences between the four sample groups, including the gut, ovary, salivary gland, and fat body tissues (Figure 3E). Moreover, ANOSIM and PERMANOVA demonstrated significant differences in bacterial communities across samples from the four distinct groups (Table 4).
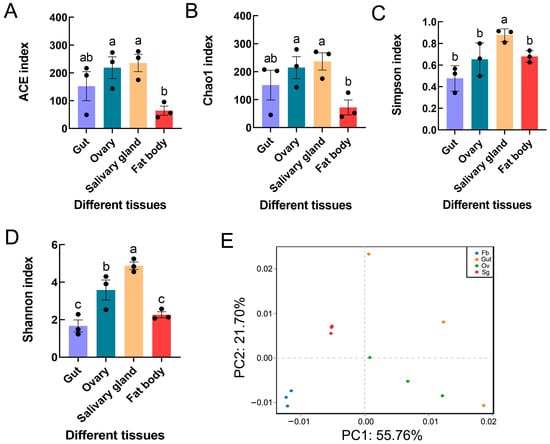
Figure 3.
Bacterial diversity and community structure in different tissues of S. frugiperda female adults: (A) ACE index; (B) Chao1 index; (C) Simpson index; (D) Shannon index. The columns and error bars represent the means ± SEs (n = 3). The different letters above the bars indicate significant differences between the tissues according to Tukey’s HSD test (one-way ANOVA, p < 0.05). (E) Principal component analysis based on operational taxonomic units (OTUs). x-axis: first principal component; y-axis: second principal component. The numbers after the quotation marks represent the contributions of the principal components to the differences between the samples. Dots represent individual samples, and dots with different colors represent different tissues.

Table 4.
Results of ANOSIM and PERMANOVA based on the Bray–Curtis index and weighted Unifrac distances.
3.3. Abundance of Wolbachia in S. frugiperda
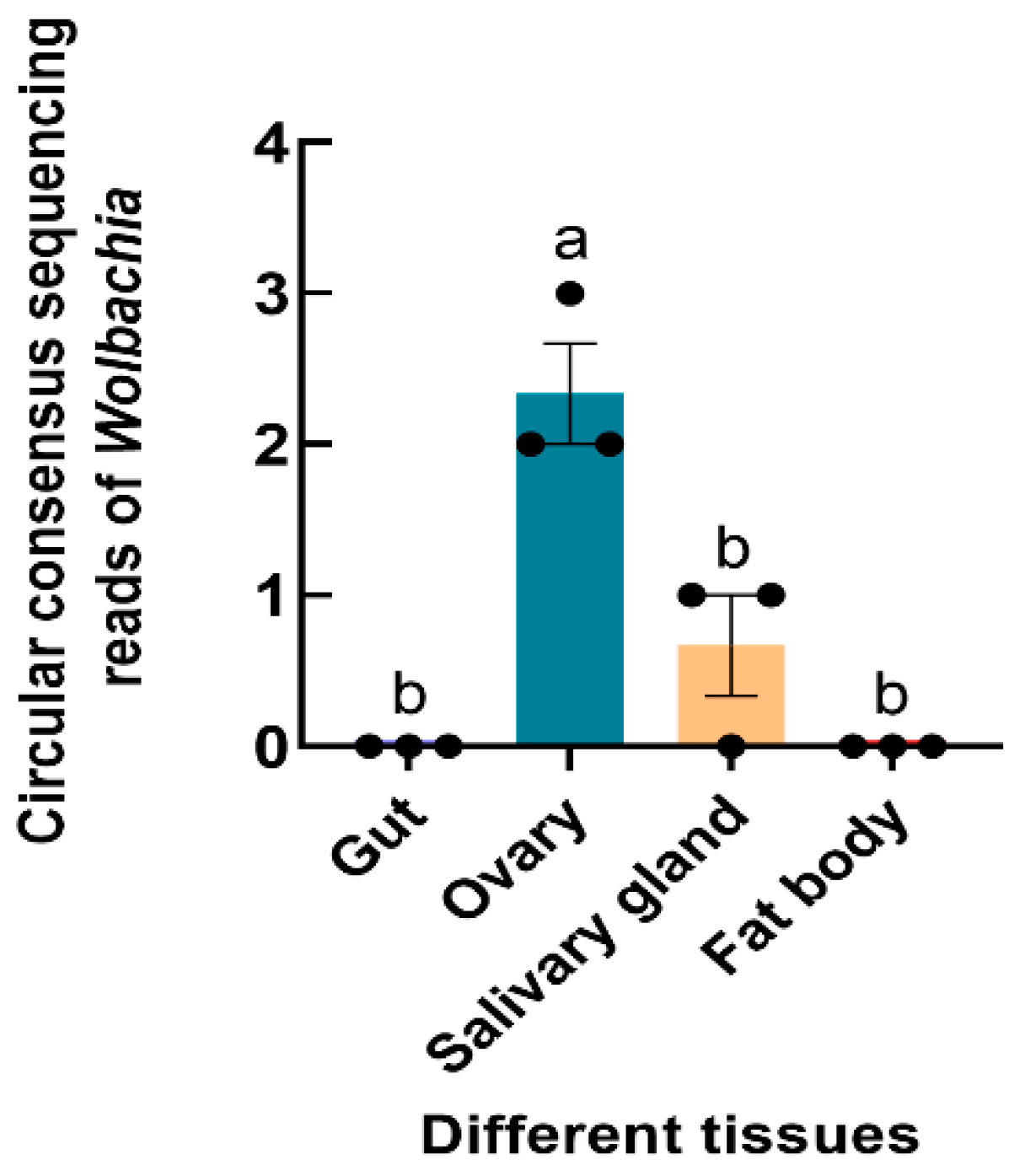
The Wolbachia 16S rRNA gene was successfully amplified from S. frugiperda, and the sequence was submitted to GenBank (accession number OR268559). 16S rRNA high-throughput sequencing revealed that Wolbachia was found in the ovaries and salivary glands of S. frugiperda female adults (F3,8 = 21.833, p < 0.001) (Figure 4).
