Feeding Appropriate Nutrients during the Adult Stage to Promote the Growth and Development of Carposina sasakii Offspring
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Methods
2.3. Data Analysis
2.4. Life Table Analysis
2.5. Population Parameters
3. Results
3.1. Developmental Duration and Longevity
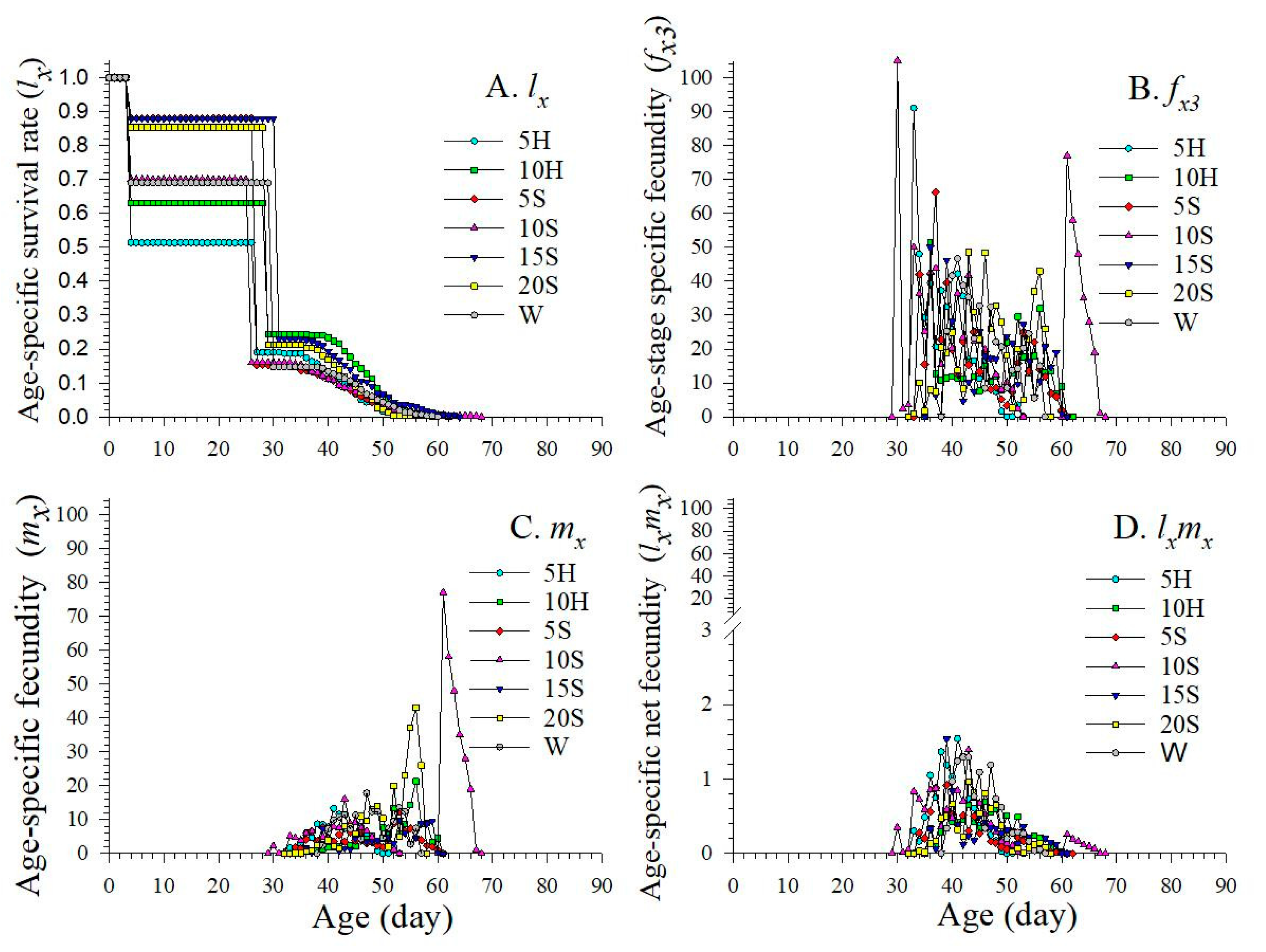
3.2. Age-Stage Specific Survival Rate
3.3. Age-Stage Specific Fecundity and Fertility
3.4. Age-Stage Life Expectancy
3.5. Age-Stage Specific Reproduction Values
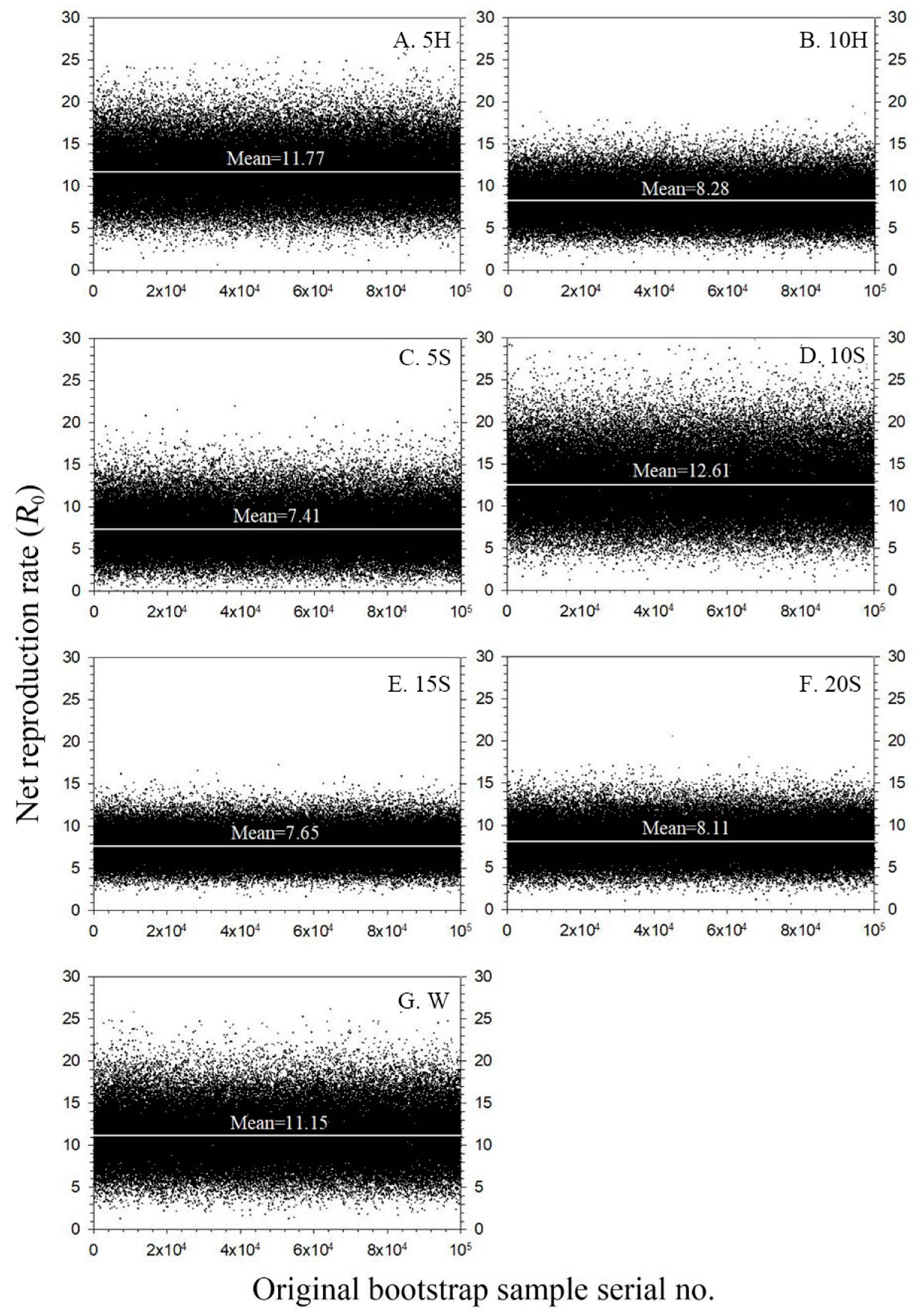
3.6. Population Dynamics Parameters
3.7. Simulation of Population Growth Dynamics
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Treatments | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | W | N | 5H | N | 10H | N | 5S | N | 10S | N | 15S | N | 20S | |
| Egg–larva duration (d) | 44 | 30.36 ± 0.66 a | 57 | 26.49 ± 0.57 c | 73 | 28.37 ± 0.45 bc | 46 | 26.83 ± 0.78 c | 48 | 26.02 ± 0.83 c | 69 | 30.33 ± 0.68 a | 64 | 28.72 ± 0.51 ab |
| Egg–larva survival rate (%) | 300 | 14.67 ± 2.05 c | 300 | 19.00 ± 2.27 abc | 300 | 24.33 ± 2.47 a | 300 | 15.33 ± 2.09 bc | 300 | 16.00 ± 2.11 bc | 300 | 23.00 ± 2.42 a | 300 | 21.33 ± 2.36 ab |
| Pupa duration (d) | 36 | 11.83 ± 0.38 c | 43 | 11.88 ± 0.46 bc | 60 | 14.42 ± 0.44 a | 31 | 12.55 ± 0.33 bc | 35 | 13.03 ± 0.41 b | 52 | 13.02 ± 0.50 bc | 45 | 12.40 ± 0.28 bc |
| Pupa survival rate (%) | 36 | 81.82 ± 5.88 a | 43 | 75.44 ± 5.75 a | 60 | 82.19 ± 4.52 a | 31 | 67.39 ± 6.98 a | 35 | 72.92 ± 6.48 a | 52 | 75.36 ± 5.22 a | 45 | 70.31 ± 5.74 a |
| Preadult survival rate sa (%) | 300 | 12.00 ± 1.87 bc | 300 | 14.33 ± 2.02 abc | 300 | 20.00 ± 2.31 a | 300 | 10.33 ± 1.76 c | 300 | 11.67 ± 1.85 c | 300 | 17.33 ± 2.17 ab | 300 | 15.00 ± 2.06 abc |
| Preadult duration (d) | 36 | 41.81 ± 0.58 a | 43 | 38.00 ± 0.60 c | 60 | 42.28 ± 0.62 a | 31 | 38.32 ± 0.80 bc | 35 | 38.34 ± 1.06 bc | 52 | 42.25 ± 0.82 a | 45 | 39.82 ± 0.57 b |
| Nf/N (%) | 300 | 6.00 ± 1.37 bc | 300 | 6.67 ± 1.43 abc | 300 | 10.67 ± 1.78 a | 300 | 3.67 ± 1.09 c | 300 | 5.67 ± 1.34 bc | 300 | 8.67 ± 1.62 ab | 300 | 7.33 ± 1.50 ab |
| Nfr/N (%) | 300 | 6.00 ± 1.37 ab | 300 | 6.33 ± 1.40 ab | 300 | 9.67 ± 1.70 a | 300 | 3.67 ± 1.09 b | 300 | 5.67 ± 1.34 ab | 300 | 8.00 ± 1.56 a | 300 | 6.33 ± 1.40 ab |
