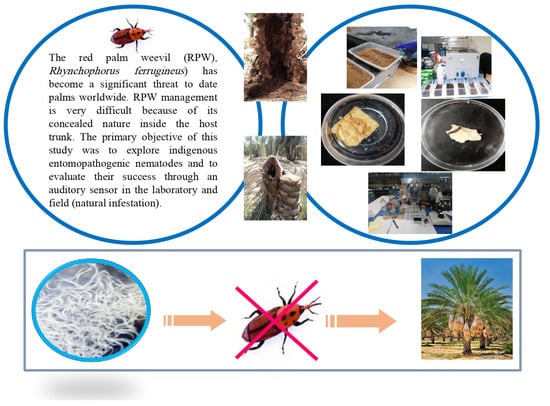
Laboratory Evaluation of Indigenous and Commercial Entomopathogenic Nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. Isolation of Entomopathogenic Nematodes
2.3. Molecular Identification of Entomopathogenic Nematodes
2.3.1. DNA Extraction and Polymerase Chain Reaction Amplification
2.3.2. DNA Sequences
2.4. The Red Palm Weevil Rearing for EPN Bioassay
2.5. Rearing of Entomopathogenic Nematodes
2.6. Pathogenicity Test of EPNs against Red Palm Weevil Eggs, Larvae, and Adults
2.7. Statistical Analysis
3. Results
3.1. Isolation and Identification of Entomopathogenic Nematodes
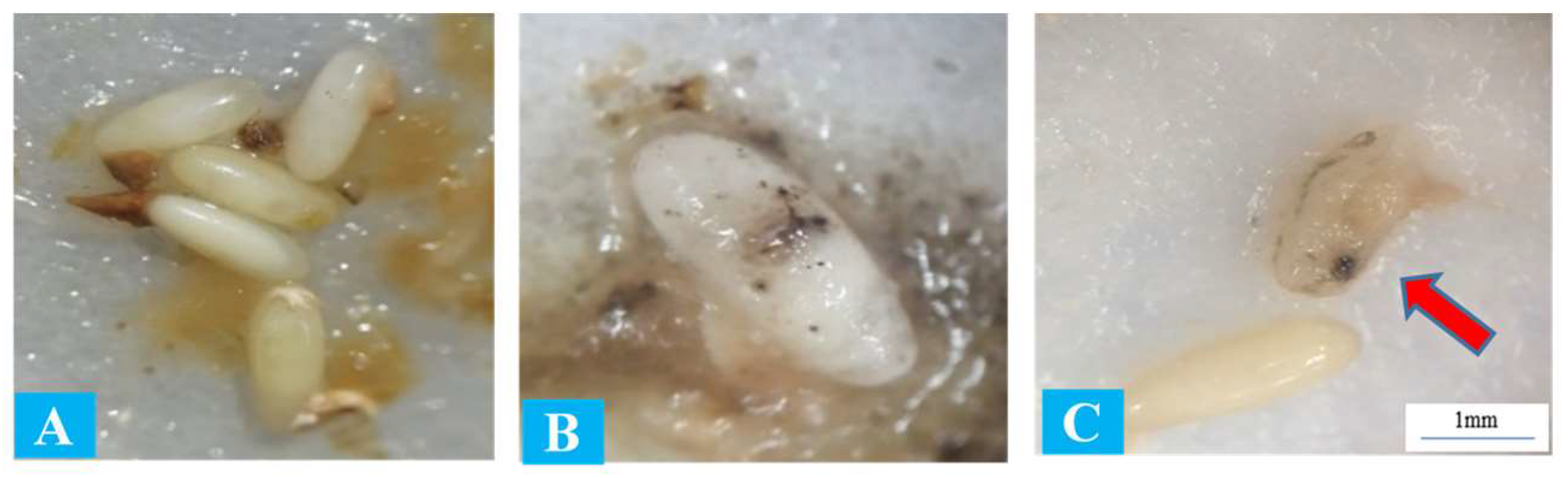
3.2. Entomopathogenic Nematodes Pathogenicity to Red Palm Weevil Eggs
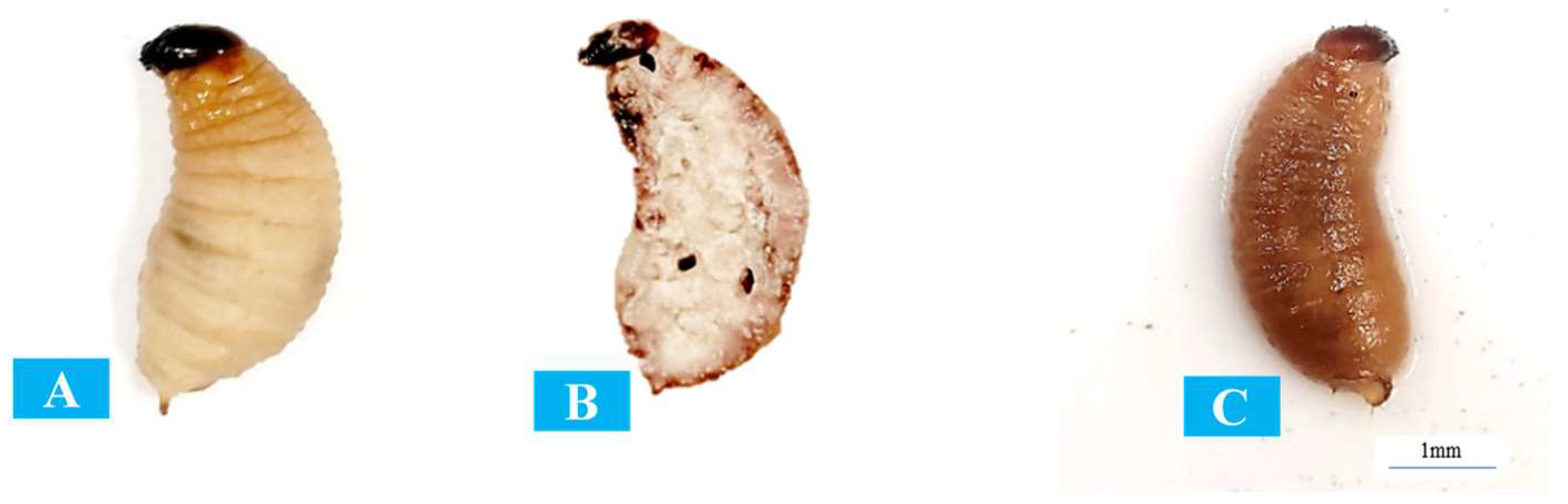
3.3. Pathogenicity against Red Palm Weevil Larvae
3.4. Pathogenicity against Red Palm Weevil Adults
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murphy, S.; Briscoe, B. The red palm weevil as an alien invasive: Biology and the prospects for biological control as a component of IPM. Biocontrol News Inf. 1999, 20, 35N–46N. [Google Scholar]
- Abraham, V.; Shuaibi, M.A.; Faleiro, J.; Abozuhairah, R.; Vidyasagar, P.S. An integrated management approach for red palm weevil Rhynchophorus ferrugineus Oliv. a key pest of date palm in the Middle East. J. Agric. Mar. Sci. 1998, 3, 77–83. [Google Scholar] [CrossRef]
- Faleiro, J. Insight into the management of red palm weevil Rhynchophorus ferrugineus Olivier: Based on experiences on coconut in India and date palm in Saudi Arabia. Fund. Agroalimed 2006, 6, 35–57. [Google Scholar]
- Faghih, A.A. The biology of red palm weevil, Rhynchophorus ferrugineus Oliv. (Coleoptera, Curculionidae) in Saravan region (Sistan & Balouchistan province, Iran). Appl. Entomol. Phytopathol. 1996, 63, 16–18. [Google Scholar]
- Vidyasagar, P.S.P.V.; Al Saihati, A.A.; Al Mohanna, O.E.; Subbei, A.I.; Abdul Mohsin, A.M. Management of red palm weevil Rhynchophorus ferrugineus Oliv., A serious pest of date palm in Al Qatif, Kingdom of Saudi Arabia. J. Plant. Crops 2000, 28, 35–43. [Google Scholar]
- Aldawood, A.; Alsagan, F.; Altuwariqi, H.; Almuteri, A.; Rasool, K. Red palm weevil chemical treatments on date palms in Saudi Arabia: Results of extensive experimentations. In Proceedings of the Colloque Méditerranéen sur les Ravageurs des Palmiers, Nice, France, 16–18 January 2013. [Google Scholar]
- Ferry, M.; Gomez, S. The red palm weevil in the Mediterranean area. Palms 2002, 46, 172–178. [Google Scholar]
- El-Shafie, H.; Faleiro, J. Optimizing components of pheromone-baited trap for the management of red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in date palm agro-ecosystem. J. Plant Dis. Prot. 2017, 124, 279–287. [Google Scholar] [CrossRef]
