The Diversity of Wolbachia and Other Bacterial Symbionts in Spodoptera frugiperda
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Plant Growth
2.2. PCR Amplification, Library Preparation, and Sequencing
2.3. Microbiome Diversity Analysis
2.4. PCR Detection of Endosymbionts from Different S. frugiperda Populations
2.5. Phylogenetic Analysis of Wolbachia in S. frugiperda
2.6. Statistical Analyses
3. Results
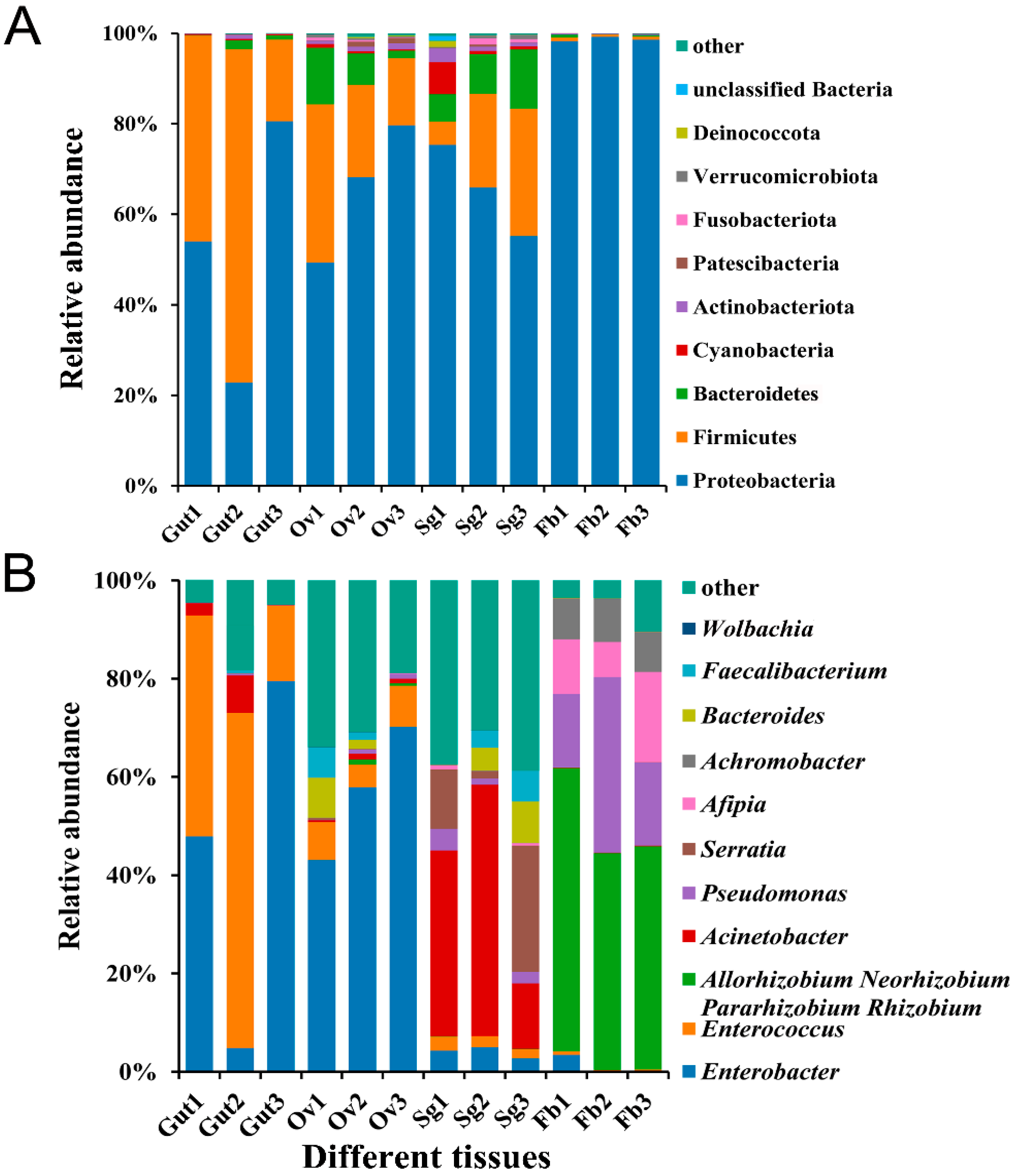
3.1. Bacterial Abundance in Different Tissues of S. frugiperda
3.2. Bacterial Diversity and Community Structure in Different Tissues of S. frugiperda
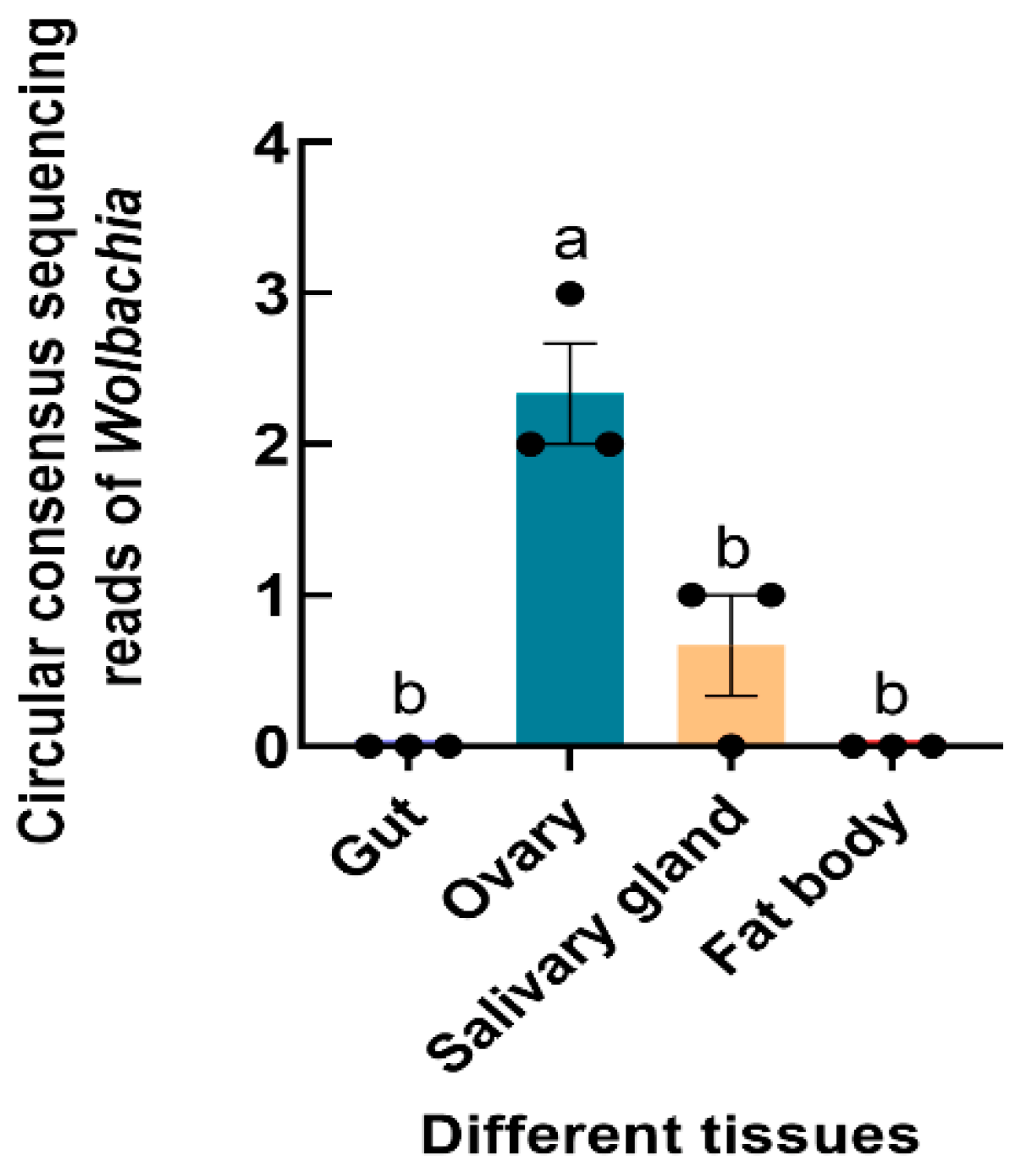
3.3. Abundance of Wolbachia in S. frugiperda
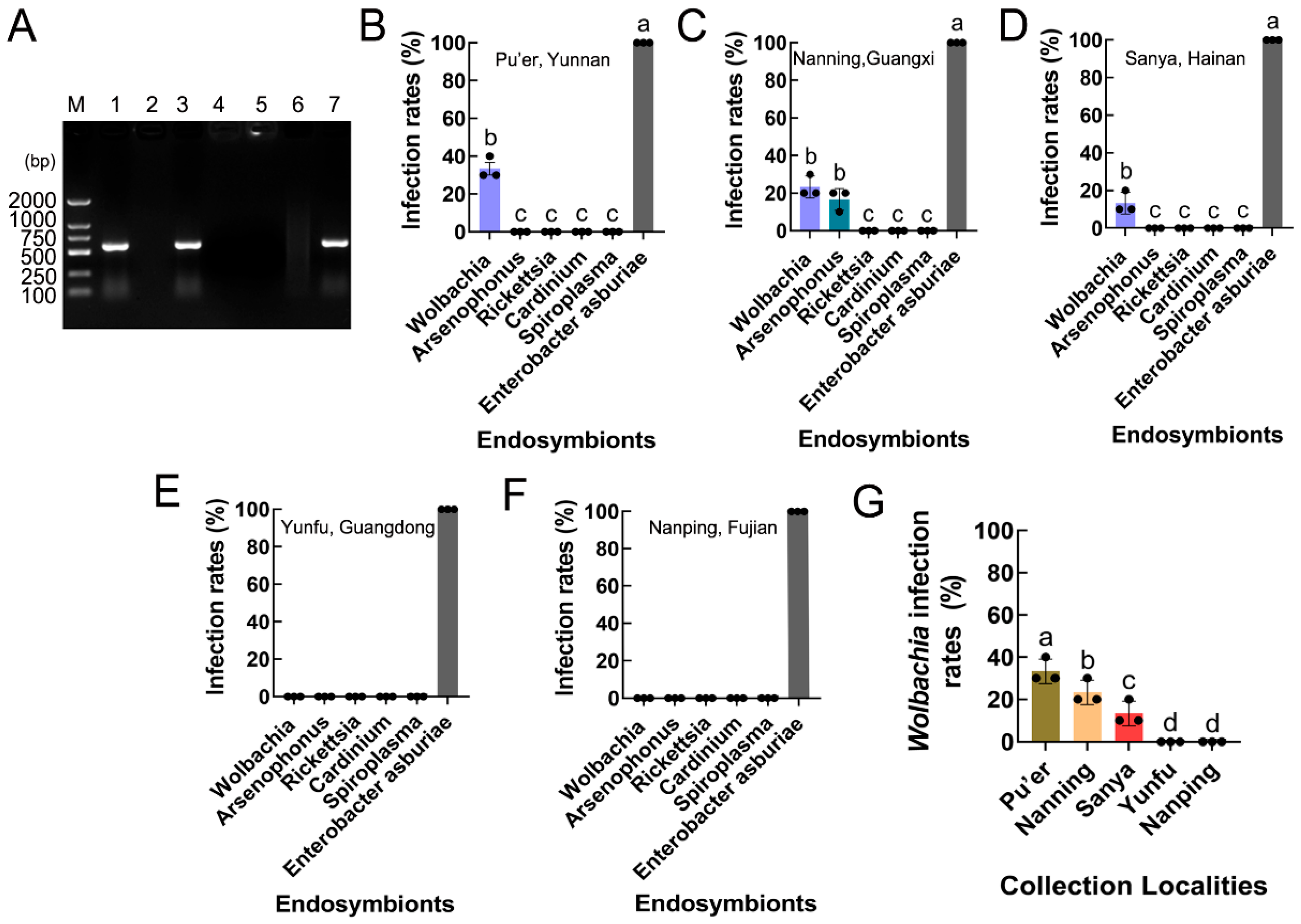
3.4. Infection and Prevalence of Endosymbionts in S. frugiperda Populations
3.5. Wolbachia Infection Rates in S. frugiperda Populations
3.6. Phylogenetic Analysis of Wolbachia in S. frugiperda Populations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Barata, R.M.; Zucchi, M.I.; Silva-Filho, M.D.C.; Omoto, C. Molecular variability of Spodoptera frugiperda (Lepidoptera: Noctuidae) populations associated to maize and cotton crops in Brazil. J. Econ. Entomol. 2006, 99, 519–526. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gomez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Xie, M.C.; Li, Y.H.; Yang, J.J.; Zhang, M.L.; Qiu, K. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant Prot. 2019, 45, 10–19. [Google Scholar] [CrossRef]
- Wang, Y.R.; Cai, X.Y.; Wang, J.D.; Tang, B.Z.; Hou, Y.M. Current opinions on the important alien invasive insect, Spodoptera frugiperda. J. Environ. Entomol. 2020, 42, 806–816. [Google Scholar] [CrossRef]
