Maternal Care Behavior and Its Consequences in Competition
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
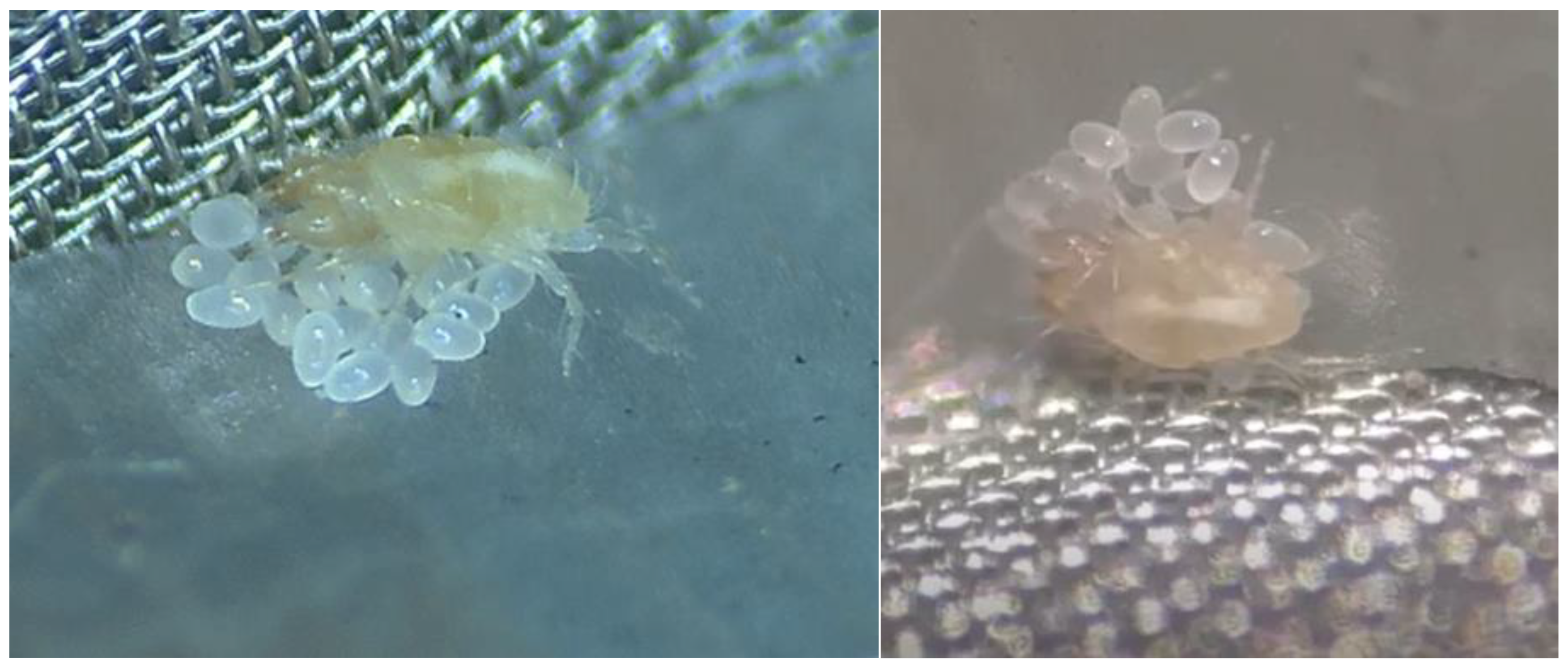
2.1. Mites Colony
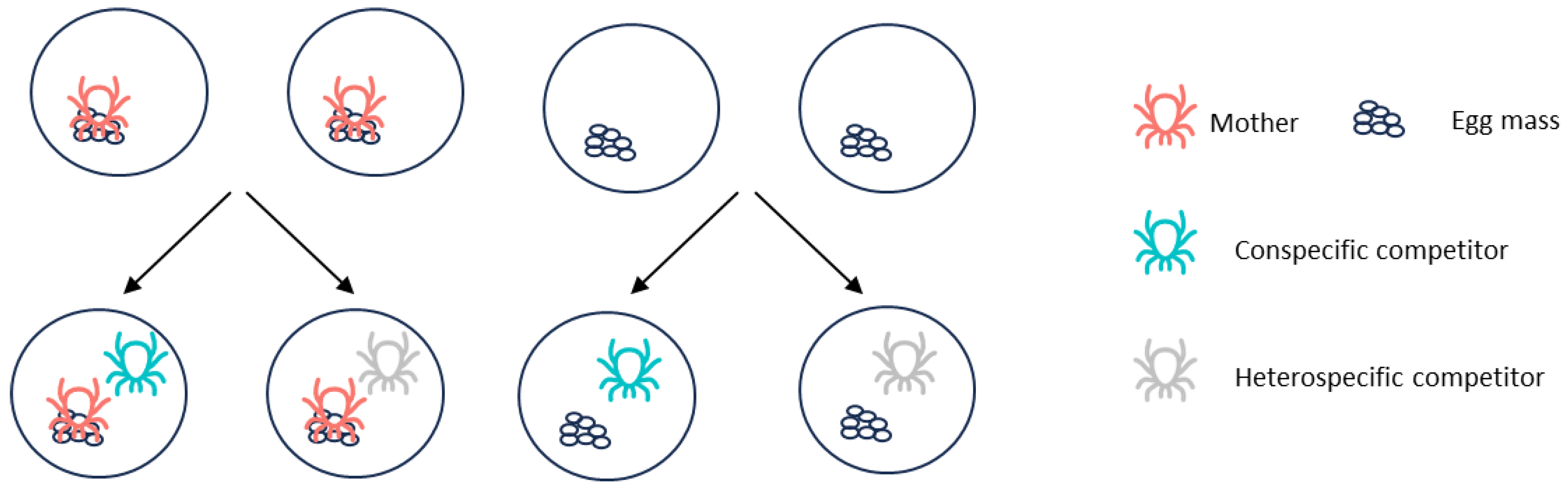
2.2. Experimental Cells
2.3. Maternal Behavior Observation
2.4. Influence of Maternal Care on Offspring and Competitors
2.5. Data Analysis
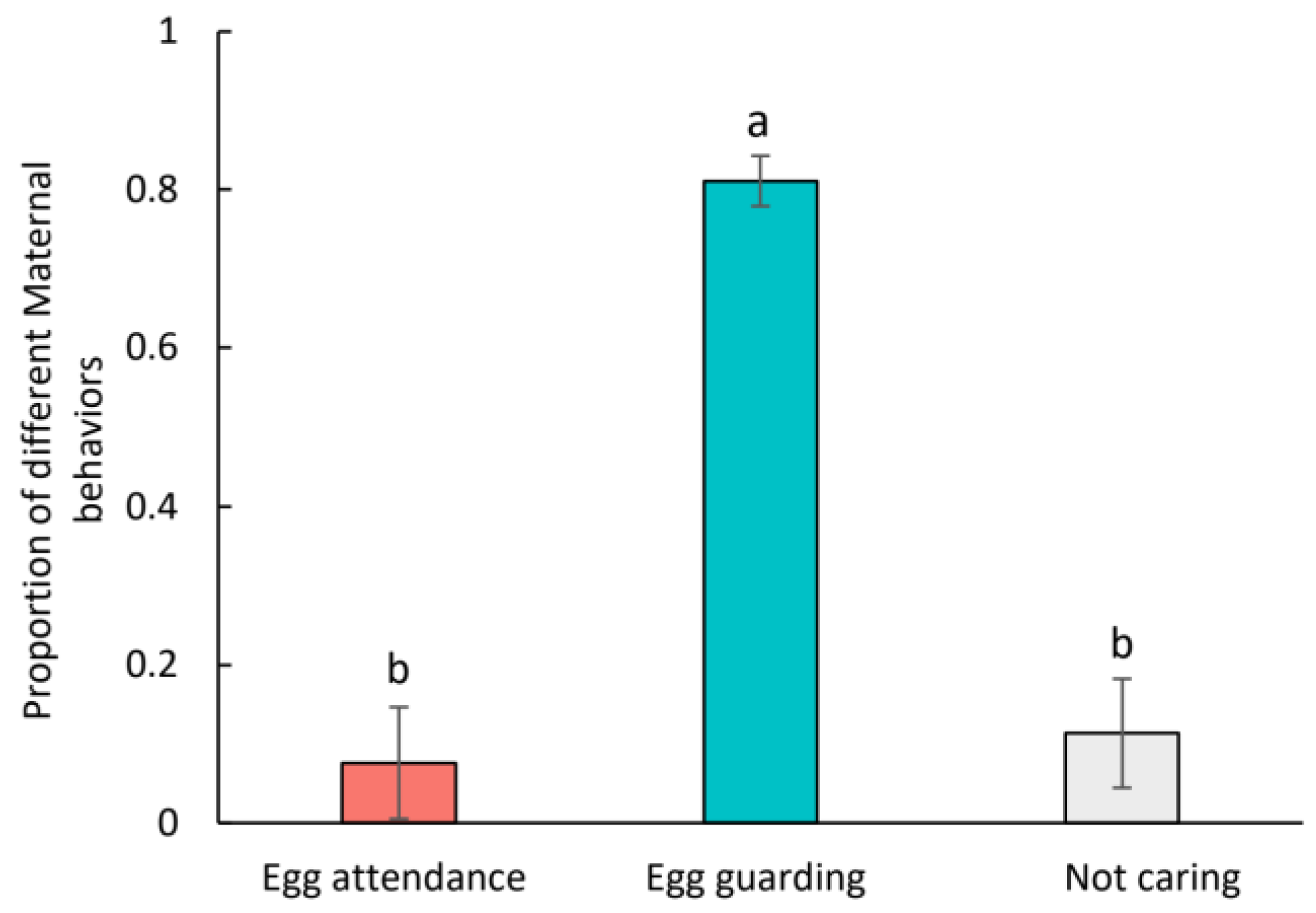
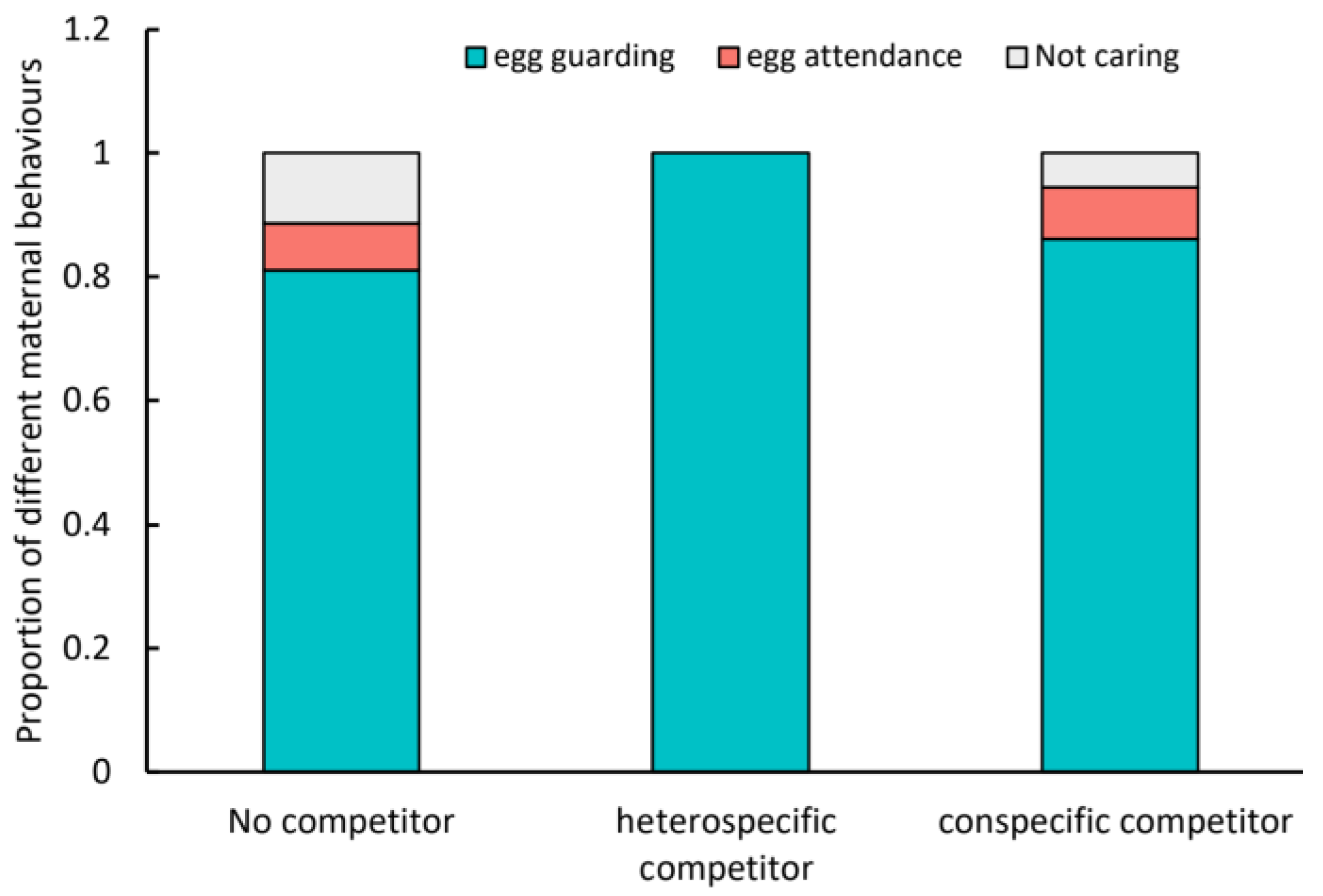
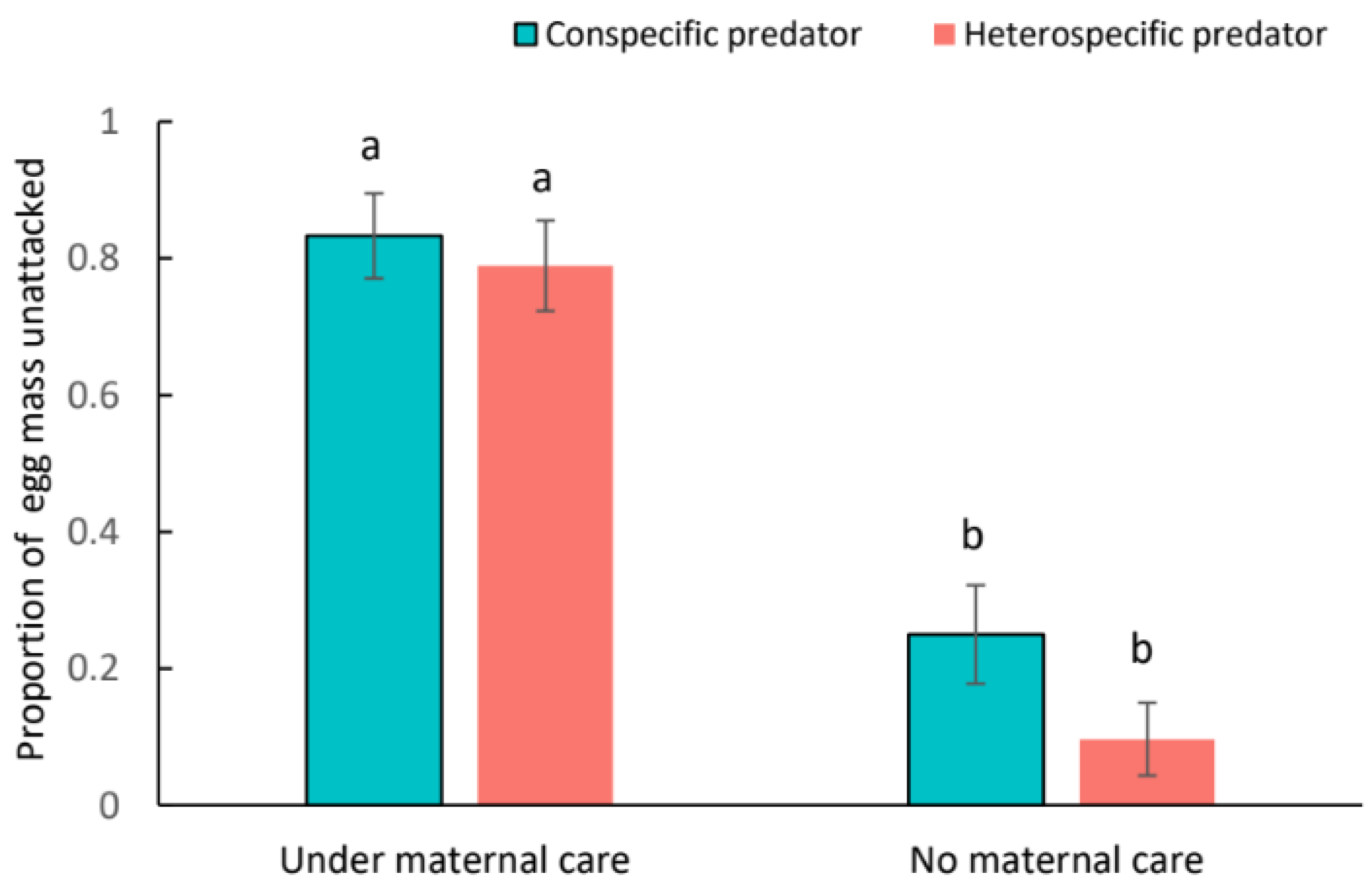
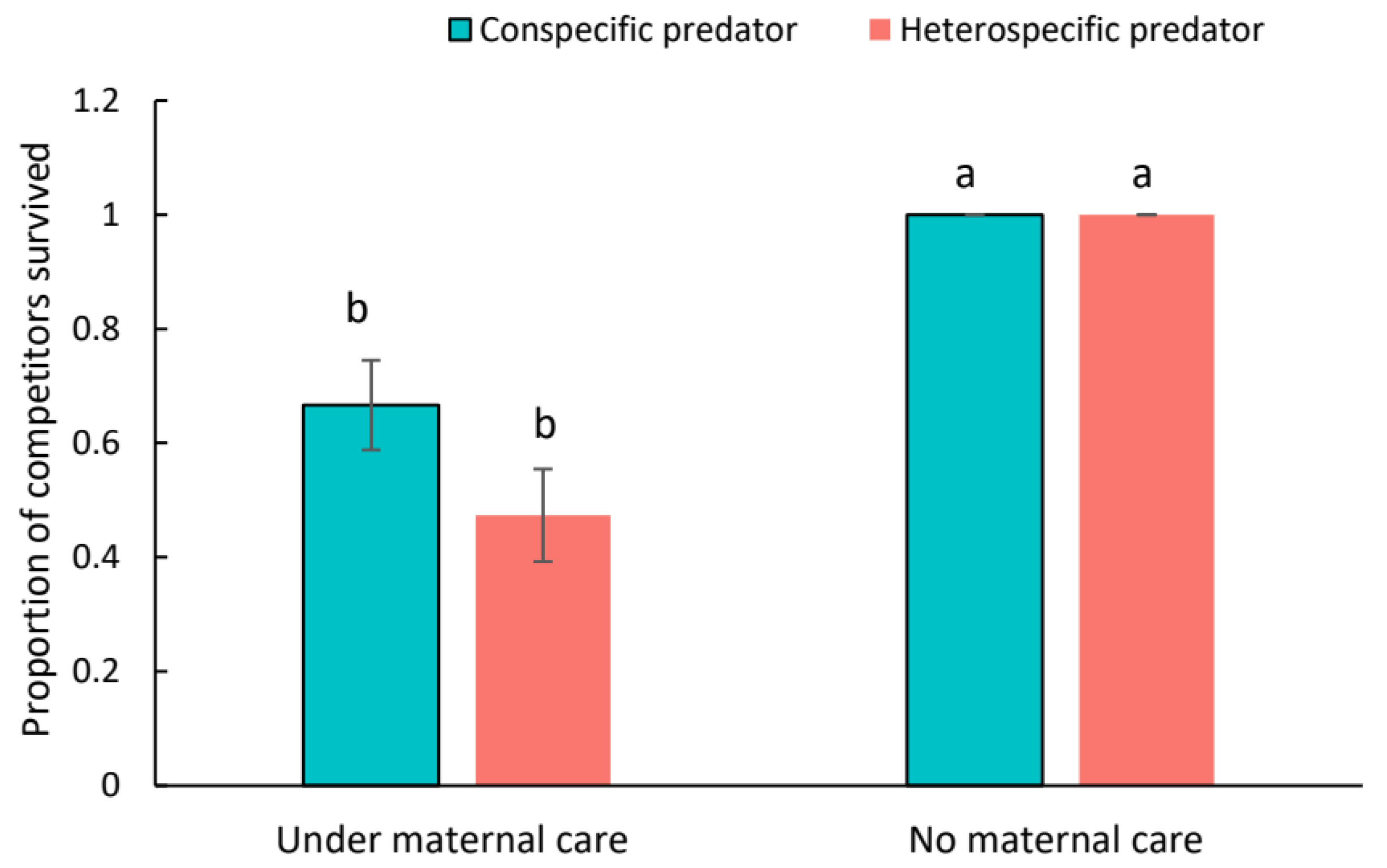
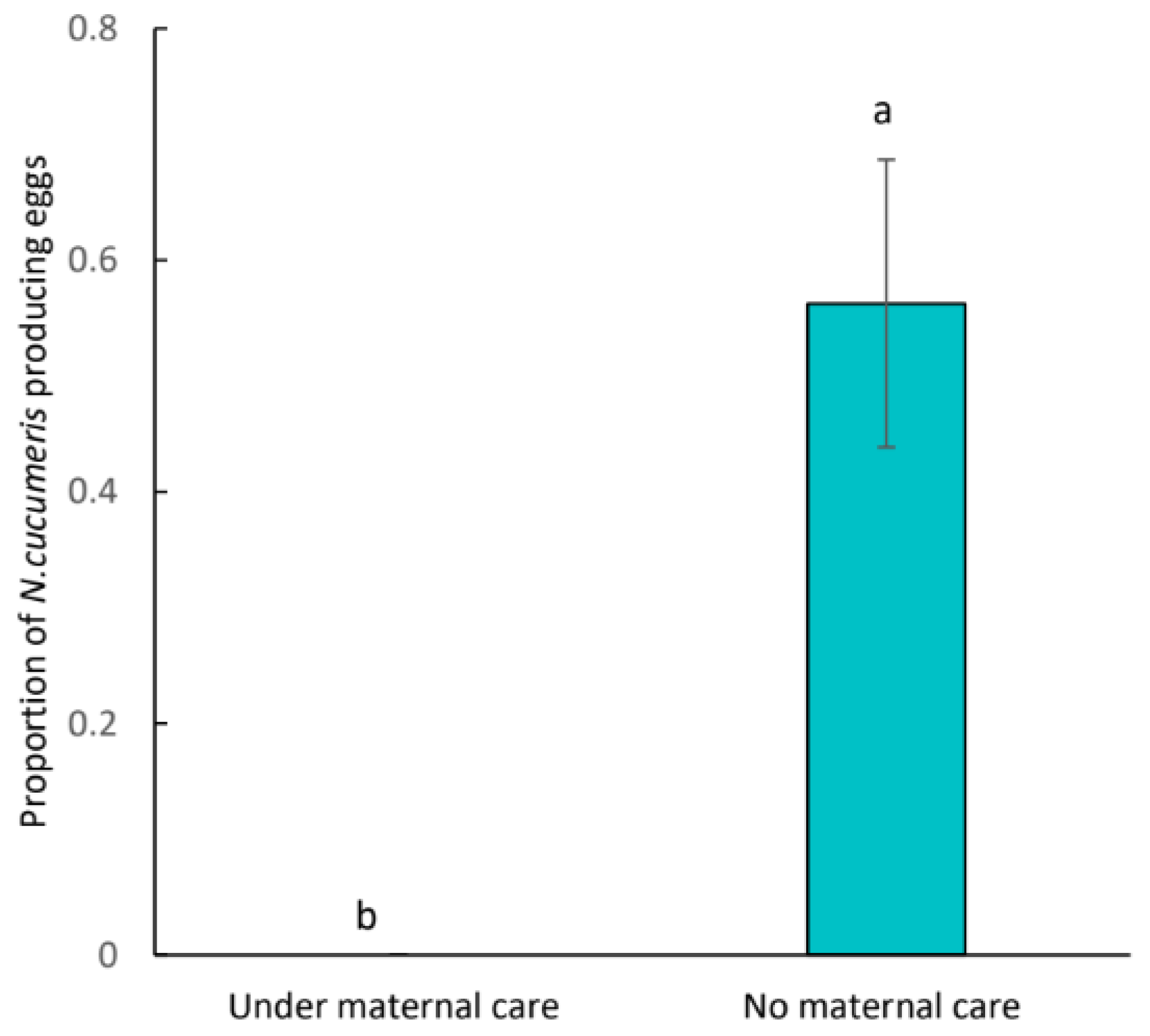
3. Results
4. Discussion
4.1. Egg Guarding Behavior and Response to Environment
4.2. Ecological Consequence of Egg Guarding
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winters, A.E.; Stevens, M.; Mitchell, C.; Blomberg, S.P.; Blount, J.D. Maternal effects and warning signal honesty in eggs and offspring of an aposematic ladybird beetle. Funct. Ecol. 2014, 28, 1187–1196. [Google Scholar] [CrossRef]
- Ghosh, D.D.; Nitabach, M.N.; Zhang, Y.; Harris, G. Multisensory integration in C. elegans. Curr. Opin. Neurobiol. 2017, 43, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, P.T.; Kölliker, M.; Royle, N.J. Parental care. In The Evolution of Insect Mating Systems; Oxford University Press: Oxford, UK, 2012; pp. 221–241. [Google Scholar]