| Female adult longevity (d) | 18 | 7.33 ± 0.74 bc | 20 | 7.50 ± 0.67 bc | 32 | 5.91 ± 0.51 cd | 11 | 10.55 ± 0.97 a | 17 | 8.94 ± 0.78 ab | 26 | 5.11 ± 0.34 d | 22 | 5.14 ± 0.41 d |
| Male adult longevity (d) | 18 | 6.00 ± 0.45 bc | 23 | 6.74 ± 0.68 abc | 28 | 6.86 ± 0.47 ab | 20 | 8.00 ± 0.74 a | 18 | 8.17 ± 0.77 a | 26 | 5.23 ± 0.43 cd | 23 | 4.26 ± 0.31 d |
| Mean longevity of all (d) | 300 | 24.53 ± 0.88 c | 300 | 18.99 ± 0.92 d | 300 | 24.36 ± 1.00 c | 300 | 27.03 ± 0.64 b | 300 | 22.48 ± 0.81 c | 300 | 31.50 ± 0.72 a | 300 | 28.66 ± 0.70 b |
References
- Boggs, C.L. Reproductive strategies of female butterflies: Variation in and constraints on fecundity. Ecol. Entomol. 2010, 11, 7–15. [Google Scholar] [CrossRef]
- Xu, G.; Lin, C.; Xiao, Z.S.; Pan, H.C. Effect of supplementary nutrition on adult lifetime of Acorn Weevils (Curculio haroldi). Chin. Bull. Entomol. 2009, 46, 718–722. [Google Scholar]
- Jones, L.C.; Rafter, M.A.; Walter, G.H. Interactions of Helicoverpa punctigera (Lepidoptera: Noctuidae) larvae and adults with four native host plants relative to field use patterns. Environ. Entomol. 2020, 50, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.; Gatehouse, A.G. Effects of the availability of food and water on reproduction in the African army worm, Spodoptera exempta. Physiol. Entomol. 1985, 10, 53–63. [Google Scholar] [CrossRef]
- Rhind, S.M.; Rae, M.T.; Brooks, A.N. Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction 2001, 122, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Gorman, H.E.; Nager, R.G. Prenatal developmental conditions have long-term effects on offspring fecundity. Proc. Biol. Sci. 2004, 271, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Bonduriansky, R.; Day, T. Nongenetic inheritance and its evolutionary implications. Ann. Rev. Ecol. Evolut. 2009, 40, 103–125. [Google Scholar] [CrossRef]
- Chavatte-Palmer, P.; Dupont, C.; Debus, N.; Camous, S. Nutritional programming and the reproductive function of the offspring. Anim. Prod. Sci. 2014, 54, 1166–1176. [Google Scholar] [CrossRef]