- Wang, Y.R.; Cai, X.Y.; Zhuang, J.X.; Hou, Y.M. Molecular identification of Spodoptera frugiperda invaded in Fujian province. Plant Prot. 2020, 46, 189–193. [Google Scholar] [CrossRef]
- Wang, H.H.; Zhao, R.; Gao, J.; Zhang, L.; Zhang, S.; Liang, P.; Gao, S.W.; Gu, S.H. Genetic architecture and insecticide resistance in Chinese populations of Spodoptera frugiperda. J. Pest Sci. 2022, 96, 1595–1610. [Google Scholar] [CrossRef]
- Banerjee, R.; Hasler, J.; Meagher, R.; Nagoshi, R.; Hietala, L.; Huang, F.N.; Narva, K.; Jurat-Fuentes, J.L. Mechanism and DNA-based detection of field-evolved resistance to transgenic Bt corn in fall armyworm (Spodoptera frugiperda). Sci. Rep. 2017, 7, 10877. [Google Scholar] [CrossRef]
- Botha, A.S.; Erasmus, A.; du Plessis, H.; Van den Berg, J. Efficacy of Bt maize for control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in South Africa. J. Econ. Entomol. 2019, 112, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Crotti, E.; Balloi, A.; Hamdi, C.; Sansonno, L.; Marzorati, M.; Gonella, E.; Favia, G.; Cherif, A.; Bandi, C.; Alma, A.; et al. Microbial symbionts: A resource for the management of insect-related problems. Microb. Biotechnol. 2012, 5, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.K.; Douglas, A.E. Hype or opportunity? using microbial symbionts in novel strategies for insect pest control. J. Insect Physiol. 2017, 103, 10–17. [Google Scholar] [CrossRef]