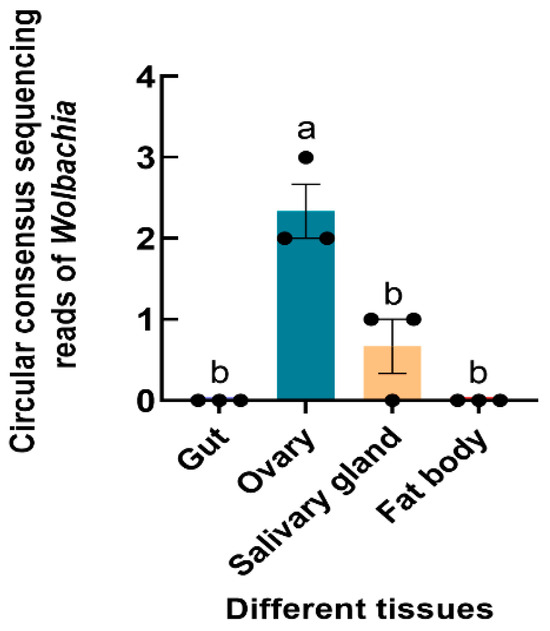
Figure 4.
Relative abundance of Wolbachia in different tissues of S. frugiperda female adults. The columns and error bars represent the means ± SEs (n = 3). The different letters above the bars indicate significant differences between the tissues according to Tukey’s HSD test (one-way ANOVA, p < 0.05).
3.4. Infection and Prevalence of Endosymbionts in S. frugiperda Populations
PCR revealed that two endosymbionts, Wolbachia (wsp gene) and Arsenophonus (16S rRNA gene), were present in S. frugiperda, while Rickettsia (16S rRNA gene), Cardinium (16S rRNA gene), and Spiroplasma (16S rRNA gene) were absent in S. frugiperda (Figure 5A). The infection and prevalence of the endosymbionts varied between the five provinces of Southeast China (including Pu’er, Yunnan; Nanning, Guangxi; Sanya, Hainan; Yunfu, Guangdong; and Nanping, Fujian). Arsenophonus was detected in only the Nanning, Guangxi populations (F5,12 = 410.900, p < 0.001), and Wolbachia was present in the Pu’er, Yunnan; Nanning, Guangxi; and Sanya, Hainan populations (F5,12 = 880.000, p < 0.001; F5,12 = 410.900, p < 0.001; F5,12 = 868.000, p < 0.001), but it was not detected in the Yunfu, Guangdong and Nanping, Fujian populations (Figure 5B–F).
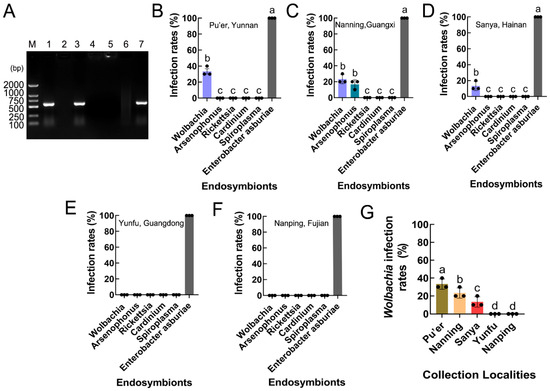
Figure 5.
Infection and prevalence of endosymbionts in S. frugiperda populations. (A) Endosymbiont species. M: DNA marker; lane 1: positive control (Wolbachia wsp); lane 2: negative control (ddH2O); lane 3: Wolbachia (wsp); lane 4: Spiroplasma (16S rDNA); lane 5: Cardinium (16S rRNA); lane 6: Rickettsia (16S rRNA); lane 7: Arsenophonus (16S rRNA). (B–F) The rates of endosymbiont infection of S. frugiperda in Pu’er, Yunnan; Nanning, Guangxi; Sanya, Hainan; Yunfu, Guangdong; and Nanping, Fujian. The columns and error bars represent the means ± SEs (n = 3). The different letters above the bars indicate significant differences between the endosymbionts according to Tukey’s HSD test (one-way ANOVA, p < 0.05). (G) Wolbachia infection rates in different S. frugiperda populations. The columns and error bars represent the means ± SEs (n = 3). The different letters above the bars indicate significant differences between the collection localities according to Tukey’s HSD test (one-way ANOVA, p < 0.05).
3.5. Wolbachia Infection Rates in S. frugiperda Populations
The Wolbachia infection rates varied between the S. frugiperda populations, being approximately 33.33% in the Pu’er, Yunnan population; 23.33% in the Nanning, Guangxi population; and 13.33% in the Sanya, Hainan population (F4,10 = 32.000, p < 0.001) (Figure 5G).