- Miller, J.S.; Rudolph, L.; Zink, A.G. Maternal nest defense reduces egg cannibalism by conspecific females in the maritime earwig Anisolabis maritima. Behav. Ecol. Sociobiol. 2011, 65, 1873–1879. [Google Scholar] [CrossRef]
- Royle, N.J.; Alonzo, S.H.; Moore, A.J. Co-evolution, conflict and complexity: What have we learned about the evolution of parental care behaviours? Curr. Opin. Behav. Sci. 2016, 12, 30–36. [Google Scholar] [CrossRef]
- Royle, N.J.; Russell, A.F.; Wilson, A.J. The evolution of flexible parenting. Science 2014, 345, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, S.T. Patterns of parental care in invertebrates. In The Evolution of Parental Care; Royle, N.J., Ed.; Oxford University Press: Oxford, UK, 2012; pp. 81–100. [Google Scholar]
- Wong, J.W.; Meunier, J.; Kölliker, M. The evolution of parental care in insects: The roles of ecology, life history and the social environment. Ecol. Entomol. 2013, 38, 123–137. [Google Scholar] [CrossRef]
- Tallamy, D.W.; Denno, R.F. Maternal care in Gargaphia solani (Hemiptera: Tingidae). Anim. Behav. 1981, 29, 771–778. [Google Scholar] [CrossRef]