- Beck, J.J.; Vannette, R.L. Harnessing insect-microbe chemical communications to control insect pests of agricultural systems. J. Agric. Food Chem. 2017, 65, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bourtzis, K.; Miller, T.A. Insect Symbiosis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume II, ISBN 978-0-8493-4194-6. [Google Scholar]
- Smith, D.C. From extracellular to intracellular: The establishment of a symbiosis. Proc. R. Soc. B-Biol. Sci. 1979, 204, 1155. [Google Scholar] [CrossRef]
- Voirol, L.R.P.; Frago, E.; Kaltenpoth, M.; Hilker, M.; Fatouros, N.E. Bacterial symbionts in lepidoptera: Their diversity, transmission, and impact on the host. Front. Microbiol. 2018, 9, 556. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Jiang, R.; Zhang, C.; Gao, T.; Wang, Y.; Liu, C.; Long, Y.; Zhang, Y.; Yang, Y. Wolbachia strain wGri from the tea geometrid moth Ectropis grisescens contributes to its host’s fecundity. Front. Microbiol. 2021, 12, 694466. [Google Scholar] [CrossRef]
- Shao, Y.Q.; Chen, B.S.; Sun, C.; Ishida, K.; Hertweck, C.; Boland, W. Symbiont-derived antimicrobials contribute to the control of the lepidopteran gut microbiota. Cell Chem. Biol. 2017, 24, 66–75. [Google Scholar] [CrossRef]
- Xia, X.F.; Gurr, G.M.; Vasseur, L.; Zheng, D.D.; Zhong, H.Z.; Qin, B.C.; Lin, J.H.; Wang, Y.; Song, F.Q.; Li, Y.; et al. Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front. Microbiol. 2017, 8, 663. [Google Scholar] [CrossRef]
- Wang, Z.B.; Li, H.; Zhou, X.G.; Tang, M.J.; Sun, L.; Zhan, S.; Xiao, Q. Comparative characterization of microbiota between the sibling species of tea geometrid moth Ectropis obliqua Prout and E. grisescens Warren. Bull. Entomol. Res. 2020, 110, 684–693. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, F.; Yun, Y.L.; Zhou, W.H.; Peng, Y. Wolbachia bacteria affect rice striped stem borer (Chilo suppressalis) susceptibility to two insecticides. Bull. Insectol. 2020, 73, 39–44. [Google Scholar]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Windsor, D.; Guo, L.R. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. B-Biol. Sci. 1995, 262, 1364. [Google Scholar] [CrossRef]
- Yen, J.H.; Barr, A.R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 1971, 232, 657–658. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F.; Bouchon, D.; Pintureau, B.; Juchault, P.; Solignac, M. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc. R. Soc. B-Biol. Sci. 1992, 250, 91–98. [Google Scholar] [CrossRef]
- Kageyama, D.; Nishimura, G.; Hoshizaki, S.; Ishikawa, Y. Feminizing Wolbachia in an insect, Ostrinia furnacalis (Lepidoptera: Crambidae). Heredity 2002, 88, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.I.; Wilson, K. Male-killing Wolbachia and mitochondrial selective sweep in a migratory African insect. BMC Evol. Biol. 2013, 13, 6. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Araujo-Jnr, E.V.; Welch, J.J.; Kawahara, A.Y. Wolbachia in butterflies and moths: Geographic structure in infection frequency. Front. Zool. 2015, 12, 16. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The Silva ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B. Package ‘vegan’: Community Ecology Package. R Package Version 2.3-0. Available online: https://github.com/vegandevs/vegan (accessed on 20 June 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. Version 3.1.1. Vienna: R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 15 July 2022).