- Wakil, W.; Yasin, M.; Qayyum, M.A.; Ghazanfar, M.U.; Al-Sadi, A.M.; Bedford, G.O.; Kwon, Y.J. Resistance to commonly used insecticides and phosphine fumigant in red palm weevil, Rhynchophorus ferrugineus (Olivier) in Pakistan. PLoS ONE 2018, 13, e0192628. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayedh, H.; Hussain, A.; Rizwan-ul-Haq, M.; Al-Jabr, A.M. Status of insecticide resistance in field-collected populations of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Int. J. Agric. Biol. 2016, 18, 103–110. [Google Scholar] [CrossRef]
- Abdel-Raheem, M.; ALghamdi, H.A.; Reyad, N.F. Nano essential oils against the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae). Entomol. Res. 2020, 50, 215–220. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.d.C.; de Brida, A.L.; Jean-Baptiste, M.C.; Leite, L.G.; Ovruski, S.M.; Lee, J.C.; Garcia, F.R.M. Compatibility of Entomopathogenic Nematodes with Chemical Insecticides for the Control of Drosophila suzukii (Diptera: Drosophilidae). Plants 2024, 13, 632. [Google Scholar] [CrossRef] [PubMed]
- Mendesil, E.; Shumeta, Z.; Anderson, P.; Rämert, B. Smallholder farmers’ knowledge, perceptions, and management of pea weevil in north and north-western Ethiopia. Crop Prot. 2016, 81, 30–37. [Google Scholar] [CrossRef]
- Sutanto, K.D.; Husain, M.; Rasool, K.G.; Mankin, R.W.; Omer, A.O.; Aldawood, A.S. Acoustic Comparisons of Red Palm Weevil (Rhynchophorus ferrugineus) Mortality in Naturally Infested Date Palms after Injection with Entomopathogenic Fungi or Nematodes, Aluminum Phosphide Fumigation, or Insecticidal Spray Treatments. Insects 2023, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Nurashikin-Khairuddin, W.; Abdul-Hamid, S.N.A.; Mansor, M.S.; Bharudin, I.; Othman, Z.; Jalinas, J. A review of entomopathogenic nematodes as a biological control agent for red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Insects 2022, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Tarasco, E.; Clausi, M.; Rappazzo, G.; Panzavolta, T.; Curto, G.; Sorino, R.; Oreste, M.; Longo, A.; Leone, D.; Tiberi, R. Biodiversity of entomopathogenic nematodes in Italy. J. Helminthol. 2015, 89, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Gözel, U.; Gözel, Ç.; Yurt, Ç.; İnci, D. Efficacy of entomopathogenic nematodes on the red palm weevil Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Curculionidae) larvae. Int. J. Bioassays 2015, 4, 4436–4439. [Google Scholar]
- Ehlers, R.-U.; Hokkanen, H. Insect biocontrol with non-endemic entomopathogenic nematodes (Steinernema and Heterorhabditis spp.): Conclusions and recommendations of a combined OECD and COST workshop on scientific and regulatory policy issues. Biocontrol. Sci. Technol. 1996, 6, 295–302. [Google Scholar] [CrossRef]