- Hardin, M.R.; Tallamy, D.W. Effect of predators and host phenology on the maternal and reproductive behaviors of Gargaphia lace bugs (Hemiptera: Tingidae). J. Insect. Behav. 1992, 5, 177–192. [Google Scholar] [CrossRef]
- Crespi, B.J. Subsociality and female reproductive success in a mycophagous thrips: An observational and experimental analysis. J. Insect. Behav. 1990, 3, 61–74. [Google Scholar] [CrossRef]
- Dickinson, J.L. Egg cannibalism by larvae and adults of the milkweed leaf beetle (Labidomera clivicollis, Coleoptera: Chrysomelidae). Ecol. Entomol. 1992, 17, 209–218. [Google Scholar] [CrossRef]
- Klug, H.; Chin, A.; St Mary, C.M. The net effects of guarding on egg survivorship in the flagfish, Jordanella floridae. Anim. Behav. 2005, 69, 661–668. [Google Scholar] [CrossRef]
- Gilbert, J.D.; Manica, A. Parental care trade-offs and life-history relationships in insects. Am. Nat. 2010, 176, 212–226. [Google Scholar] [CrossRef]
- Günther, R.; Richards, S.J.; Bickford, D.; Johnston, G.R. A new egg-guarding species of Oreophryne (Amphibia, Anura, Microhylidae) from southern Papua New Guinea. Zoosystematics Evol. 2012, 88, 223–230. [Google Scholar]