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). In Wiley Statsref: Statistics Reference Online; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- Duplouy, A.; Hornett, E.A. Uncovering the hidden players in lepidoptera biology: The heritable microbial endosymbionts. Peer J. 2018, 6, e4629. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Ren, S.X.; Xue, X.; Li, X.X.; Jin, G.H.; Qiu, B.L. Prevalence of endosymbionts in Bemisia tabaci populations and their in vivo sensitivity to antibiotics. Curr. Microbiol. 2010, 61, 322–328. [Google Scholar] [CrossRef]
- Shi, P.Q.; Wang, L.; Liu, Y.; An, X.; Chen, X.S.; Ahmed, M.Z.; Qiu, B.L.; Sang, W. Infection dynamics of endosymbionts reveal three novel localization patterns of Rickettsia during the development of whitefly Bemisia tabaci. FEMS Microbiol. Ecol. 2018, 94, fiy165. [Google Scholar] [CrossRef]
- Ren, W.B.; Wei, H.Y.; Yang, Y.; Shao, S.X.; Wu, H.X.; Chen, X.M.; Yang, Z. Molecular detection and phylogenetic analyses of Wolbachia in natural populations of nine galling aphid species. Sci. Rep. 2020, 10, 12025. [Google Scholar] [CrossRef]
- Zhou, W.; Rousset, F.; O’Neil, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. B-Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Thao, M.L.; Baumann, P. Evolutionary relationships of primary prokaryotic endosymbionts of whiteflies and their hosts. Appl. Environ. Microbiol. 2004, 70, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, Y.; Ghanim, M.; Chiel, E.; Gerling, D.; Portnoy, V.; Steinberg, S.; Tzuri, G.; Horowitz, R.; Belausov, E.; Mozes-Daube, N.; et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl. Environ. Microbiol. 2006, 72, 3646–3652. [Google Scholar] [CrossRef] [PubMed]
- Weeks, A.R.; Velten, R.; Stouthamer, R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc. R. Soc. B-Biol. Sci. 2003, 270, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Herren, J.K.; Gordon, I.; Holland, P.W.H.; Smith, D. The butterfly Danaus chrysippus (Lepidoptera: Nymphalidae) in Kenya is variably infected with respect to genotype and body size by a maternally transmitted male-killing endosymbiont (Spiroplasma). Int. J. Trop. Insect Sci. 2007, 27, 62–69. [Google Scholar] [CrossRef]
- Ledbetter, R.N.; Connon, S.A.; Neal, A.L.; Dohnalkova, A.; Magnuson, T.S. Biogenic mineral production by a novel arsenic-metabolizing thermophilic bacterium from the Alvord Basin, Oregon. Appl. Environ. Microbiol. 2007, 73, 5928–5936. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. Partitionfinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Tang, X.S.; Freitak, D.; Vogel, H.; Ping, L.Y.; Shao, Y.Q.; Cordero, E.A.; Andersen, G.; Westermann, M.; Heckel, D.G.; Boland, W. Complexity and variability of gut commensal microbiota in polyphagous lepidopteran larvae. PLoS ONE 2012, 7, e36978. [Google Scholar] [CrossRef]
- Xia, X.F.; Zheng, D.D.; Zhong, H.Z.; Qin, B.C.; Gurr, G.M.; Vasseur, L.; Lin, H.L.; Bai, J.L.; He, W.Y.; You, M.S. DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS ONE 2013, 8, e68852. [Google Scholar] [CrossRef]
