Larval Frass of Hermetia illucens as Organic Fertilizer: Composition and Beneficial Effects on Different Crops
Abstract
:Simple Summary
Abstract
1. Introduction
Insect Rearing
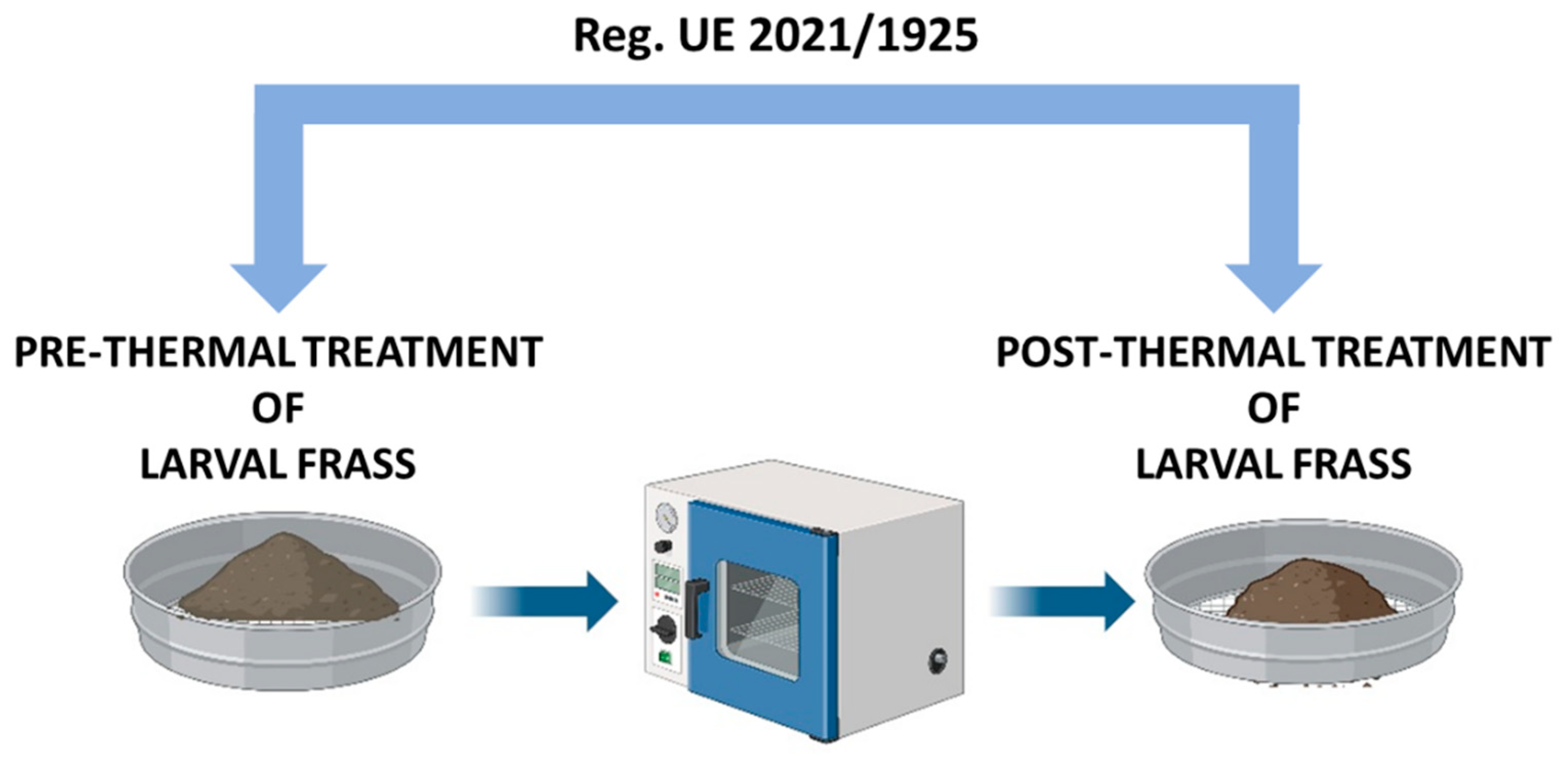
2. Frass
2.1. Frass Composition
2.1.1. Macronutrients
2.1.2. Micronutrients
2.2. Microbiological Composition of BSFL Frass
Impact of BSFL Frass Microorganisms
3. Frass Application
4. Conclusions
5. Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X. Managing land carrying capacity: Key to achieving sustainable production systems for food security. Land 2022, 11, 484. [Google Scholar] [CrossRef]
- Liang, G. Nitrogen fertilization mitigates global food insecurity by increasing cereal yield and its stability. Glob. Food Secur. 2022, 34, 100652. [Google Scholar] [CrossRef]
- Bebber, D.P.; Richards, V.R. A meta-analysis of the effect of organic and mineral fertilizers on soil microbial diversity. Appl. Soil Ecol. 2022, 175, 104450. [Google Scholar] [CrossRef]
- Gärttling, D.; Kirchner, S.M.; Schulz, H. Assessment of the N-and P-fertilization effect of black soldier fly (Diptera: Stratiomyidae) by-products on maize. J. Insect Sci. 2020, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect rearing: Potential, challenges, and circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]
- Madau, F.A.; Arru, B.; Furesi, R.; Pulina, P. Insect farming for feed and food production from a circular business model perspective. Sustainability 2020, 12, 5418. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Müller, A.; Wolf, D.; Gutzeit, H.O. The black soldier fly, Hermetia illucens—A promising source for sustainable production of proteins, lipids and bioactive substances. Z. Naturforschung C 2017, 72, 351–363. [Google Scholar] [CrossRef]
- Smetana, S.; Palanisamy, M.; Mathys, A.; Heinz, V. Sustainability of insect use for feed and food: Life Cycle Assessment perspective. J. Clean. Prod. 2016, 137, 741–751. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Scieuzo, C.; Franco, A.; Salvia, R.; Triunfo, M.; Addeo, N.F.; Vozzo, S.; Piccolo, G.; Bovera, F.; Ritieni, A.; Francia, A.D.; et al. Enhancement of fruit byproducts through bioconversion by Hermetia illucens (Diptera: Stratiomyidae). Insect Sci. 2023, 30, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Scieuzo, C.; Salvia, R.; Mancini, I.M.; Caniani, D.; Masi, S.; Falabella, P. A mobile black soldier fly farm for on-site disposal of animal dairy manure. Bull. Insectol. 2022, 75, 75–82. [Google Scholar]
- Fowles, T.M.; Nansen, C. Insect-based bioconversion: Value from food waste. In Food Waste Management: Solving the Wicked Problem; Palgrave Macmillan: Cham, Switzerland, 2020; pp. 321–346. [Google Scholar]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Schmitt, E.; Falabella, P. Lipids from Hermetia illucens, an innovative and sustainable source. Sustainability 2021, 13, 10198. [Google Scholar] [CrossRef]
- Franco, A.; Salvia, R.; