Nuclear Receptor FTZ-F1 Controls Locust Molt by Regulating the Molting Process of Locusta migratoria
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Gene Identification of LmFTZ-F1
2.3. Tissue- and Stage-Dependent Expression Analysis of LmFTZ-F1
2.4. Functional Analysis of LmFTZ-F1 by RNAi
2.5. Microsection and Hematoxylin-Eosin (H&E) Staining of the Cuticle
2.6. Microsection and Chitin Staining
2.7. RNA-Seq Analysis
2.8. Data Analysis
3. Results
3.1. Bioinformatic Analysis of LmFTZ-F1
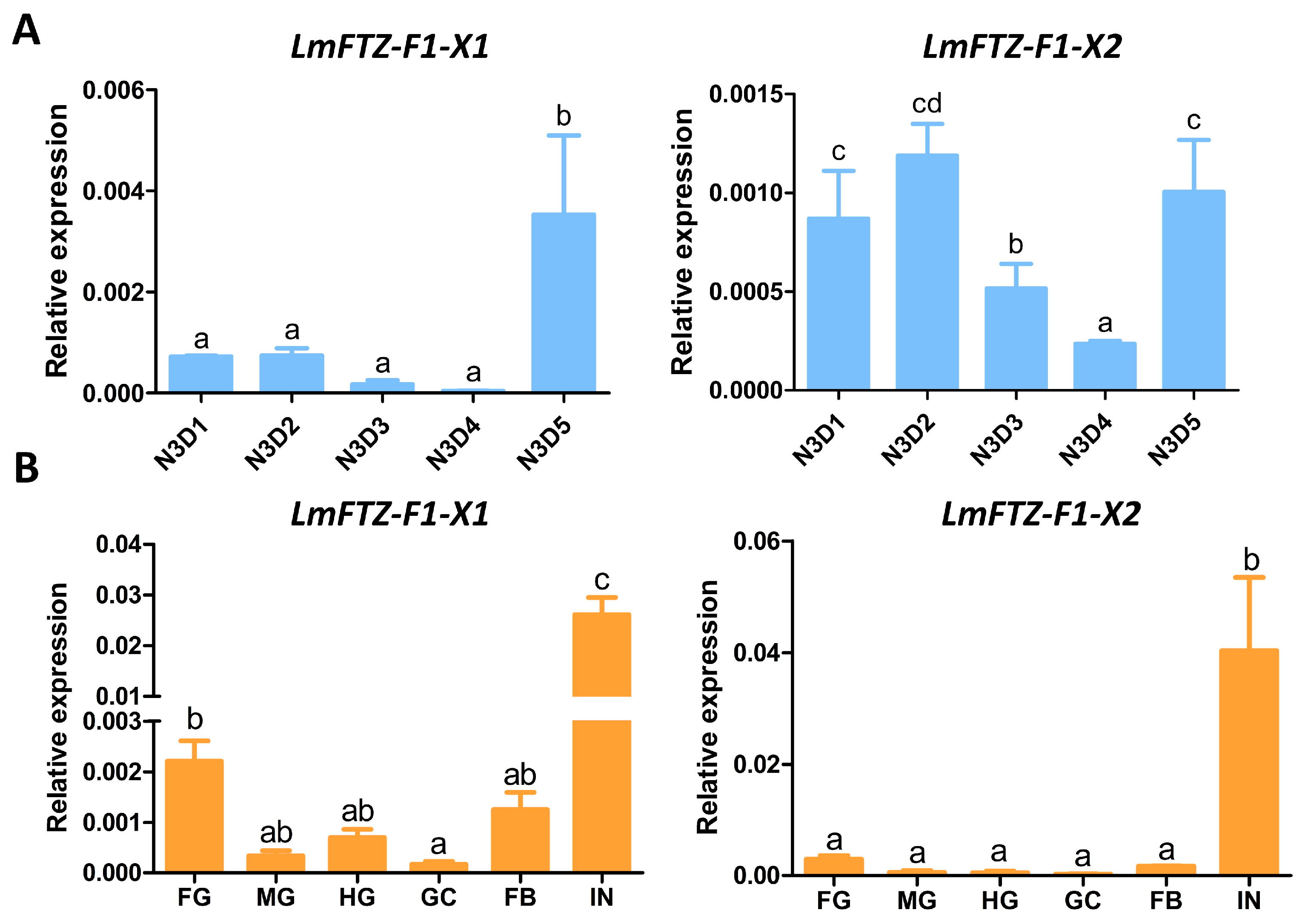
3.2. Tissue and Developmental Expression Patterns of LmFTZ-F1
3.3. Effect on Nymphal Survival after LmFTZ-F1 RNAi
3.4. Effects of LmFTZ-F1s RNAi on Cuticle Formation of L. migratoria
3.5. Differentially Expressed Genes after LmFTZ-F1s RNAi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, L.; Yu, Z.; Jia, Q.; Zhang, X.; Ma, E.; Li, S.; Zhu, K.Y.; Feyereisen, R.; Zhang, J. Knockdown of LmCYP303A1 alters cuticular hydrocarbon profiles and increases the susceptibility to desiccation and insecticides in Locusta migratoria. Pestic. Biochem. Phys. 2020, 168, 104637. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, X.; Wang, Y.; Moussian, B.; Zhu, K.Y.; Li, S.; Ma, E.; Zhang, J. LmCYP4G102: An oenocyte-specific cytochrome P450 gene required for cuticular waterproofing in the Migratory locust, Locusta migratoria. Sci. Rep. 2016, 6, 29980. [Google Scholar] [CrossRef] [PubMed]
- Truman, J.W. The evolution of insect metamorphosis. Curr. Biol. 2019, 29, R1252–R1268. [Google Scholar] [CrossRef] [PubMed]
- Nestel, D.; Tolmasky, D.; Rabossi, A.; Quesada-Allué, L.A. Lipid, Carbohydrates and Protein Patterns During Metamorphosis of the Mediterranean Fruit Fly, Ceratitis capitata (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2003, 96, 237–244. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, Turnover, and Functions of Chitin in Insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.H.; Papandreou, N.C.; Iconomidou, V.A.; Smith, R.F.; Hamodrakas, S.J. Cuticular proteins. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 134–166. [Google Scholar]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Le Gof, G.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14858–14863. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.F.; Mu, L.L.; Chen, X.; Guo, W.C.; Li, G.Q. Rna interference of chitin synthase genes inhibits chitin biosynthesis and affects larval performance in Leptinotarsa decemlineata (say). Int. J. Biol. Sci. 2016, 12, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Arakane, Y.; Beeman, R.W.; Kramer, K.J.; Muthukrishnan, S. Functional Specialization Among Insect Chitinase Family Genes Revealed by RNA Interference. Proc. Natl. Acad. Sci. USA 2008, 105, 6650–6655. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Zhang, J.; Li, D.; Sun, Y.; Guo, Y.; Ma, E.; Zhu, K.Y. Silencing of two alternative splicing-derived mrna variants of chitin synthase 1 gene by rnai is lethal to the oriental migratory locust, Locusta migratoria manilensis (meyen). Insect Biochem. Mol. Biol. 2010, 40, 824–833. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Wang, Y.; Liu, X.; Ma, E.; Sun, Y.; Li, S.; Zhu, K.Y.; Zhang, J. Two chitinase 5 genes from Locusta migratoria: Molecular characteristics and functional differentiation. Insect Biochem. Mol. Biol. 2015, 58, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Noh, M.Y.; Dittmer, N.T.; Muthukrishnan, S.; Kramer, K.J.; Kanost, M.R.; Arakane, Y. Cuticular protein with a low complexity sequence becomes cross-linked during insect cuticle sclerotization and is required for the adult molt. Sci. Rep. 2015, 5, 10484. [Google Scholar] [CrossRef] [PubMed]
- Fahrbach, E.S.; Smagghe, G.; Velarde, A.R. Insect Nuclear Receptors. Annu. Rev. Entomol. 2012, 57, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Qin, Z.Y.; Liu, W.M.; Liu, X.J.; Moussian, B.; Ma, E.B.; Li, S.; Zhang, J.Z. Nuclear receptor HR3 controls locust molt by regulating chitin synthesis and degradation genes of Locusta migratoria. Insect Biochem. Mol. Biol. 2018, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Sun, Y.; Liang, X.; Zhang, R.; Zhao, X.; Zhang, M.; Zhang, J. A nuclear receptor HR4 is essential for the formation of epidermal cuticle in the migratory locust, Locusta migratoria. Insect Biochem. Mol. Biol. 2022, 143, 103740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Qin, Z.Y.; Zhang, J.; Yang, Y.; Jia, P.; Yang, Q.; Ma, E.B.; Zhang, J.Z. Nuclear receptor hormone receptor 39 is required for locust moulting by regulating the chitinase and carboxypeptidase genes. Insect Mol. Biol. 2019, 28, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Pick, L.; Anderson, W.R.; Shultz, J.; Woodard, C.T. The Ftz-F1 family: Orphan nuclear receptors regulated by novel protein–protein interactions. Adv. Dev. Biol. 2006, 16, 255–296. [Google Scholar]
- Zhang, W.; Ma, L.; Liu, X.; Peng, Y.; Liang, G.; Xiao, H. Dissecting the roles of FTZ-F1 in larval molting and pupation, and the sublethal effects of methoxyfenozide on Helicoverpa armigera. Pest. Manag. Sci. 2021, 77, 1328–1338. [Google Scholar] [CrossRef]
- Li, X.; Shan, C.; Li, F.; Liang, P.; Smagghe, G.; Gao, X. Transcription factor FTZ-F1 and cis-acting elements mediate expression of CYP6BG1 conferring resistance to chlorantraniliprole in Plutella xylostella. Pest. Manag. Sci. 2019, 75, 1172–1180. [Google Scholar] [CrossRef]
- Cruz, J.; Nieva, C.; Mané-Padrós, D.; Martín, D.; Bellés, X. Nuclear receptor BgFTZ-F1 regulates molting and the timing of ecdysteroid production during nymphal development in the hemimetabolous insect Blattella germanica. Dev. Dyn. 2008, 237, 3179–3191. [Google Scholar] [CrossRef]
- Bernardo, T.J.; Dubrovsky, E.B. The Drosophila juvenile hormone receptor candidates methoprene -tolerant (MET) and germ cell-expressed (GCE) utilize a conserved LIXXL motif to bind the FTZ-F1 nuclear receptor. J. Biol. Chem. 2012, 287, 7821–7833. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M.; Hiruma, K.; Zhou, X.; Nelson, C.A. Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster. Insect Biochem. Mol. Biol. 2003, 33, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, L.; Raikhel, A.S. Posttranscriptional control of the competence factor betaFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2003, 100, 13338–13343. [Google Scholar] [CrossRef]