- Goldberg, R.L.; Downing, P.A.; Griffin, A.S.; Green, J.P. The costs and benefits of paternal care in fish: A meta-analysis. Proc. R. Soc. B 2020, 287, 20201759. [Google Scholar] [CrossRef] [PubMed]
- Porcel, X.; Dubos, N.; Nöel, J.; Lava, H.; Velo, J.H.; Melo, M.; Rosa, G.M.; Andreone, F.; Crottini, A.; Crottini, A. Male parental care in Malagasy stream-dwelling frogs of the Mantidactylus femoralis group (Anura: Mantellidae: Ochthomantis): Egg guarding in Ochthomantis. Herpetol. Notes 2022, 15, 55–61. [Google Scholar]
- Hale, R.E.; St Mary, C.M. Nest tending increases reproductive success, sometimes: Environmental effects on paternal care and mate choice in flagfish. Anim. Behav. 2007, 74, 577–588. [Google Scholar] [CrossRef]
- Klug, H.; Bonsall, M.B. When to care for, abandon, or eat your offspring: The evolution of parental care and filial cannibalism. Am. Nat. 2007, 170, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Furness, A.I.; Capellini, I. The reproductive ecology drivers of egg attendance in amphibians. Ecol. Lett. 2022, 25, 2500–2512. [Google Scholar] [CrossRef] [PubMed]
- Mouret, N.; Lécureuil, C.; Meunier, J. The costs and benefits of maternal egg care in the earwig Forficula pubescens. Insectes Soc. 2023, 70, 69–79. [Google Scholar] [CrossRef]
- Summers, F.M.; Witt, R.L. Nesting behavior of Cheyletus eruditus. (Acarina: Cheyletidae). Pan-Pac. Entomol. 1972, 48, 261–269. [Google Scholar]
- Thind, B.B.; Ford, H.L. Laboratory studies on the use of two new arenas to evaluate the impact of the predatory mites Blattisocius tarsalis and Cheyletus eruditus on residual populations of the stored product mite Acarus siro. Exp. Appl. Acarol. 2006, 38, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Athanassion, C.G.; Palyvos, N.E. The Cheyletoidea (Prostigmata), with special reference to the potential of Cheyletus malaccensis Oudemans as biological control agent of post-harvest pests. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Springer: Berlin/Heidelberg, Germany, 2015; pp. 241–249. [Google Scholar]
- Pulpan, J.; Verner, P.H. Control of tyroglyphoid mites in stored grain by the predatory mite Cheyletus eruditus (Schrank). Can. J. Zool. 1965, 43, 417–432. [Google Scholar] [CrossRef]
- Halmai, Z. The phenomenon ‘cannibalism’ in Dermatophagoides farinae (Acari) populations. Parasitol. Hungar 1994, 27, 69–72. [Google Scholar]