- Han, R.; Ehlers, R.-U. Pathogenicity, development, and reproduction of Heterorhabditis bacteriophora and Steinernema carpocapsae under axenic in vivo conditions. J. Invertebr. Pathol. 2000, 75, 55–58. [Google Scholar] [CrossRef]
- Abbas, M.; Saleh, M.; Akil, A. Laboratory and field evaluation of the pathogenicity of entomopathogenic nematodes to the red palm weevil, Rhynchophorus ferrugineus (Oliv.) (Col.: Curculionidae). Anz. Für Schädlingskunde 2001, 74, 167–168. [Google Scholar] [CrossRef]
- El Roby, A.; Rezk, M.; Shamseldean, M. Compartive Efficiency of Egyptian Entomopathogenic Nematodes and Imidacloprid on the Red Palm Weevil, Rhynchophorus ferrugineus Oliv. J. Plant Prot. Pathol. 2018, 9, 447–451. [Google Scholar] [CrossRef]
- Ansari, M.A.; Butt, T.M. Influence of the application methods and doses on the susceptibility of black vine weevil larvae Otiorhynchus sulcatus to Metarhizium anisopliae in field-grown strawberries. BioControl 2013, 58, 257–267. [Google Scholar] [CrossRef]
- Gupta, S.; Kaul, V.; Srivastava, K.; Monobrlillah, M. Pathogenicity and in vivo culturing of a local isolate of Steinernema carpocapsae against Spodoptera litura (Fab.). Indian J. Entomol. 2008, 70, 346–349. [Google Scholar]
- Razia, M.; Sivaramakrishnan, S. Isolation and identification of entomopathogenic nematodes of Kodaikanal hills of South India. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 693–699. [Google Scholar]
- Cimen, H.; Půža, V.; Nermuť, J.; Hatting, J.; Ramakuwela, T.; Faktorova, L.; Hazir, S. Steinernema beitlechemi n. sp., a new entomopathogenic nematode (Nematoda: Steinernematidae) from South Africa. Nematology 2016, 18, 439–453. [Google Scholar] [CrossRef]
- Mráček, Z.; Půža, V.; Nermut, J. Addendum to the description of Steinernema carpocapsae (Weiser, 1955) Wouts, Mráček, Gerdin & Bedding, 1982. Russ. J. Nematol. 2014, 22, 109–120. [Google Scholar]
- Khashaba, E.H.; Abd El Azim, A. Isolation, identification, and study of the genetic diversity between three entomopathogenic nematodes belonging to Heterorhabditis sp. using ISSR technique. Egypt. J. Biol. Pest Control 2021, 31, 1–7. [Google Scholar] [CrossRef]
- Aldawood, A.S.; Rasool, K.G.; Sukirno, S.; Husain, M.; Sutanto, K.D.; Alduailij, M.A. Semi-artificial diet developed for the successful rearing of red palm weevil: Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) in the laboratory. J. King Saud Univ. Sci. 2022, 34, 102272. [Google Scholar] [CrossRef]
- Al-Ayedh, H. Evaluating a semi-synthetic diet for rearing the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Int. J. Trop. Insect Sci. 2011, 31, 20–28. [Google Scholar] [CrossRef]
- Kasuya, E. Angular transformation–another effect of different sample sizes. Ecol. Res. 2004, 19, 165–167. [Google Scholar] [CrossRef]
- SAS. SAS/STAT 9.2 User’s Guide, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2009. [Google Scholar]