3.6. Phylogenetic Analysis of Wolbachia in S. frugiperda Populations
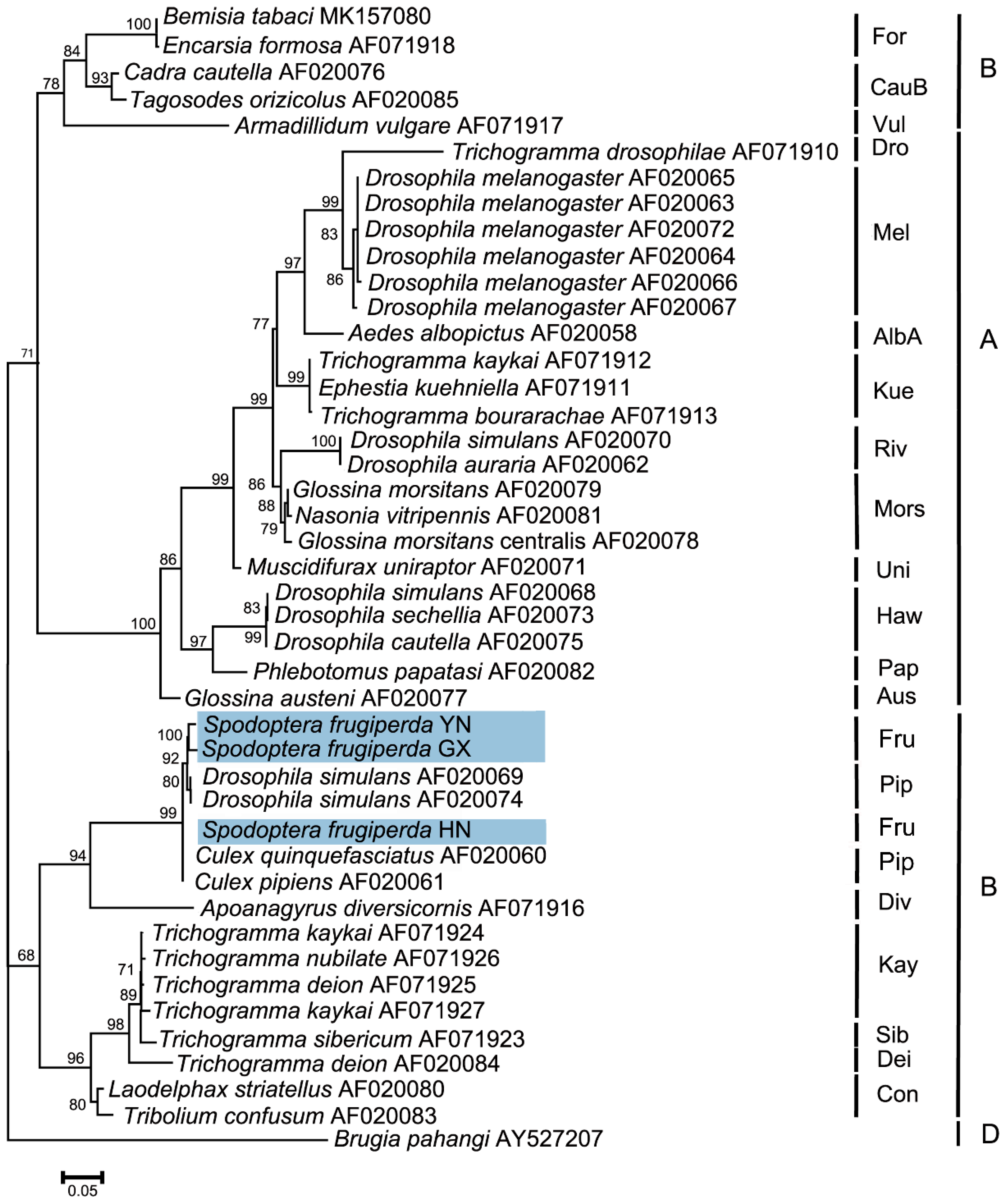
The wsp sequences from different S. frugiperda populations were deposited in the GenBank database (accession numbers OR282569–OR282571). Phylogenetic analyses of Wolbachia based on the wsp gene indicated that the tested Wolbachia strains from three S. frugiperda populations belonged to the supergroup B and were named the wFru strain. In addition, S. frugiperda from the Pu’er, Yunnan and Nanning, Guangxi populations were first clustered into one branch, and then clustered with the Sanya, Hainan population to form one peripheral branch (Figure 6).
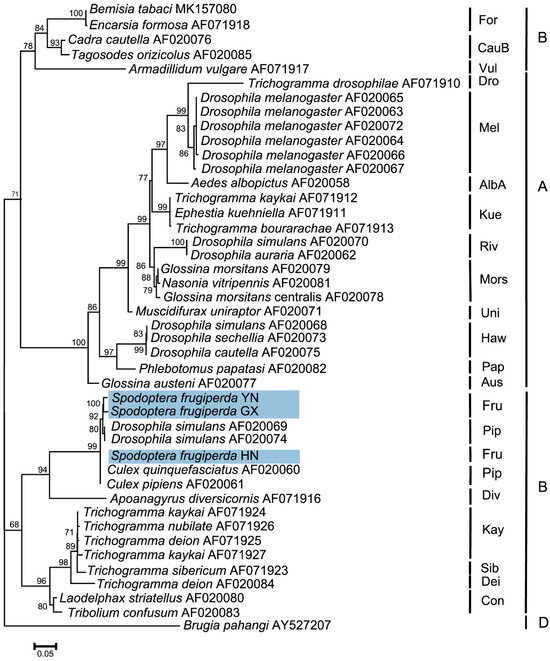
Figure 6.