- Žd’árková, E. Biological control of storage mites by Cheyletus eruditus. Integr. Pest Manag. Rev. 1998, 3, 111–116. [Google Scholar] [CrossRef]
- Knapp, M.; van Houten, Y.; van Baal, E.; Groot, T. Use of predatory mites in commercial biocontrol: Current status and future prospects. Acarologia 2018, 58, 72–82. [Google Scholar] [CrossRef]
- Vangansbeke, D.; Duarte, M.V.; Pekas, A.; Wäckers, F.; Bolckmans, K. Mass production of predatory mites: State of the art and future challenges. In Mass Production of Beneficial Organisms; Elsevier: Amsterdam, The Netherlands, 2023; pp. 195–232. [Google Scholar]
- Pekar, S.; Žďárková, E. A model of the biological control of Acarus siro by Cheyletus eruditus (Acari: Acaridae, Cheyletidae) on grain. J. Pest Sci. 2004, 77, 1–10. [Google Scholar] [CrossRef]
- Danso, J.K.; Opit, G.P.; Giles, K.L.; Noden, B.H. Numerical responses of the predatory mites, Cheyletus eruditus (Trombidiformes: Cheyletidae) and Cheyletus malaccensis, to Liposcelis decolor (Psocodea: Liposcelididae). J. Econ. Entomol. 