- Khan, Y.; Javed, N. Entomopathogenic nematodes survey, persistence in soil, reproductive potential and their effects on Meloidogyne incognita. Egypt. J. Agronematology 2018, 17, 109–120. [Google Scholar] [CrossRef]
- Dzięgielewska, M.; Skwiercz, A. The Influence of SELECTED abiotic factors on the occurrence of entomopathogenic nematodes (Steinernematidae, Heterorhabditidae) in soil. Pol. J. Soil Sci. 2018, 1, 12–21. [Google Scholar] [CrossRef]
- Cheruiyot, H.K.; Ochuodho, J.; Njira, P. Assessment of the prevalence of entomopathogenic nematodes in vegetable fields in Uasin Gishu county, Kenya. Afr. Crop Sci. Conf. Proc. 2013, 11, 251–255. [Google Scholar]
- Campos-Herrera, R.; Pathak, E.; El-Borai, F.E.; Stuart, R.J.; Gutiérrez, C.; Rodríguez-Martín, J.A.; Graham, J.H.; Duncan, L.W. Geospatial patterns of soil properties and the biological control potential of entomopathogenic nematodes in Florida citrus groves. Soil Biol. Biochem. 2013, 66, 163–174. [Google Scholar] [CrossRef]
- Al-Zaidawi, J.B.; Karimi, J.; Moghadam, E.M. Molecular characterizations of the entomopathogenic nematodes, Heterorhabditis bacteriophora and Oscheius myriophilus from Iraq. Egypt J. Biol. Pest Control 2019, 29, 38. [Google Scholar] [CrossRef]
- Chaerani; Suryadi, Y.; Priyatno, T.P.; Koswanudin, D.; Rahmat, U.; Sujatmo; Yusuf; Griffin, C.T. Isolasi nematoda patogen serangga Steinernema dan Heterorhabditis. J. Hama Dan Penyakit Tumbuh. Trop. 2007, 1, 1–9. [Google Scholar] [CrossRef]
- Hominick, W.M. Biogeography. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 115–143. [Google Scholar]
- El-Kholy, M.; Abdelzaher, H.; Abdel-Moniem, A.; Ibraheem, M. Isolation of entomopathogenic nematodes from soil of olive orchards and evaluation as a biological control of the olive scale, Parlatoria oleae Colvée (Homoptera: Diaspididae) in Saudi Arabia. Int. J. Geol. Agric. Environ. Sci. 2014, 2, 23–26. [Google Scholar]
- Delgado-Gamboa, J.R.; Ruíz-Vega, J.; Ibarra-Rendón, J.E.; Aquino-Bolaños, T.; Giron-Pablo, S. Isolation and identification of native entomopathogenic nematodes (Nematoda: Rhabditidae) and potential for controlling Scyphophorus acupunctatus in a laboratory. Southwest. Entomol. 2015, 40, 731–739. [Google Scholar] [CrossRef]
- Ma, J.; Chen, S.; Zou, Y.; Li, X.; Han, R.; de Clercq, P.; Moens, M. Natural occurrence of entomopathogenic nematodes in North China. Russ. J. Nematol. 2010, 18, 117–126. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Foelkel, E.; Voss, M.; Monteiro, L.; Nishimura, G. Isolation of entomopathogenic nematodes in an apple orchard in Southern Brazil and its virulence to Anastrepha fraterculus (Diptera: Tephritidae) larvae, under laboratory conditions. Braz. J. Biol. 2016, 77, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Girón-Pablo, S.; Ruiz-Vega, J.; Pérez-Pacheco, R.; Sánchez-García, J.; Aquino-Bolaños, T. Isolation of entomopathogenic nematodes and control of Phyllophaga vetula Horn in Oaxaca, Mexico. Afr. J. Biotechnol. 2012, 11, 16525–16531. [Google Scholar]