- Colombo, V.; Pettigrove, V.J.; Golding, L.A.; Hoffmann, A.A. Transgenerational effects of parental nutritional status on offspring development time, survival, fecundity, and sensitivity to zinc in Chironomus tepperi Midges. Ecotoxicol. Environ. Saf. 2014, 110, 1–7. [Google Scholar] [CrossRef]
- Berndt, L.A.; Wratten, S.D.; Hassan, P.G. Effects of buckwheat flowers on leafroller (Lepidoptera: Tortricidae) parasitoids in a New Zealand vineyard. Agric. For. Entomol. 2002, 4, 39–45. [Google Scholar] [CrossRef]
- Romeis, J.; Wäckers, F.L. Feeding responses by female pieris brassicae butterflies to carbohydrates and amino acids. Physiol. Entomol. 2000, 25, 247–253. [Google Scholar] [CrossRef]
- Li, C.M.; Xu, J.; Liu, Q.; Han, G.J.; Xu, B.; Yang, Y.Z.; Liu, X.-J. Potential influence of carbohydrate and amino acid intake by adults on the population dynamics of Cnaphalocrocis medinalis (Lepidoptera: Crambidae). J. Integr. Agric. 2021, 20, 1889–1897. [Google Scholar] [CrossRef]
- Bao, X.W.; Zheng, F.; Cai, M.F.; Wu, J.X. Effect of complementary nutrients on adult’s reproduction and longevity of oriental fruit moth, Grapholitha molesta Busck. J. Northw. A F Univ. 2010, 38, 119–123. [Google Scholar]
- Liu, M.; Lu, Y.; Zhang, J.M. Influence of different nutritional conditions on diamondback moth lifetime and Spawning. J. Zhejang Agric. Sci. 2018, 59, 2202–2203+2207. [Google Scholar]
- Gu, X.; Zhang, L.; Chen, H.; Ke, Y. Effects of different supplementary foods on longevity of Diglyphus isaea. Plant Prot. 2010, 36, 89–92. [Google Scholar]
- Ishiguri, Y.; Shirai, Y. Flight activity of the peach fruit moth, Carposina sasakii (Lepidoptera: Carposinidae), measured by a flight mill. Appl. Entomol. Zool. 2004, 39, 127–131. [Google Scholar] [CrossRef]
- Xue, Y.; Ma, R.; Li, X.; Li, L. Research and application on the sex pheromone of Carposina sasakii Matsumura (Lepidoptera: Carposinidae). Chin. J. Biol. Control 2010, 26, 211–216. [Google Scholar]
- Hua, B.; Zeng, X.; Zhang, H. Diapause of Carposina sasakii Mastasumura (Lepidoptera Carposinidae) on various host plants. Acta Agric. Boreal.-Occident. Sin. 1998, 5, 28–32. [Google Scholar]
- Kim, D.; Lee, J. Oviposition model of Carposina sasakii (Lepidoptera: Carposinidae). Ecol. Model. 2003, 162, 145–153. [Google Scholar] [CrossRef]
