Silkworm Hemolymph and Cocoon Metabolomics Reveals Valine Improves Feed Efficiency of Silkworm Artificial Diet
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Insects and Diets
2.2. Cocoon and Hemolymph Samples Collection
2.3. Feeding with Different Concentrations of Methionine
2.4. Measurement of Developmental Parameters
2.5. GC-MS Sample Pretreatment
- (1)
- Freeze-dried samples of silkworm cocoons and hemolymph were redissolved in 80 μL methoxyamine solution (20 mg/mL).
- (2)
- The sample was vortexed for 2 min and treated with ultrasonic wave for 15 min after instant separation. Derivatization was carried out at 37 °C for 1.5 h.
- (3)
- MSTFA (60 μL) was added with instantaneous centrifugation. The vortex instantaneously dissociated at 37 °C for 1 h.
- (4)
- After immediate separation, 10 μL n-heptane was added. The mixture was vortexed, which resulted in transient dissociation.
- (5)
- Centrifugation was performed at 14,000× g for 20 min at 10 °C.
- (6)
- The supernatant (100 μL) was sucked into the loading bottle.
2.6. GC-MS Data Processing
3. Results
3.1. Cocoon and Hemolymph Extract Metabolic Profiles of Silkworms Reared on Fresh Mulberry Leaf and Artificial Diet
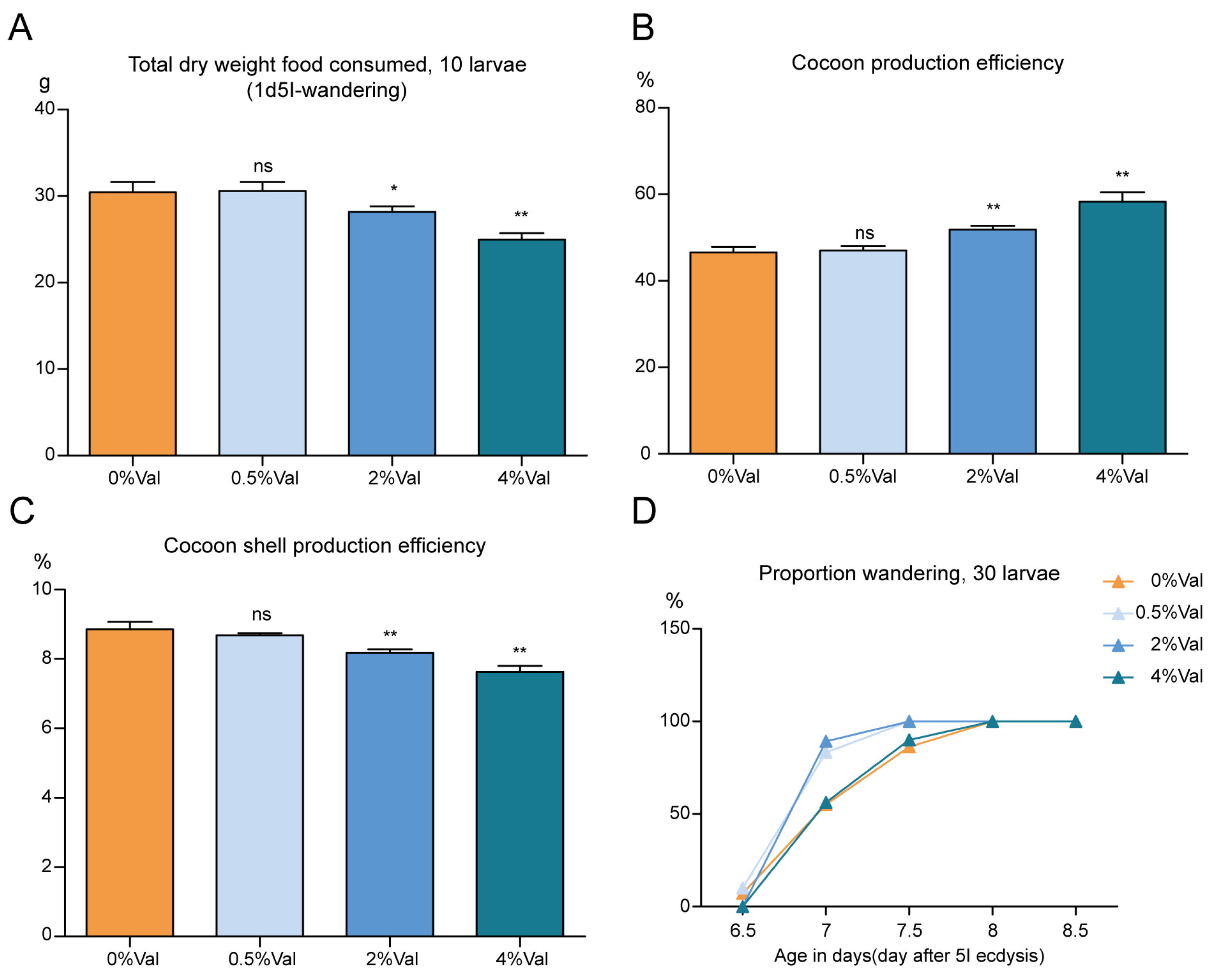
3.2. Effect of Valine on Feed Efficiency of Silkworm, Bombyx Mori
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, Q.; Zhou, Z.; Lu, C.; Cheng, D.; Dai, F.; Li, B.; Zhao, P.; Zha, X.; Cheng, T.; Chai, C.; et al. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 2004, 306, 1937–1940. [Google Scholar] [CrossRef]
- Li, Y.; Ni, M.; Li, F.; Zhang, H.; Xu, K.; Zhao, X.; Tian, J.; Hu, J.; Wang, B.; Shen, W.; et al. Effects of TiO2 NPs on Silkworm Growth and Feed Efficiency. Biol. Trace Elem. Res. 2016, 169, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Shyamala, M.B.; Sharada, K.; Bhat, M.G.; Bhat, J.V. Chloromycetin in the nutrition of the silkworm Bombyx mori L.-II. Influence on digestion and utilization of protein, fat, and minerals. J. Insect Physiol. 1960, 4, 229–234. [Google Scholar] [CrossRef]
- Zhang, H.; Ni, M.; Li, F.; Xu, K.; Wang, B.; Hong, F.; Shen, W.; Li, B. Effects of feeding silkworm with nanoparticulate anatase TiO2 (TiO2 NPs) on its feed efficiency. Biol. Trace Elem. Res. 2014, 159, 224–232. [Google Scholar] [CrossRef]
- Saoi, M.; Britz-McKibbin, P. New Advances in Tissue Metabolomics: A Review. Metabolites 2021, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, X.; Ye, A.; Cao, J.; He, R.; Pan, M.; Jin, F.; Ma, H.; Zhou, W. Multi-tissue metabolomic profiling reveals potential mechanisms of cocoon yield in silkworms (Bombyx mori) fed formula feed versus mulberry leaves. Front. Mol. Biosci. 2022, 9, 977047. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.L.; Zhang, S.X.; Tao, H.; Chen, Z.H.; Li, X.; Qiu, J.F.; Cui, W.Z.; Sima, Y.H.; Cui, W.Z.; Xu, S.Q. Metabolomics differences between silkworms (Bombyx mori) reared on fresh mulberry (Morus) leaves or artificial diets. Sci. Rep. 2017, 7, 10972. [Google Scholar] [CrossRef]