2023, 116, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Danso, J.K.; Opit, G.P.; Goad, C.L.; Noden, B.H.; Giles, K.L. Functional responses of predatory mites, Cheyletus eruditus (Schrank) and Cheyletus malaccensis Oudemans (Trombidiformes: Cheyletidae) to Liposcelis decolor (Pearman) (Psocodea: Liposcelididae). J. Stored Prod. Res. 2023, 103, 102141. [Google Scholar] [CrossRef]
- Sun, W.; Xia, L.; Wu, Y. Life histories and functional responses of two predatory mites feeding on the stored-grain pest Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae). Insects 2023, 14, 478. [Google Scholar] [CrossRef]
- Maurer, V. The dynamics of Dermanyssus gallinae (Acari: Dermanyssidae) Populations Interacting with Laying Hens and the Predatory Mite Cheyletus eruditus (Acari: Cheyletidae). Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 1993. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Rohwer, S. Parent cannibalism of offspring and egg raiding as a courtship strategy. Am. Nat. 1978, 112, 429–440. [Google Scholar] [CrossRef]
- Chen, X.; Sun, L.; Zhang, Y.P.; Zhang, Y.X.; Lin, J.Z. Responses of avermectin-resistant and susceptible strains of Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) to Tetranychus urticae Koch (Acari: Tetranychidae) on sweet potato. Syst. Appl. Acarol. 2020, 25, 2286–2299. [Google Scholar]