- Hua, B.; Zeng, X.; Zhang, H. Influences of apple maturity on the development and diapause of Carposina sasakii Matsumura. Acta Univ. Agric. Boreal.-Occident. 1996, 24, 42–45. [Google Scholar]
- Li, X.; Feng, D.; Xue, Q.; Meng, T.; Ma, R.; Deng, A.; Chi, H.; Wu, Z.; Atlıhan, R.; Men, L.; et al. Density-dependent demography and mass-rearing of Carposina sasakii (Lepidoptera: Carposinidae) incorporating life table variability. J. Econ. Entomol. 2018, 112, 255–265. [Google Scholar] [CrossRef]
- Huang, K.; Wu, W. Serial lecture—The big pest in china (xi)—The peach fruit Borer. Chin. Bull. Entomol. 1957, 1, 34–41. [Google Scholar]
- Chi, H.; Fu, J.; You, M. Age-stage, two-sex life table and its application in population ecology and integrated pest managemant. Acta Entomol. Sin. 2019, 62, 255–262. [Google Scholar]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.; Fu, J.; Xu, Y.; et al. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2019, 40, 102–123. [Google Scholar] [CrossRef]
- Roya, T.; Chi, H. Demography of Tetranychus urticae (Acari: Tetranychidae) under different nitrogen regimes with estimations of confidence intervals. Crop Prot. 2022, 155, 105920–105929. [Google Scholar]
- Chang, L.; Zhang, N.; Shu, Z.; Huang, K. Observation on mating and oviposition habits of adults of peach fruit moth (Carposina niponensis Wal.). Insect Knowl. 1964, 6, 271–273. [Google Scholar]
- Chang, N.-X. Studies on the blology of the apple fruit moth—Influences of the fruits on the establishment, growth and diapause of the larvae. Acta Entomol. Sin. 1977, 20, 170–176. [Google Scholar]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2023. Available online: http://140.120.197.173/Ecology (accessed on 15 December 2023).
- Chi, H.; Liu, H. Two New Methods for the Study of Insect Population Ecology. IEEE 1985, 24, 225–240. [Google Scholar]
- Chi, H.; Getz, W.M. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Guncan, A.; Kavousi, A.; Gharakhani, G.; Atlihan, R.; Ozgokce, M.S.; Shirazi, J.; Amir-Maafi, M.; Maroufpoor, M.; Roya, T. Twosex-mschart: The key tool for life table research and education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Chi, H.; Su, H. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Lotka, A.J. Studies on the mode of growth of material aggregates. Am. J. Sci. 1907, 24, 199–216. [Google Scholar] [CrossRef]