- de Almeida, L.G.; de Moraes, L.A.B.; Trigo, J.R.; Omoto, C.; Cnsoli, F.L. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation. PLoS ONE 2017, 12, e0174754. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Flores, A.A.; Valadez-Lira, J.A.; Oppert, B.; Gomez-Flores, R.; Tamez-Guerra, R.; Rodríguez-Padilla, C.; Tamez-Guerra, P. Regulation by gut bacteria of immune response, Bacillus thuringiensis susceptibility and hemolin expression in Plodia interpunctella. J. Insect Physiol. 2017, 98, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Sree, K.S.; Varma, A. Biocontrol of Lepidopteran Pests: Use of Soil Microbes and Their Metabolites; CRC Press: Cham, Switzerland, 2015; Volume 43, ISBN 978-3-319-14498-6. [Google Scholar]
- Zaspel, J.M.; Hoy, M.A. Microbial diversity associated with the fruit-piercing and blood-feeding moth Calyptra thalictri (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 2008, 101, 1050–1055. [Google Scholar] [CrossRef]
- Robinson, C.J.; Schloss, P.; Ramos, Y.; Raffa, K.; Handelsman, J. Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microb. Ecol. 2010, 59, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Mcmillan, W.O.; Fierer, N. Metamorphosis of a butterfly-associated bacterial community. PLoS ONE 2014, 9, e86995. [Google Scholar] [CrossRef] [PubMed]
- Dantur, K.I.; Enrique, R.; Welin, B.; Castagnaro, A.P. Isolation of cellulolytic bacteria from the intestine of Diatraea saccharalis larvae and evaluation of their capacity to degrade sugarcane biomass. AMB Express 2015, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Wei, G.F.; Jia, S.H.; Huang, J.H.; Miao, X.X.; Zhou, Z.H.; Zhao, L.P.; Huang, Y.P. Microbial communities in the larval midgut of laboratory and field populations of cotton bollworm (Helicoverpa armigera). Can. J. Microbiol. 2007, 52, 1085–1092. [Google Scholar] [CrossRef]
- Brinkmann, N.; Martens, R.; Tebbe, C.C. Origin and diversity of metabolically active gut bacteria from laboratory-bred larvae of Manduca sexta (Sphingidae, Lepidoptera, Insecta). Appl. Environ. Microbiol. 2008, 74, 7189–7196. [Google Scholar] [CrossRef]
- Mason, C.J.; Raffa, K.F. Acquisition and structuring of midgut bacterial communities in gypsy moth (Lepidoptera: Erebidae) larvae. Environ. Entomol. 2014, 43, 595–604. [Google Scholar] [CrossRef]
- Chen, B.S.; Du, K.Q.; Sun, C.; Vimalanathan, A.; Liang, X.L.; Li, Y.; Wang, B.H.; Lu, X.M.; Li, L.J.; Shao, Y.Q. Gut bacterial and fungal communities of the domesticated silkworm (Bombyx mori) and wild mulberry-feeding relatives. ISME J. 2018, 12, 2252–2262. [Google Scholar] [CrossRef]
- Gao, X.; Li, W.; Luo, J.; Zhang, L.; Ji, J.; Zhu, X.; Wang, L.; Zhang, S.; Cui, J. Biodiversity of the microbiota in Spodoptera exigua (Lepidoptera: Noctuidae). J. Appl. Microbiol. 2018, 126, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Xing, D.F.; Wagner, R.; Regan, J.M.; Richard, T.L.; Logan, B.E. Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell. Appl. Environ. Microbiol. 2009, 75, 3673–3678. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.F.; Sun, B.T.; Gurr, G.M.; Vasseur, L.; Xue, M.Q.; You, M.S. Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Raff, K.F.; Goodman, R.M.; Handelsman, J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl. Environ. Microbiol. 2004, 70, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.R.; Crickmore, N. Gut bacteria are not required for the insecticidal activity of bacillus thuringiensis toward the tobacco Hornworm, Manduca sexta. Appl. Environ. Microbiol. 2009, 75, 5094–5099. [Google Scholar] [CrossRef] [PubMed]