- Schöller, M.E.; Flinn, P.W.; Grieshop, M.J.; Zd’árková, E. Biological control of stored product pests. In Insect Management for Food Storage and Processing; Elsevier: Amsterdam, The Netherlands, 2006; pp. 67–87. [Google Scholar]
- Li, G.Y.; Pattison, N.; Zhang, Z.Q. Immature development and survival of Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) on eggs of Tyrophagus curvipenis (Fain & Fauvel) (Acari: Acaridae). Acarologia 2021, 61, 84–93. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.-Y.; Li, Y.-C.; Liu, H. Maternal Care Behavior and Its Consequences in Competition. Insects 2024, 15, 236. https://doi.org/10.3390/insects15040236
Li G-Y, Li Y-C, Liu H. Maternal Care Behavior and Its Consequences in Competition. Insects. 2024; 15(4):236. https://doi.org/10.3390/insects15040236
Chicago/Turabian StyleLi, Guang-Yun, Yu-Chuang Li, and Huai Liu. 2024. "Maternal Care Behavior and Its Consequences in Competition" Insects 15, no. 4: 236. https://doi.org/10.3390/insects15040236
APA StyleLi, G.-Y., Li, Y.-C., & Liu, H. (2024). Maternal Care Behavior and Its Consequences in Competition. Insects, 15(4), 236. https://doi.org/10.3390/insects15040236