- Tuan, S.; Lee, C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on Peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 107, 805–813. [Google Scholar] [CrossRef]
- Tuan, S.; Li, N.; Yeh, C.; Tang, L.; Chi, H. Effects of green manure cover crops on Spodoptera litura (Lepidoptera: Noctuidae) populations. J. Econ. Entomol. 2014, 107, 897–905. [Google Scholar] [CrossRef]
- Gholamhossein, G.; Hamideh, S.; Chi, H. Demography of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) reared on elicitor-treated tomato plants with an innovative comparison of projected population sizes and application of the multinomial theorem for population survival. Pest Manag. Sci. 2023, 79, 4964–4976. [Google Scholar]
- Triggs, A.M.; Knell, R.J. Parental diet has strong transgenerational effects on offspring immunity. Funct. Ecol. 2012, 26, 1409–1417. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Cui, F. Effect of adult foods on fecundity and ovary development of beet armyworm Spodoptera exigua (Hübner). Southw. China J. Agric. Sci. 2004, 1, 34–37. [Google Scholar]
- Ashok, K.; Balasubramani, V.; Kennedy, J.; Geethalakshmi, V.; Jeyakumar, P.; Sathiah, N. Evaluating artificial diets for the fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) through nutritional indices and an age-stage, two-sex life table approach. Afr. Entomol. 2021, 29, 620–634. [Google Scholar] [CrossRef]
- Li, C.; Hu, C.; Zhi, J.; Yue, W.; Li, H. Effects of nano-graphene oxide on the growth and reproductive dynamics of Spodoptera frugiperda based on an age-stage, two-sex life table. Insects 2022, 13, 929. [Google Scholar] [CrossRef]
- Tahmasebi, M.; Shakarami, J.; Mardani-Talaee, M.; Serrão, J.E. Evaluation of resistance of six chickpea cultivars to the cowpea weevil, Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae: Bruchinae) with age-stage, two-sex life table. J. Stored Prod. Res. 2022, 99, 102014–102023. [Google Scholar] [CrossRef]