- Cornwallis, C.K.; van’t Padje, A.; Ellers, J.; Klein, M.; Jackson, R.; Kiers, E.T.; West, S.A.; Henry, L.M. Symbioses shape feeding niches and diversification across insects. Nat. Ecol. Evol. 2023, 7, 1022–1044. [Google Scholar] [CrossRef]
- Lange, C.; Boyer, S.; Bezemer, T.M.; Lefort, M.C.; Dhami, M.K.; Biggs, E.; Groenteman, R.; Fowler, S.V.; Paynter, Q.; Mogena, A.M.V.; et al. Impact of intraspecific variation in insect microbiomes on host phenotype and evolution. ISME J. 2023, 17, 1798–1807. [Google Scholar] [CrossRef]
- Ou, D.; Qiu, J.H.; Su, Z.Q.; Wang, L.; Qiu, B.L. The phylogeny and distribution of Wolbachia in two pathogen vector insects, Asian citrus psyllid and Longan psyllid. Front. Cell. Infect. Microbiol. 2023, 13, 1121186. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Wang, Z.P.; Chen, L.M.; Wang, Y.R.; Hou, Y.M. Research progress of endosymbionts in lepidopteran insects. Acta Entomol. Sin. 2021, 64, 1465–1477. [Google Scholar] [CrossRef]
- Charlat, S.; Hornett, E.A.; Dyson, E.A.; Ho, P.P.Y.; Loc, N.T.; Schilthuizen, M.; Davies, N.; Roderick, G.K.; Hurst, G.D.D. Prevalence and penetrance variation of male-killing Wolbachia across Indo-Pacific populations of the butterfly Hypolimnas bolina. Mol. Ecol. 2005, 14, 3525–3530. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Liu, T.S.; He, A.; Zhang, L.; Li, J.Y.; Li, T.P.; Miao, X.; You, M.S.; You, S.J. Diversity of Wolbachia infection and its influence on mitochondrial DNA variation in the diamondback moth, Plutella xylostella. Mol. Phylogenet. Evol. 2023, 182, 107751. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R.; Bordenstein, S.R. Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia, and cytoplasmic incompatibility. PLoS ONE 2011, 6, e29106. [Google Scholar] [CrossRef] [PubMed]
- Hague, M.T.J.; Mavengere, H.; Matute, D.R.; Cooper, B.S. Environmental and genetic contributions to imperfect wMel-like Wolbachia transmission and frequency variation. Genetics 2020, 215, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Sumi, T.; Miura, K.; Miyatake, T. Wolbachia density changes seasonally amongst populations of the pale grass blue butterfly, Zizeeria maha (Lepidoptera: Lycaenidae). PLoS ONE 2017, 12, e0175373. [Google Scholar] [CrossRef]
- Toju, H.; Fukatsu, T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: Relevance of local climate and host plants. Mol. Ecol. 2011, 20, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Breinholt, J.W.; Kawahara, A.Y. Evidence for common horizontal transmission of Wolbachia among butterflies and moths. BMC Evol. Biol. 2016, 16, 118. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kageyama, D.; Hoshizaki, S.; Ishikawa, Y. Sex-specific death in the Asian corn borer moth (Ostrinia furnacalis) infected with Wolbachia occurs across larval development. Genome 2007, 50, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Sicard, M.; Namias, A.; Perriat-Sanguinet, M.; Carron, E.; Unal, S.; Altinli, M.; Landmann, F.; Weill, M. Cytoplasmic incompatibility variations in relation with Wolbachia cid genes divergence in Culex pipiens. mBio 2021, 12, e02797-20. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.J.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Duan, X.Z.; Sun, J.T.; Wang, L.T.; Shu, X.H.; Guo, Y.; Keiichiro, M.; Keiichiro, M.; Zhu, Y.X.; Bing, X.L.; Hoffmann, A.A.; et al. Recent infection by Wolbachia alters microbial communities in wild Laodelphax striatellus populations. Microbiome 2020, 8, 104. [Google Scholar] [CrossRef]