Phylogenetic analysis of Wolbachia based on the wsp gene in S. frugiperda populations. This phylogenetic tree was constructed and analyzed via the maximum likelihood (ML) method using 1000 bootstrap replicates. The numbers at the nodes indicate the percentages of reliability for each branch of the tree. The branch length is drawn proportionally to the estimated sequence divergence. The blue-shaded parts show the different populations of S. frugiperda. “YN”, “GX”, and “HN” represent Pu’er, Yunnan; Nanning, Guangxi; and Sanya, Hainan, respectively. Brugia pahangi was used as an outgroup.
4. Discussion
Insects harbor many symbiotic bacteria, and these bacteria and their hosts have formed complex symbiotic relationships via coevolution [53,54]. Growing evidence has revealed that manipulating the bacterial communities of insects could be a feasible strategy for reducing the incidence of improper and excessive application of insecticides [55,56]. Thus, in this study, 16S rRNA high-throughput sequencing and polymerase chain reaction (PCR) methods were used to determine the composition, diversity, and potential function of bacterial symbionts, especially Wolbachia, of S. frugiperda.
Lepidopteran insects comprise the second most diverse insect order, with some of the most devastating agricultural pests worldwide [57]. Recently, extensive evidence has shown that developmental stage, diet, host phylogeny, and habitat environmental conditions determine insect bacterial diversity and community structure [17]. However, the composition of the bacterial microbiota is relatively simple in lepidopteran insects, including S. frugiperda [58,59,60]. At the phylum level, Xia et al. [20] reported that Proteobacteria was the dominant taxon (from 70.42% to 97.44% in larvae, 97.44% to 99.58% in pupae, and 99.64% to 100% in adults), followed by Firmicutes (from 2.45% to 29.48% in larvae, 0.42% to 1% in pupae, and 0 to 0.36% in adults), in the P. xylostella gut. Dantur et al. [61] suggested that the bacterial genes of Proteobacteria and Firmicutes can degrade cellulose, hemicellulose, and pectin in Spodoptera litura (Lepidoptera: Noctuidae). Similarly, our study demonstrates that the bacterial communities in different female tissues of S. frugiperda mostly comprised Proteobacteria, Firmicutes, and Bacteroidetes, which are considered constituents of the core microbiota. These findings are consistent with those of some studies describing the microbiota in other lepidopteran insects, such as Lymantria dispar, Helicoverpa armigera, Manduca sexta, Bombyx mori, and Spodoptera exigua [62,63,64,65,66].
In general, at the genus level, the predominant microbiota members include Enterobacter, Enterococcus, Pseudomonas, Bacillus, and Staphylococcus, which are present in more than 70% of the studied lepidopteran insects [17,20,66]. Among these bacteria, Enterococcus and Enterobacter have been implicated in the degradation of the plant cell wall, which can release available nutrients in P. xylostella [67]. In addition, a high abundance of Enterobacter can accelerate the development of resistance to insecticides in P. xylostella [68]. In previous studies, Enterococcus was found to diminish the insecticidal efficacy of Bacillus thuringiensis (Bt) in Lymantria dispar (Lepidoptera: Liparidae) larvae [69]. Similarly, the gut microbiota of Manduca sexta (Lepidoptera: Sphingidae) and P. xylostella was reported to reduce the effectiveness of the Cry1Ac toxin [70]. In line with these findings, our results also showed that the dominant bacterial genera were Enterobacter and Enterococcus in different tissues, which may offer new insights into the chemical resistance mechanism of S. frugiperda in the future. Alpha and beta diversity analyses revealed significant differences in microbes from different tissues of S. frugiperda, and these findings underscore the complexity of microbial ecosystems. In addition, growing evidence has shown that a range of ecological interactions, evolutionary histories, and environmental factors may contribute to differences in insect diversity [71,72].
16S rRNA high-throughput sequencing suggested that the intracellular bacteria endosymbiont Wolbachia was found in the ovaries and salivary glands of S. frugiperda female adults. Moreover, our results are similar to the findings of a previous study by Ou et al. [73], in which Wolbachia was detected in the midgut, salivary glands, testes, and ovaries of Diaphorina citri (Hemiptera: Psyllidae) and Cornegenapsylla sinica (Hemiptera: Psyllidae). To date, approximately 80% of lepidopteran insects are estimated to be infected by Wolbachia [74]. However, the mean infection prevalence (proportion of infection within a population) in many species of lepidoptera is relatively low, at approximately 27% [17]. Some of these studies have revealed that Wolbachia infection rates may be related to geographical distribution [29,41,75]. For instance, Wang et al. [21] reported that Wolbachia infection rates differed between all tested E. grisescens samples collected from eight geographical tea-producing areas (Xinchang, Yuhang, Liyang, Langxi, Nanchang, Enshi, Guiyang, and Guiyang) in China. Zhu et al. [76] reported that 7% of P. xylostella was infected with Wolbachia, but only in individuals from South America, North America, Africa, and Asia, as no infections were detected in populations from Europe and Oceania. Furthermore, different environmental factors strongly influence the Wolbachia titer [77,78]. In the butterfly Zizeeria maha (Lepidoptera: Lycaenidae), the Wolbachia density decreased across several host generations from spring/early summer to autumn [79]. In the present study, we showed that Wolbachia was present in only Pu’er, Yunnan; Nanning, Guangxi; and Sanya, Hainan, with infection rates of 33.33%, 23.33%, and 13.33%, respectively, and no infection was detected in populations from Yunfu, Guangdong and Nanping, Fujian. This difference may be correlated to the host’s genetic variation, diet, temperature, and other abiotic factors [80].