- Tao, S.; Wang, J.; Liu, M.; Sun, F.; Li, B.; Ye, C. Haemolymph metabolomic differences in silkworms (Bombyx mori L.) under mulberry leaf and two artificial diet rearing methods. Arch. Insect Biochem. Physiol. 2022, 109, e21851. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, J.; Deng, X.; Liu, L.; Zha, X. Metabonomic Analysis of Silkworm Midgut Reveals Differences between the Physiological Effects of an Artificial and Mulberry Leaf Diet. Insects 2023, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Wang, G.; Dong, Z.; Xia, Q.; Zhao, P. Comparative Fecal Metabolomes of Silkworms Being Fed Mulberry Leaf and Artificial Diet. Insects 2020, 11, 851. [Google Scholar] [CrossRef]
- Meng, X.; Abdlli, N.; Wang, N.; Lu, P.; Nie, Z.; Dong, X.; Lu, S.; Chen, K. Effects of Ag Nanoparticles on Growth and Fat Body Proteins in Silkworms (Bombyx mori). Biol. Trace Elem. Res. 2017, 180, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Zhang, H.; Li, F.C.; Wang, B.B.; Xu, K.Z.; Shen, W.D.; Li, B. Nanoparticulate anatase TiO2 (TiO2 NPs) upregulates the expression of silkworm (Bombyx mori) neuropeptide receptor and promotes silkworm feeding, growth, and silking. Peptides 2015, 68, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, X.; Zhao, P.; Sun, Y.; Zhao, X.; Xiong, Y.; Xu, G.; Xia, Q. GC/MS-based metabolomic studies reveal key roles of glycine in regulating silk synthesis in silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2015, 57, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ye, A.; Wu, X.; Qu, Z.; Xu, S.; Sima, Y.; Wang, Y.; He, R.; Jin, F.; Zhan, P.; et al. Combined analysis of silk synthesis and hemolymph amino acid metabolism reveal key roles for glycine in increasing silkworm silk yields. Int. J. Biol. Macromol. 2022, 209, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Liu, Q.; Tan, X.; Xie, X.; Xia, Q.; Zhao, P. GC/MS-based metabolomics analysis reveals active fatty acids biosynthesis in the Filippi’s gland of the silkworm, Bombyx mori, during silk spinning. Insect Biochem. Mol. Biol. 2018, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, D.; Meng, Z.; Dong, Z.; Lin, Y.; Chen, S.; Xia, Q.; Zhao, P. Wild Silkworm Cocoon Contains More Metabolites Than Domestic Silkworm Cocoon to Improve Its Protection. J. Insect Sci. 2017, 17, 105. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Wang, X.; Chang, L.; Liu, Q.; Xia, Q.; Zhao, P. BmSLC7A5 is essential for silk protein synthesis and larval development in Bombyx mori. Insect Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zheng, H.S.; Zhang, R.; Xu, Y.P.; Pan, H.; Li, S.; Liu, C.; Cheng, T.C. Dimmed gene knockout shortens larval growth and reduces silk yield in the silkworm, Bombyx mori. Insect Mol. Biol. 2023, 32, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, F.; Guo, K.; Xu, J.; Zhao, P.; Xia, Q. CRISPR/Cas9-mediated gene editing of the let-7 seed sequence improves silk yield in the silkworm, Bombyx mori. Int. J. Biol. Macromol. 