- Cho, K.H.; Daubnerová, I.; Park, Y.; Zitnan, D.; Adams, M.E. Secretory competence in a gateway endocrine cell conferred by the nuclear receptor βFTZ-F1 enables stage-specific ecdysone responses throughout development in Drosophila. Dev. Biol. 2014, 385, 253–262. [Google Scholar] [CrossRef]
- Shu, Y.Y.; Li, X.M.; Kang, K.X.; Li, C.; Yang, W.J. Nuclear receptor FTZ-F1 is required for larval-pupal molting by regulating ecdysteroidogenesis and chitin metabolism in Lasioderma serricorne. J. Stored Prod. Res. 2023, 101, 102096. [Google Scholar]
- Wang, X.; Fang, X.; Yang, P.; Jiang, X.; Jiang, F.; Zhao, D.; Li, B.; Cui, F.; Wei, J.; Ma, C.; et al. The locust genome provides insight into swarm formation and long-distance flight. Nat. Commun. 2014, 5, 2957. [Google Scholar] [CrossRef]
- Nicholas, K.B. GeneDoc, analysis and visualization of genetic variation. Embnew. News. 1997, 4, 14. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5, Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cui, M.; Liu, X.; Li, T.; Guo, Y.; Ma, E.; Zhang, J. Selection of reference genes on different days during the development of the fifth-instar nymph of Locusta migratoria with quantitative real-time PCR. Chin. J. Appl. Entomol. 2014, 51, 733–740. [Google Scholar]
- Liu, W.M.; Xie, X.P.; Xue, J.L.; Gao, Y.; Zhang, Y.F.; Zhang, X.X.; Tan, J.S. Histopathological changes of Ceroplastes japonicus infected by Lecanicillium lecanii. J. Invertebr. Pathol. 2009, 101, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Song, T.Q.; Yang, M.L.; Wang, Y.L.; Liu, Q.; Wang, H.M.; Zhang, J.; Li, T. Cuticular protein LmTwdl1 is involved in molt development of the Migratory locust. Insect Sci. 2016, 23, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Liu, W.M.; Zhao, X.M.; Yu, Z.T.; Guo, H.F.; Yang, Y.; Moussian, B.; Zhu, K.Y.; Zhang, J.Z. Lipophorin receptor is required for the accumulations of cuticular hydrocarbons and ovarian neutral lipids in Locusta migratoria. Int. J. Biol. Macromol. 2023, 236, 123746. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods. 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Liu, X.P.; Fu, K.Y.; Lü, F.G.; Meng, Q.W.; Guo, W.C.; Li, G.Q. Involvement of FTZ-F1 in the regulation of pupation in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2014, 55, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Cheng, M.D.; Ze, L.J.; Shen, C.H.; Jin, L.; Li, G.Q. Dissecting the Isoform-Specific Roles of FTZ-F1 in the Larval-Larval and Larval-Pupal Ecdyses in Henosepilachna vigintioctopunctata. Insects 2022, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Eichner, C.; Male, R. The ftz-f1 gene encodes two functionally distinct nuclear receptor isoforms in the ectoparasitic copepod salmon louse (Lepeophtheirus salmonis). PLoS ONE 2021, 16, e0251575. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.C. Circadian organization of chitin in some insect skeletons. Q. J. Microsc. Sci. 1965, 106, 315. [Google Scholar] [CrossRef]