- Salama, H.; Abd-Elgawad, M.M. Isolation of heterorhabditid nematodes from palm tree planted areas and their implications in the red palm weevil control. Anz. Für Schädlingskunde=J. Pest Sci. 2001, 74, 43–45. [Google Scholar] [CrossRef]
- Bhat, A.H.; Chaubey, A.K.; Askary, T.H. Global distribution of entomopathogenic nematodes, Steinernema and Heterorhabditis. Egypt. J. Biol. Pest Control 2020, 30, 1–15. [Google Scholar] [CrossRef]
- Chaerani; Prabowo, H.; Indrayani, I.G.A.A. Isolation and Molecular Identification of Entomopathogenic Nematodes (Steinernema and Heterorhabditis) from East Java and Bali (Isolasi dan Identifikasi Molekuler Nematoda Entomopatogen [Steinernema dan Heterorhabditis] dari Jawa Timur dan Bali). J. AgroBiogen 2018, 14, 85–96. [Google Scholar] [CrossRef]
- Shahina, F.; Salma, J.; Mehreen, G.; Bhatti, M.; Tabassum, K. Rearing of Rhynchophorus ferrugineus in laboratory and field conditions for carrying out various efficacy studies using EPNs. Pak. J. Nematol. 2009, 27, 219–228. [Google Scholar]
- Triggiani, O.; Tarasco, E. Evaluation of the effects of autochthonous and commercial isolates of Steinernematidae and Heterorhabditidae on Rhynchophorus ferrugineus. Bull. Insectology 2011, 64, 175–180. [Google Scholar]
- El Sobki, A.; El-Ashry, R.; Arafa, O.E. Joint toxicity of Insecticides and some entomopathogenic nematode species against Rhynchophorus ferrugineus (Olivier) insect in vitro. J. Plant Prot. Pathol. 2020, 11, 161–168. [Google Scholar] [CrossRef]
- Llácer, E.; Martínez de Altube, M.M.; Jacas, J. Evaluation of the efficacy of Steinernema carpocapsae in a chitosan formulation against the red palm weevil, Rhynchophorus ferrugineus, in Phoenix canariensis. BioControl 2009, 54, 559–565. [Google Scholar] [CrossRef]
- Atakan, E.; Elekçioğlu, I.; Gözel, U.; Güneş, Ç.; Yüksel, O. First report of Heterorhabditis bacteriophora (Poinar, 1975) (Nematoda: Heterorhabditidae) isolated from the red palm weevil, Rhynchophorus ferrugineus (Oliver, 1790) (Coleoptera: Curculionidae) in Turkey. EPPO Bull. 2009, 39, 189–193. [Google Scholar] [CrossRef]
- Indriyanti, D.R.; Widiyaningrum, P.; Slamet, M.; Maretta, Y.A. Effectiveness of Metarhizium anisopliae and Entomopathogenic Nematodes to Control Oryctes rhinoceros Larvae in the Rainy Season. Pak. J. Biol. Sci. 2017, 20, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Ahmad, J.N.; Sharif, M.Z.; Majeed, D.; Kiran, H.; Jafir, M.; Ali, H. Comparative effectiveness of entomopathogenic nematodes against red palm weevil (Rhynchophorus ferrugineus) in Pakistan. J. Entomol. Zool. Stud. 2017, 5, 756–760. [Google Scholar]