2023, 243, 124793. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Fu, X.; Wang, G.; Qi, W.; Mao, D.; Shi, Z.; Shen, W.L.; Wang, L. A post-ingestive amino acid sensor promotes food consumption in Drosophila. Cell Res. 2018, 28, 1013–1025. [Google Scholar] [CrossRef]
- Qu, J.; Feng, P.; Zhu, Q.; Ren, Y.; Li, B. Study on the Effect of Stretching on the Strength of Natural Silk Based on Different Feeding Methods. ACS Biomater. Sci. Eng. 2022, 8, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Nicodemo, D.; Oliveira, J.E.; Sedano, A.A.; Marconcini, J.M.; Tonoli, G.H.D. Impact of different silkworm dietary supplements on its silk performance. J. Mater. Sci. 2014, 49, 6302–6310. [Google Scholar] [CrossRef]
- Goto, S.; Nagao, K.; Bannai, M.; Takahashi, M.; Nakahara, K.; Kangawa, K.; Murakami, N. Anorexia in rats caused by a valine-deficient diet is not ameliorated by systemic ghrelin treatment. Neuroscience 2010, 166, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Jia, H.; He, P.; Mao, X.; Qiao, S.; Zeng, X. Valine Supplementation in a Reduced Protein Diet Regulates Growth Performance Partially through Modulation of Plasma Amino Acids Profile, Metabolic Responses, Endocrine, and Neural Factors in Piglets. J. Agric. Food Chem. 2018, 66, 3161–3168. [Google Scholar] [CrossRef]
- Meyer, F.; Jansen van Rensburg, C.; Gous, R.M. The response of weaned piglets to dietary valine and leucine. Animal 2017, 11, 1279–1286. [Google Scholar] [CrossRef][Green Version]




| Metabolite | Category | p | AC/MC |
|---|---|---|---|
| urea | Organic acid | 3 × 10−12 | 819.98 |
| D-pinitol | Sugar | 3 × 10−18 | 136.78 |
| glycerol | Alcohol | 9 × 10−13 | 66.77 |
| D-gluconic acid | Organic acid | 7 × 10−14 | 8.61 |
| (R*, S*)-3,4-dihydroxybutanoic acid | Organic acid | 2 × 10−12 | 5.96 |
| benzoic acid | Organic acid | 4 × 10−16 | 4.88 |
| propanoic acid | Organic acid | 7 × 10−15 | 4.36 |
| 2,3,4-trihydroxybutyric acid | Organic acid | 1 × 10−6 | 3.09 |
| D-(+)-talose | Sugar | 6 × 10−3 | 1.60 |
| butanedioic acid | Organic acid | 1 × 10−3 | 1.22 |
| L-threonic acid | Organic acid | 2 × 10−9 | 0.42 |
| citric acid | Organic acid | 4 × 10−10 | 0.40 |
| L-tyrosine | Amino acid | 4 × 10−4 | 0.37 |
| 2-butenedioic acid(E) | Organic acid | 1 × 10−12 | 0.24 |
| phenylalanine | Amino acid | 4 × 10−4 | 0.15 |
| L-threonine | Amino acid | 9 × 10−5 | 0.10 |
| L-leucine | Amino acid | 5 × 10−13 | 0.10 |
| L-isoleucine | Amino acid | 1 × 10−13 | 0.07 |
| L-valine | Amino acid | 1 × 10−12 | 0.05 |
| glycine | Amino acid | 2 × 10−10 | 0.05 |
| serine | Amino acid | 1 × 10−10 | 0.04 |
| L-asparagine | Amino acid | 6 × 10−5 | 0.03 |
| Metabolite | Category | p | AH/MH |
|---|---|---|---|