- Ju, J.F.; Bing, X.L.; Zhao, D.S.; Guo, Y.; Xi, Z.Y.; Hoffmann, A.A.; Zhang, K.J.; Huang, H.J.; Gong, J.T.; Zhang, X.; et al. Wolbachia supplement biotin and riboflavin to enhance reproduction in planthoppers. ISME J. 2020, 14, 676–687. [Google Scholar] [CrossRef]



| Development Stage | Number of Samples | Host | Collection Date | Collection Location | Latitude | Longitude |
|---|---|---|---|---|---|---|
| Adult | 30 | Corn | 8 July 2021 | Pu’er, Yunnan | 22°50′38″ N | 99°89′05″ E |
| Adult | 30 | Corn | 5 July 2021 | Nanning, Guangxi | 22°60′76″ N | 108°23′55″ E |
| Adult | 30 | Corn | 1 December 2021 | Sanya, Hainan | 18°37′78″ N | 109°15′16″ E |
| Adult | 30 | Corn | 18 August 2021 | Yunfu, Guangdong | 22°72′09″ N | 111°46′46″ E |
| Adult | 30 | Corn | 20 August 2021 | Nanping, Fujian | 26°97′11″ N | 117°73′25″ E |
| Target Gene | Primer Sequence (5′-3′) | Reference |
|---|---|---|
| Wolbachia | Forward: 5′-CTATAGCTGATCTGAGAGGAT-3′ | [44] |
| (16S rRNA) | Reverse: 5′-YGCTTCGAGTGAAACCAATTC-3′ | |
| Wolbachia | Forward: 5′-TGGTCCAATAAGTGATGAAGAAAC-3′ | [45] |
| (wsp) | Reverse: 5′-AAAAATTAAACGCTACTCCA-3′ | |
| Arsenophonus | Forward: 5′-CGTTTGATGAATTCATAGTCAAA-3′’ | [46] |
| (16S rRNA) | Reverse: 5′-GGTCCTCCAGTTAGTGTTACCCAAC-3′ | |
| Rickettsia | Forward: 5′-GCTCAGAACGAACGCTATC-3′ | [47] |
| (16S rRNA) | Reverse: 5′-GAAGGAAAGCATCTCTGC-3′ | |
| Cardinium | Forward: 5′-GCGGTGTAAAATGAGCGTG-3′ | [48] |
| (16S rRNA) | Reverse: 5′-ACCTMTTCTTAACTCAAGCCT-3′ | |
| Spiroplasma | Forward: 5′-GAGAGTTTGATCCTGGCTCAG-3′ | [49] |
| (16S rDNA) | Reverse: 5′-TTCCCTTACAACAGACCTTTACAATCC-3′ | |
| Bacterial | Forward: 5′-AGAGTTTGATCCTGGCTCAG-3′ | [50] |
| (16S rRNA) | Reverse: 5′-GGTTACCTTGTTACGACTT-3′ |
| Sample Name | Clean CCS Readings | Effective CCS Readings | OTU Numbers | Good’s Coverage |
|---|---|---|---|---|
| Gut | 6892.67 ± 473.32 | 6881.00 ± 472.02 | 110.00 ± 35.38 | 0.9935 ± 0.0025 |
| Ovary | 5588.67 ± 398.40 | 5528.00 ± 427.79 | 194.33 ± 33.11 | 0.9916 ± 0.0041 |
| Salivary gland | 6981.00 ± 249.03 | 6828.33 ± 270.84 | 209.33 ± 18.80 | 0.9922 ± 0.0031 |
| Fat body | 7375.00 ± 83.74 | 7274.33 ± 71.42 | 44.33 ± 6.69 | 0.9975 ± 0.0007 |
| Beta Diversity Distance | ANOSIM (R, p) | PERMANOVA (R2, p) |
|---|---|---|
| Bray–Curtis | 0.899, 0.001 | 0.761, 0.001 |
| Weighted Unifrac | 0.787, 0.001 | 0.754, 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, L.; Cai, X.; Rutikanga, A.; Qiu, B.; Hou, Y. The Diversity of Wolbachia and Other Bacterial Symbionts in Spodoptera frugiperda. Insects 2024, 15, 217. https://doi.org/10.3390/insects15040217
Liu Y, Zhang L, Cai X, Rutikanga A, Qiu B, Hou Y. The Diversity of Wolbachia and Other Bacterial Symbionts in Spodoptera frugiperda. Insects. 2024; 15(4):217. https://doi.org/10.3390/insects15040217
Chicago/Turabian StyleLiu, Yuan, Lina Zhang, Xiangyun Cai, Alexandre Rutikanga, Baoli Qiu, and Youming Hou. 2024. "The Diversity of Wolbachia and Other Bacterial Symbionts in Spodoptera frugiperda" Insects 15, no. 4: 217. https://doi.org/10.3390/insects15040217