- Xu, H.; Yang, N.; Chi, H.; Ren, G.; Wan, F. Comparison of demographic fitness and biocontrol effectiveness of two parasitoids, Encarsia sophia and Eretmocerus hayati (Hymenoptera: Aphelinidae), against Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2018, 74, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Men, L.; Peng, Y.; Li, J.; Deng, A.; Chen, Y.; Liu, X.; Ma, R. Morphological differences of the reproductive system could be used to predict the optimum Grapholita molesta (Busck) Control Period. Sci. Rep. 2017, 7, 8198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yao, R.; Zhang, L.; Cheng, Y.; Liu, Y.; Luo, L. Effects of supplementary nutrition on adult reproduction and longevity of Athetis lepigone (Maschler). J. Plant Prot. 2015, 42, 1004–1008. [Google Scholar]
- Feng, B.; Zhu, X.; Zhong, L.; Wang, X.; Liang, S.; Liu, W.; Guo, Q.S.; Du, Y. Effects of supplemental nutrition on the survival and reproduction of Spodoptera frugiperda (Smith). Chin. J. Biol. Control 2021, 37, 1172–1178. [Google Scholar]
- Milano, P.; Filho, E.B.; Parra, J.R.P.; Oda, M.L.; Cnsoli, L.L. Effects of adult feeding on the reproduction and longevity of Noctuidae, Crambidae, Tortricidae and Elachistidae species. Neotrop. Entomol. 2010, 39, 172–180. [Google Scholar] [CrossRef]
- Dussutour, A.; Simpson, S.J. Ant workers die young and colonies collapse when fed a high-protein diet. Proc. R. Soc. B Biol. Sci. 2012, 279, 2402–2408. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.P. The effects of a juvenile hormone analogue on laboratory colonies of pharaoh’s ant, Monomorium Pharaonis (L.) (Hymenoptera, Formicidae). Bull. Entomol. Res. 1975, 65, 75–80. [Google Scholar] [CrossRef]
- Shah, R.M.; Ali, S.S.; Naeem, A. Methoxyfenozide resistance of the housefly, Musca domestica L. (Diptera: Muscidae): Cross-resistance patterns, stability and associated fitness costs. Pest Manag. Sci. 2017, 73, 254–261. [Google Scholar] [CrossRef]
- Hu, L.; He, Z.; Zhang, X. Age-stage two-sex life tables of the experimental population of Problepsis superans (Lepidoptera: Geometridae) on three ligustrum species. Acta Entomol. Sin. 2014, 57, 1408–1417. [Google Scholar]






| Parameter | Treatment | ||||||
|---|---|---|---|---|---|---|---|
| W | 5H | 10H | 5S | 10S | 15S | 20S | |
| F1 Female Adult Fecundity (eggs/female) | 185.89 ± 30.33 a | 176.55 ± 26.25 a | 77.63 ± 16.08 b | 202.18 ± 40.52 a | 222.53 ± 35.80 a | 88.23 ± 13.35 b | 110.64 ± 18.67 b |