- Noh, M.Y.; Kramer, K.J.; Muthukrishnan, S.; Kanost, M.R.; Beeman, R.W.; Arakane, Y. Two major cuticular proteins are required for assembly of horizontal laminae and vertical pore canals in rigid cuticle of Tribolium castaneum. Insect Biochem. Mol. Biol. 2014, 53, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wigglesworth, V.B. The epidermal cell and the metamorphosis of insects. Nature 1960, 188, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Eastburn, D.J.; Han, M. The Caenorhabditis elegans nuclear receptor gene nhr-25 regulates epidermal cell development. Mol. Cell Biol. 2004, 24, 7345–7358. [Google Scholar] [CrossRef] [PubMed]
- Burmrster, T. Evolution and function of the insect hexamerins. Eur. J. Entomol. 1999, 96, 213–225. [Google Scholar]
- Zhou, X.G.; Wheeler, M.M.; Oi, F.M.; Scharf, M.E. RNA interference in the termite Reticulitermes flavipes through ingestion of double-stranded RNA. Insect Biochem. Mol. Biol. 2008, 38, 805–815. [Google Scholar] [CrossRef]
- Tang, B.; Wei, P.; Zhao, L.; Shi, Z.; Shen, Q.; Yang, M.; Xie, G.; Wang, S. Knockdown of five trehalase genes using RNA interference regulates the gene expression of the chitin biosynthesis pathway in Tribolium castaneum. BMC Biotechnol. 2016, 16, 67. [Google Scholar] [CrossRef]
- Liebl, M.; Nelius, V.; Günter, K.; Ando, O.; Wegener, G. Fate and effects of the trehalase inhibitor trehazolin in the migratory locust (Locusta migratoria). J. Insect Physiol. 2010, 56, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Yang, Y.; Liu, W.; Zhang, J. BTB domain-containing protein 6 is involved in the development of locust wings during the nymph to adult transition. Int. J. Biol. Macromol. 2020, 150, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Jia, Q.; Zhang, X.; Zhang, X.; Liu, S.; Park, Y.; Feyereisen, R.; Zhu, K.Y.; Ma, E.; Zhang, J.; et al. CYP303A1 has a conserved function in adult eclosion in Locusta migratoria and Drosophila melanogaster. Insect Biochem. Mol. Biol. 2019, 113, 103210. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, H.; Liu, X.; Li, H.; Lan, Q.; Wu, H.; Wang, Y.; Zhang, J.; Zhao, X. Nuclear Receptor FTZ-F1 Controls Locust Molt by Regulating the Molting Process of Locusta migratoria. Insects 2024, 15, 237. https://doi.org/10.3390/insects15040237
Zhang Y, Li H, Liu X, Li H, Lan Q, Wu H, Wang Y, Zhang J, Zhao X. Nuclear Receptor FTZ-F1 Controls Locust Molt by Regulating the Molting Process of Locusta migratoria. Insects. 2024; 15(4):237. https://doi.org/10.3390/insects15040237
Chicago/Turabian StyleZhang, Yichao, Hongjing Li, Xiaoman Liu, Hongli Li, Qiuyan Lan, Haihua Wu, Yanli Wang, Jianzhen Zhang, and Xiaoming Zhao. 2024. "Nuclear Receptor FTZ-F1 Controls Locust Molt by Regulating the Molting Process of Locusta migratoria" Insects 15, no. 4: 237. https://doi.org/10.3390/insects15040237
APA StyleZhang, Y., Li, H., Liu, X., Li, H., Lan, Q., Wu, H., Wang, Y., Zhang, J., & Zhao, X. (2024). Nuclear Receptor FTZ-F1 Controls Locust Molt by Regulating the Molting Process of Locusta migratoria. Insects, 15(4), 237. https://doi.org/10.3390/insects15040237