Wolbachia strains are highly diverse among lepidopteran insects, consisting of 90 different strains [81]. For example, Zhu et al. [76] reported three Wolbachia strains infecting P. xylostella, with plutWA1 and plutWA2 belonging to supergroup A and plutWB1 belonging to supergroup B. Sakamoto et al. [82] reported that infection with the Wolbachia strain wFur in Ostrinia scapulalis (Lepidoptera: Crambidae) caused feminization of genetic males. In addition, molecular typing analyses demonstrated that these Wolbachia strains in all of the tested E. grisescens samples were of the same wGri strain, and could strongly induce CI and enhance the fecundity of its female hosts [18]. In our study, phylogenetic analyses based on the wsp gene revealed that all of the Wolbachia strains from different S. frugiperda populations belonged to the supergroup B and were named the wFru strain. Since these were the Wolbachia strains inducing CI in supergroup B [83], this possibility may also occur in the Wolbachia strain wFru of S. frugiperda. Moreover, Wolbachia can also help the host synthesize molecules, which is essential for growth and development, alter host microbial communities, and protect hosts against a variety of pathogens and abiotic stresses [84,85,86]; thus, the functions of the Wolbachia strain wFru in S. frugiperda are worthy of further investigation.
In conclusion, our results revealed that Proteobacteria, Firmicutes, and Bacteroidetes were the three most dominant bacterial phyla in different tissues of S. frugiperda female adults. The dominant bacterial genera were Enterobacter and Enterococcus, which varied in abundance between tissues. In addition, Wolbachia was found in the ovaries and salivary glands of S. frugiperda female adults. Although the infection and abundance of Wolbachia varied among different geographical populations, they all belonged to the supergroup B and were named the wFru strain, which has been considered to potentially induce cytoplasmic incompatibility. Further studies are needed to elucidate the host phenotypes of Wolbachia infection and its usefulness as a biological control agent for S. frugiperda management.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/insects15040217/s1, Table S1: The reference sequences of the Wolbachia wsp genes used in the phylogenetic analysis.
Author Contributions
This article was originally designed by Y.L. and Y.H.; Y.L., L.Z. and X.C. carried out the experiments; B.Q. contributed to the phenotyping and biological data analyses; Y.L. and L.Z. also participated in the data analysis; Y.L., A.R., B.Q. and Y.H. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2022YFC2601400), the project of Guangdong Laboratory for Lingnan Modern Agriculture (NT2021003), the National Key Research and Development Programme of China (2017YFC1200605), the Science and Technology Major Project of Fujian (2017NZ0003-1-6), and the Guangzhou Basic and Applied Basic Research Program, China (202102021290).
Data Availability Statement
The Wolbachia 16S rRNA gene sequence of the S. frugiperda deposit GenBank was OR268559. The Wolbachia wsp gene sequences in the Yunnan, Guangxi, and Hainan S. frugiperda deposit GenBank were OR282569–OR282571.The raw sequencing data were deposited in the NCBI Sequence Read Archive (SRA) database under accession number PRJNA995535.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Barata, R.M.; Zucchi, M.I.; Silva-Filho, M.D.C.; Omoto, C. Molecular variability of Spodoptera frugiperda (Lepidoptera: Noctuidae) populations associated to maize and cotton crops in Brazil. J. Econ. Entomol. 2006, 99, 519–526. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gomez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Xie, M.C.; Li, Y.H.; Yang, J.J.; Zhang, M.L.; Qiu, K. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant Prot. 2019, 45, 10–19. [Google Scholar] [CrossRef]
- Wang, Y.R.; Cai, X.Y.; Wang, J.D.; Tang, B.Z.; Hou, Y.M. Current opinions on the important alien invasive insect, Spodoptera frugiperda. J. Environ. Entomol. 2020, 42, 806–816. [Google Scholar] [CrossRef]
- Wang, Y.R.; Cai, X.Y.; Zhuang, J.X.; Hou, Y.M. Molecular identification of Spodoptera frugiperda invaded in Fujian province. Plant Prot. 2020, 46, 189–193. [Google Scholar] [CrossRef]
- Wang, H.H.; Zhao, R.; Gao, J.; Zhang, L.; Zhang, S.; Liang, P.; Gao, S.W.; Gu, S.H. Genetic architecture and insecticide resistance in Chinese populations of Spodoptera frugiperda. J. Pest Sci. 2022, 96, 1595–1610. [Google Scholar] [CrossRef]