| Od (days) | 5.44 ± 0.75 ab | 5.89 ± 0.75 a | 4.03 ± 0.57 bc | 7.64 ± 1.12 a | 6.71 ± 0.87 a | 3.13 ± 0.26 c | 4.16 ± 0.44 b |
| APOP (days) | 1.28 ± 0.25 ab | 1.00 ± 0.15 bc | 1.62 ± 0.24 a | 1.09 ± 0.09 b | 1.18 ± 0.26 ab | 1.38 ± 0.18 ab | 0.63 ± 0.14 c |
| TPOP (days) | 43.72 ± 0.86 ab | 39.42 ± 1.07 c | 45.10 ± 0.93 a | 39.91 ± 1.47 c | 39.53 ± 1.87 c | 44.96 ± 1.29 a | 41.05 ± 1.07 bc |
| Parameter | Treatment | ||||||
|---|---|---|---|---|---|---|---|
| W | 5H | 10H | 5S | 10S | 15S | 20S | |
| r (d−1) | 0.0531 ± 0.0067 a | 0.0606 ± 0.0069 a | 0.0457 ± 0.0065 a | 0.0476 ± 0.0099 a | 0.0615 ± 0.0076 a | 0.0449 ± 0.0058 a | 0.0467 ± 0.0061 a |
| λ (d−1) | 1.0546 ± 0.0071 a | 1.0624 ± 0.0073 a | 1.0467 ± 0.0068 a | 1.0488 ± 0.0103 a | 1.0634 ± 0.0081 a | 1.0459 ± 0.0060 a | 1.0478 ± 0.0064 a |
| R0 (offspring/individual) | 11.15 ± 3.11 a | 11.77 ± 3.05 a | 8.28 ± 2.18 a | 7.41 ± 2.62 a | 12.61 ± 3.57 a | 7.65 ± 1.82 a | 8.11 ± 2.12 a |
| T (days) | 45.40 ± 0.88 a | 40.71 ± 0.74 c | 46.29 ± 1.56 a | 42.05 ± 1.60 abc | 41.21 ± 1.61 bc | 45.36 ± 1.40 ab | 44.83 ± 1.03 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, T.; Li, Y.; Ren, X.; Liu, Q.; Wu, L.; Deng, A.; Gao, R.; Zhang, Y.; Men, L.; Zhang, Z. Feeding Appropriate Nutrients during the Adult Stage to Promote the Growth and Development of Carposina sasakii Offspring. Insects 2024, 15, 283. https://doi.org/10.3390/insects15040283
Fu T, Li Y, Ren X, Liu Q, Wu L, Deng A, Gao R, Zhang Y, Men L, Zhang Z. Feeding Appropriate Nutrients during the Adult Stage to Promote the Growth and Development of Carposina sasakii Offspring. Insects. 2024; 15(4):283. https://doi.org/10.3390/insects15040283
Chicago/Turabian StyleFu, Tong, Yiran Li, Xinrun Ren, Qiao Liu, Ling Wu, Angie Deng, Ruihe Gao, Yuhong Zhang, Lina Men, and Zhiwei Zhang. 2024. "Feeding Appropriate Nutrients during the Adult Stage to Promote the Growth and Development of Carposina sasakii Offspring" Insects 15, no. 4: 283. https://doi.org/10.3390/insects15040283
APA StyleFu, T., Li, Y., Ren, X., Liu, Q., Wu, L., Deng, A., Gao, R., Zhang, Y., Men, L., & Zhang, Z. (2024). Feeding Appropriate Nutrients during the Adult Stage to Promote the Growth and Development of Carposina sasakii Offspring. Insects, 15(4), 283. https://doi.org/10.3390/insects15040283