| D-glucopyranoside | Glycosides | 2 × 10−16 | 0.06 |
| L-valine | Amino acid | 2 × 10−9 | 0.08 |
| L-leucine | Amino acid | 2 × 10−9 | 0.10 |
| butanoic acid | Organic acid | 2 × 10−6 | 0.10 |
| 2-keto-L-gluconic acid | Organic acid | 1 × 10−10 | 0.14 |
| L-isoleucine | Amino acid | 2 × 10−7 | 0.14 |
| Myo-inositol | Sugar | 9 × 10−7 | 0.14 |
| methylmaleic acid | Organic acid | 2 × 10−13 | 0.23 |
| DL-arabinose | Sugar | 3 × 10−12 | 0.28 |
| 2-hydroxymandelic acid | Organic acid | 3 × 10−7 | 0.30 |
| L-threonine | Amino acid | 2 × 10−6 | 0.32 |
| L-alanine | Amino acid | 1 × 10−6 | 0.35 |
| L-asparagine | Amino acid | 4 × 10−7 | 0.42 |
| D-mannose | Sugar | 1 × 10−8 | 0.44 |
| glycine | Amino acid | 3 × 10−5 | 0.48 |
| phenylalanine | Amino acid | 7 × 10−4 | 0.49 |
| hexanedioic acid | Organic acid | 9 × 10−8 | 0.56 |
| butanedioic acid | Organic acid | 3 × 10−8 | 0.58 |
| 2-butenedioic acid | Organic acid | 2 × 10−8 | 0.59 |
| phosphoric acid | Organic acid | 3 × 10−3 | 0.70 |
| serine | Amino acid | 3 × 10−2 | 0.74 |
| L-proline | Amino acid | 1 × 10−2 | 0.81 |
| 1,4-butanediamine | Biogenic amine | 3 × 10−4 | 1.23 |
| hexadecanoic acid | Fatty acid | 3 × 10−3 | 1.40 |
| β-sitosterol | Sterol and lipid | 6 × 10−4 | 1.41 |
| glycerol | Alcohol | 1 × 10−3 | 1.41 |
| citric acid | Organic acid | 6 × 10−5 | 1.46 |
| D-(+)-cellobiose | Sugar | 2 × 10−2 | 1.55 |
| D-(+)-trehalose | Sugar | 6 × 10−10 | 1.68 |
| β-alanine | Amino acid | 5 × 10−5 | 1.71 |
| N,N-dimethylglycine | Amino acid | 3 × 10−6 | 1.92 |
| urea | Organic acid | 1 × 10−10 | 2.08 |
| D-sorbitol | Sugar alcohol | 3 × 10−5 | 2.13 |
| pentanedioic acid | Organic acid | 6 × 10−9 | 2.14 |
| ritalinic acid | Benzene | 1 × 10−9 | 2.16 |
| mannitol | Sugar | 2 × 10−7 | 2.20 |
| aminomalonic acid | Organic acid | 7 × 10−4 | 2.59 |
| pantothenic acid | Vitamin | 1 × 10−13 | 4.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Li, L.; Qin, D.; Chen, H.; Liu, Y.; Shen, G.; Zhao, P. Silkworm Hemolymph and Cocoon Metabolomics Reveals Valine Improves Feed Efficiency of Silkworm Artificial Diet. Insects 2024, 15, 291. https://doi.org/10.3390/insects15040291
Wu J, Li L, Qin D, Chen H, Liu Y, Shen G, Zhao P. Silkworm Hemolymph and Cocoon Metabolomics Reveals Valine Improves Feed Efficiency of Silkworm Artificial Diet. Insects. 2024; 15(4):291. https://doi.org/10.3390/insects15040291
Chicago/Turabian StyleWu, Jinxin, Lingyi Li, Daoyuan Qin, Han Chen, Yuanlin Liu, Guanwang Shen, and Ping Zhao. 2024. "Silkworm Hemolymph and Cocoon Metabolomics Reveals Valine Improves Feed Efficiency of Silkworm Artificial Diet" Insects 15, no. 4: 291. https://doi.org/10.3390/insects15040291
APA StyleWu, J., Li, L., Qin, D., Chen, H., Liu, Y., Shen, G., & Zhao, P. (2024). Silkworm Hemolymph and Cocoon Metabolomics Reveals Valine Improves Feed Efficiency of Silkworm Artificial Diet. Insects, 15(4), 291. https://doi.org/10.3390/insects15040291