- Banerjee, R.; Hasler, J.; Meagher, R.; Nagoshi, R.; Hietala, L.; Huang, F.N.; Narva, K.; Jurat-Fuentes, J.L. Mechanism and DNA-based detection of field-evolved resistance to transgenic Bt corn in fall armyworm (Spodoptera frugiperda). Sci. Rep. 2017, 7, 10877. [Google Scholar] [CrossRef]
- Botha, A.S.; Erasmus, A.; du Plessis, H.; Van den Berg, J. Efficacy of Bt maize for control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in South Africa. J. Econ. Entomol. 2019, 112, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Crotti, E.; Balloi, A.; Hamdi, C.; Sansonno, L.; Marzorati, M.; Gonella, E.; Favia, G.; Cherif, A.; Bandi, C.; Alma, A.; et al. Microbial symbionts: A resource for the management of insect-related problems. Microb. Biotechnol. 2012, 5, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.K.; Douglas, A.E. Hype or opportunity? using microbial symbionts in novel strategies for insect pest control. J. Insect Physiol. 2017, 103, 10–17. [Google Scholar] [CrossRef]
- Beck, J.J.; Vannette, R.L. Harnessing insect-microbe chemical communications to control insect pests of agricultural systems. J. Agric. Food Chem. 2017, 65, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bourtzis, K.; Miller, T.A. Insect Symbiosis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume II, ISBN 978-0-8493-4194-6. [Google Scholar]
- Smith, D.C. From extracellular to intracellular: The establishment of a symbiosis. Proc. R. Soc. B-Biol. Sci. 1979, 204, 1155. [Google Scholar] [CrossRef]
- Voirol, L.R.P.; Frago, E.; Kaltenpoth, M.; Hilker, M.; Fatouros, N.E. Bacterial symbionts in lepidoptera: Their diversity, transmission, and impact on the host. Front. Microbiol. 2018, 9, 556. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Jiang, R.; Zhang, C.; Gao, T.; Wang, Y.; Liu, C.; Long, Y.; Zhang, Y.; Yang, Y. Wolbachia strain wGri from the tea geometrid moth Ectropis grisescens contributes to its host’s fecundity. Front. Microbiol. 2021, 12, 694466. [Google Scholar] [CrossRef]
- Shao, Y.Q.; Chen, B.S.; Sun, C.; Ishida, K.; Hertweck, C.; Boland, W. Symbiont-derived antimicrobials contribute to the control of the lepidopteran gut microbiota. Cell Chem. Biol. 2017, 24, 66–75. [Google Scholar] [CrossRef]
- Xia, X.F.; Gurr, G.M.; Vasseur, L.; Zheng, D.D.; Zhong, H.Z.; Qin, B.C.; Lin, J.H.; Wang, Y.; Song, F.Q.; Li, Y.; et al. Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front. Microbiol. 2017, 8, 663. [Google Scholar] [CrossRef]
- Wang, Z.B.; Li, H.; Zhou, X.G.; Tang, M.J.; Sun, L.; Zhan, S.; Xiao, Q. Comparative characterization of microbiota between the sibling species of tea geometrid moth Ectropis obliqua Prout and E. grisescens Warren. Bull. Entomol. Res. 2020, 110, 684–693. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, F.; Yun, Y.L.; Zhou, W.H.; Peng, Y. Wolbachia bacteria affect rice striped stem borer (Chilo suppressalis) susceptibility to two insecticides. Bull. Insectol. 2020, 73, 39–44. [Google Scholar]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Windsor, D.; Guo, L.R. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. B-Biol. Sci. 1995, 262, 1364. [Google Scholar] [CrossRef]
- Yen, J.H.; Barr, A.R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 1971, 232, 657–658. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F.; Bouchon, D.; Pintureau, B.; Juchault, P.; Solignac, M. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc. R. Soc. B-Biol. Sci. 1992, 250, 91–98. [Google Scholar] [CrossRef]
- Kageyama, D.; Nishimura, G.; Hoshizaki, S.; Ishikawa, Y. Feminizing Wolbachia in an insect, Ostrinia furnacalis (Lepidoptera: Crambidae). Heredity 2002, 88, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.I.; Wilson, K. Male-killing Wolbachia and mitochondrial selective sweep in a migratory African insect. BMC Evol. Biol. 2013, 13, 6. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Araujo-Jnr, E.V.; Welch, J.J.; Kawahara, A.Y. Wolbachia in butterflies and moths: Geographic structure in infection frequency. Front. Zool. 2015, 12, 16. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The Silva ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B. Package ‘vegan’: Community Ecology Package. R Package Version 2.3-0. Available online: https://github.com/vegandevs/vegan (accessed on 20 June 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. Version 3.1.1. Vienna: R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 15 July 2022).