- Cappa, F.; Torrini, G.; Mazza, G.; Inghilesi, A.F.; Benvenuti, C.; Viliani, L.; Roversi, P.F.; Cervo, R. Assessing immunocompetence in red palm weevil adult and immature stages in response to bacterial challenge and entomopathogenic nematode infection. Insect Sci. 2020, 27, 1031–1042. [Google Scholar] [CrossRef] [PubMed]


| Exposure Time (Days) | Treatments | Egg Mortality (%) | Statistical Analysis |
|---|---|---|---|
| 3 | H. indica | 55.3 ± 8 a | F = 17.7; df = 3, 8; p = 0.0007 |
| S. carpocapsae | 38.8 ± 6.98 a | ||
| Palmanem | 43 ± 3.86 a | ||
| Control (H2O) | 0 ± 0 b | ||
| 5 | H. indica | 90 ± 0 a | F = 215, df = 3, 8; p ≤ 0.0001 |
| S. carpocapsae | 90 ± 0 a | ||
| Palmanem | 90 ± 0 a | ||
| Control (H2O) | 1.9 ± 1.9 b |
| Exposure Time (Days) | Treatments | Larval Mortality (%) | Statistical Analysis |
|---|---|---|---|
| 7 | H. indica | 66.2 ± 11.9 a | F = 14.56, df = 3, 8; p ≤ 0.0013 |
| S. carpocapsae | 47.9 ± 6.4 a | ||
| Palmanem | 78.1 ± 11.9 a | ||
| Control (H2O) | 0 ± 0 b | ||
| 10 | H. indica | 90 ± 0 a | F = 215, df = 3, 8; p ≤ 0.0001 |
| S. carpocapsae | 90 ± 0 a | ||
| Palmanem | 90 ± 0 a | ||
| Control (H2O) | 1.9 ± 1.9 b |
| Exposure Time (Days) | Treatments | Adult Mortality (%) | Statistical Analysis |
|---|---|---|---|
| 7 | H. indica | 59.4 ± 16.1 ab | F = 18.50, df = 3, 8; p ≤ 0.0034 |
| S. carpocapsae | 47.9 ± 6.4 b | ||
| Palmanem | 90 ± 0 a | ||
| Control (H2O) | 0 ± 0 c | ||
| 10 | H. indica | 90 ± 0 a | F = 215, df = 3, 8; p ≤ 0.0001 |
| S. carpocapsae | 90 ± 0 a | ||
| Palmanem | 90 ± 0 a | ||
| Control (H2O) | 1.9 ± 1.9 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husain, M.; Rasool, K.G.; Sutanto, K.D.; Omer, A.O.; Tufail, M.; Aldawood, A.S. Laboratory Evaluation of Indigenous and Commercial Entomopathogenic Nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Insects 2024, 15, 290. https://doi.org/10.3390/insects15040290
Husain M, Rasool KG, Sutanto KD, Omer AO, Tufail M, Aldawood AS. Laboratory Evaluation of Indigenous and Commercial Entomopathogenic Nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Insects. 2024; 15(4):290. https://doi.org/10.3390/insects15040290
Chicago/Turabian StyleHusain, Mureed, Khawaja G. Rasool, Koko D. Sutanto, Abdalsalam O. Omer, Muhammad Tufail, and Abdulrahman S. Aldawood. 2024. "Laboratory Evaluation of Indigenous and Commercial Entomopathogenic Nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae)" Insects 15, no. 4: 290. https://doi.org/10.3390/insects15040290
APA StyleHusain, M., Rasool, K. G., Sutanto, K. D., Omer, A. O., Tufail, M., & Aldawood, A. S. (2024). Laboratory Evaluation of Indigenous and Commercial Entomopathogenic Nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Insects, 15(4), 290. https://doi.org/10.3390/insects15040290