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). In Wiley Statsref: Statistics Reference Online; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- Duplouy, A.; Hornett, E.A. Uncovering the hidden players in lepidoptera biology: The heritable microbial endosymbionts. Peer J. 2018, 6, e4629. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Ren, S.X.; Xue, X.; Li, X.X.; Jin, G.H.; Qiu, B.L. Prevalence of endosymbionts in Bemisia tabaci populations and their in vivo sensitivity to antibiotics. Curr. Microbiol. 2010, 61, 322–328. [Google Scholar] [CrossRef]
- Shi, P.Q.; Wang, L.; Liu, Y.; An, X.; Chen, X.S.; Ahmed, M.Z.; Qiu, B.L.; Sang, W. Infection dynamics of endosymbionts reveal three novel localization patterns of Rickettsia during the development of whitefly Bemisia tabaci. FEMS Microbiol. Ecol. 2018, 94, fiy165. [Google Scholar] [CrossRef]
- Ren, W.B.; Wei, H.Y.; Yang, Y.; Shao, S.X.; Wu, H.X.; Chen, X.M.; Yang, Z. Molecular detection and phylogenetic analyses of Wolbachia in natural populations of nine galling aphid species. Sci. Rep. 2020, 10, 12025. [Google Scholar] [CrossRef]
- Zhou, W.; Rousset, F.; O’Neil, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. B-Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Thao, M.L.; Baumann, P. Evolutionary relationships of primary prokaryotic endosymbionts of whiteflies and their hosts. Appl. Environ. Microbiol. 2004, 70, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, Y.; Ghanim, M.; Chiel, E.; Gerling, D.; Portnoy, V.; Steinberg, S.; Tzuri, G.; Horowitz, R.; Belausov, E.; Mozes-Daube, N.; et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl. Environ. Microbiol. 2006, 72, 3646–3652. [Google Scholar] [CrossRef] [PubMed]
- Weeks, A.R.; Velten, R.; Stouthamer, R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc. R. Soc. B-Biol. Sci. 2003, 270, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Herren, J.K.; Gordon, I.; Holland, P.W.H.; Smith, D. The butterfly Danaus chrysippus (Lepidoptera: Nymphalidae) in Kenya is variably infected with respect to genotype and body size by a maternally transmitted male-killing endosymbiont (Spiroplasma). Int. J. Trop. Insect Sci. 2007, 27, 62–69. [Google Scholar] [CrossRef]
- Ledbetter, R.N.; Connon, S.A.; Neal, A.L.; Dohnalkova, A.; Magnuson, T.S. Biogenic mineral production by a novel arsenic-metabolizing thermophilic bacterium from the Alvord Basin, Oregon. Appl. Environ. Microbiol. 2007, 73, 5928–5936. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. Partitionfinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Tang, X.S.; Freitak, D.; Vogel, H.; Ping, L.Y.; Shao, Y.Q.; Cordero, E.A.; Andersen, G.; Westermann, M.; Heckel, D.G.; Boland, W. Complexity and variability of gut commensal microbiota in polyphagous lepidopteran larvae. PLoS ONE 2012, 7, e36978. [Google Scholar] [CrossRef]
- Xia, X.F.; Zheng, D.D.; Zhong, H.Z.; Qin, B.C.; Gurr, G.M.; Vasseur, L.; Lin, H.L.; Bai, J.L.; He, W.Y.; You, M.S. DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS ONE 2013, 8, e68852. [Google Scholar] [CrossRef]
- de Almeida, L.G.; de Moraes, L.A.B.; Trigo, J.R.; Omoto, C.; Cnsoli, F.L. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation. PLoS ONE 2017, 12, e0174754. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Flores, A.A.; Valadez-Lira, J.A.; Oppert, B.; Gomez-Flores, R.; Tamez-Guerra, R.; Rodríguez-Padilla, C.; Tamez-Guerra, P. Regulation by gut bacteria of immune response, Bacillus thuringiensis susceptibility and hemolin expression in Plodia interpunctella. J. Insect Physiol. 2017, 98, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Sree, K.S.; Varma, A. Biocontrol of Lepidopteran Pests: Use of Soil Microbes and Their Metabolites; CRC Press: Cham, Switzerland, 2015; Volume 43, ISBN 978-3-319-14498-6. [Google Scholar]
- Zaspel, J.M.; Hoy, M.A. Microbial diversity associated with the fruit-piercing and blood-feeding moth Calyptra thalictri (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 2008, 101, 1050–1055. [Google Scholar] [CrossRef]
- Robinson, C.J.; Schloss, P.; Ramos, Y.; Raffa, K.; Handelsman, J. Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microb. Ecol. 2010, 59, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Mcmillan, W.O.; Fierer, N. Metamorphosis of a butterfly-associated bacterial community. PLoS ONE 2014, 9, e86995. [Google Scholar] [CrossRef] [PubMed]
- Dantur, K.I.; Enrique, R.; Welin, B.; Castagnaro, A.P. Isolation of cellulolytic bacteria from the intestine of Diatraea saccharalis larvae and evaluation of their capacity to degrade sugarcane biomass. AMB Express 2015, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Wei, G.F.; Jia, S.H.; Huang, J.H.; Miao, X.X.; Zhou, Z.H.; Zhao, L.P.; Huang, Y.P. Microbial communities in the larval midgut of laboratory and field populations of cotton bollworm (Helicoverpa armigera). Can. J. Microbiol. 2007, 52, 1085–1092. [Google Scholar] [CrossRef]
- Brinkmann, N.; Martens, R.; Tebbe, C.C. Origin and diversity of metabolically active gut bacteria from laboratory-bred larvae of Manduca sexta (Sphingidae, Lepidoptera, Insecta). Appl. Environ. Microbiol. 2008, 74, 7189–7196. [Google Scholar] [CrossRef]
- Mason, C.J.; Raffa, K.F. Acquisition and structuring of midgut bacterial communities in gypsy moth (Lepidoptera: Erebidae) larvae. Environ. Entomol. 2014, 43, 595–604. [Google Scholar] [CrossRef]
- Chen, B.S.; Du, K.Q.; Sun, C.; Vimalanathan, A.; Liang, X.L.; Li, Y.; Wang, B.H.; Lu, X.M.; Li, L.J.; Shao, Y.Q. Gut bacterial and fungal communities of the domesticated silkworm (Bombyx mori) and wild mulberry-feeding relatives. ISME J. 2018, 12, 2252–2262. [Google Scholar] [CrossRef]
- Gao, X.; Li, W.; Luo, J.; Zhang, L.; Ji, J.; Zhu, X.; Wang, L.; Zhang, S.; Cui, J. Biodiversity of the microbiota in Spodoptera exigua (Lepidoptera: Noctuidae). J. Appl. Microbiol. 2018, 126, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Xing, D.F.; Wagner, R.; Regan, J.M.; Richard, T.L.; Logan, B.E. Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell. Appl. Environ. Microbiol. 2009, 75, 3673–3678. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.F.; Sun, B.T.; Gurr, G.M.; Vasseur, L.; Xue, M.Q.; You, M.S. Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Raff, K.F.; Goodman, R.M.; Handelsman, J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl. Environ. Microbiol. 2004, 70, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.R.; Crickmore, N. Gut bacteria are not required for the insecticidal activity of bacillus thuringiensis toward the tobacco Hornworm, Manduca sexta. Appl. Environ. Microbiol. 2009, 75, 5094–5099. [Google Scholar] [CrossRef] [PubMed]
- Cornwallis, C.K.; van’t Padje, A.; Ellers, J.; Klein, M.; Jackson, R.; Kiers, E.T.; West, S.A.; Henry, L.M. Symbioses shape feeding niches and diversification across insects. Nat. Ecol. Evol. 2023, 7, 1022–1044. [Google Scholar] [CrossRef]
- Lange, C.; Boyer, S.; Bezemer, T.M.; Lefort, M.C.; Dhami, M.K.; Biggs, E.; Groenteman, R.; Fowler, S.V.; Paynter, Q.; Mogena, A.M.V.; et al. Impact of intraspecific variation in insect microbiomes on host phenotype and evolution. ISME J. 2023, 17, 1798–1807. [Google Scholar] [CrossRef]
- Ou, D.; Qiu, J.H.; Su, Z.Q.; Wang, L.; Qiu, B.L. The phylogeny and distribution of Wolbachia in two pathogen vector insects, Asian citrus psyllid and Longan psyllid. Front. Cell. Infect. Microbiol. 2023, 13, 1121186. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Wang, Z.P.; Chen, L.M.; Wang, Y.R.; Hou, Y.M. Research progress of endosymbionts in lepidopteran insects. Acta Entomol. Sin. 2021, 64, 1465–1477. [Google Scholar] [CrossRef]
- Charlat, S.; Hornett, E.A.; Dyson, E.A.; Ho, P.P.Y.; Loc, N.T.; Schilthuizen, M.; Davies, N.; Roderick, G.K.; Hurst, G.D.D. Prevalence and penetrance variation of male-killing Wolbachia across Indo-Pacific populations of the butterfly Hypolimnas bolina. Mol. Ecol. 2005, 14, 3525–3530. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Liu, T.S.; He, A.; Zhang, L.; Li, J.Y.; Li, T.P.; Miao, X.; You, M.S.; You, S.J. Diversity of Wolbachia infection and its influence on mitochondrial DNA variation in the diamondback moth, Plutella xylostella. Mol. Phylogenet. Evol. 2023, 182, 107751. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R.; Bordenstein, S.R. Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia, and cytoplasmic incompatibility. PLoS ONE 2011, 6, e29106. [Google Scholar] [CrossRef] [PubMed]
- Hague, M.T.J.; Mavengere, H.; Matute, D.R.; Cooper, B.S. Environmental and genetic contributions to imperfect wMel-like Wolbachia transmission and frequency variation. Genetics 2020, 215, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Sumi, T.; Miura, K.; Miyatake, T. Wolbachia density changes seasonally amongst populations of the pale grass blue butterfly, Zizeeria maha (Lepidoptera: Lycaenidae). PLoS ONE 2017, 12, e0175373. [Google Scholar] [CrossRef]
- Toju, H.; Fukatsu, T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: Relevance of local climate and host plants. Mol. Ecol. 2011, 20, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Breinholt, J.W.; Kawahara, A.Y. Evidence for common horizontal transmission of Wolbachia among butterflies and moths. BMC Evol. Biol. 2016, 16, 118. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kageyama, D.; Hoshizaki, S.; Ishikawa, Y. Sex-specific death in the Asian corn borer moth (Ostrinia furnacalis) infected with Wolbachia occurs across larval development. Genome 2007, 50, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Sicard, M.; Namias, A.; Perriat-Sanguinet, M.; Carron, E.; Unal, S.; Altinli, M.; Landmann, F.; Weill, M. Cytoplasmic incompatibility variations in relation with Wolbachia cid genes divergence in Culex pipiens. mBio 2021, 12, e02797-20. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.J.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Duan, X.Z.; Sun, J.T.; Wang, L.T.; Shu, X.H.; Guo, Y.; Keiichiro, M.; Keiichiro, M.; Zhu, Y.X.; Bing, X.L.; Hoffmann, A.A.; et al. Recent infection by Wolbachia alters microbial communities in wild Laodelphax striatellus populations. Microbiome 2020, 8, 104. [Google Scholar] [CrossRef]
- Ju, J.F.; Bing, X.L.; Zhao, D.S.; Guo, Y.; Xi, Z.Y.; Hoffmann, A.A.; Zhang, K.J.; Huang, H.J.; Gong, J.T.; Zhang, X.; et al. Wolbachia supplement biotin and riboflavin to enhance reproduction in planthoppers. ISME J. 2020, 14, 676–687. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).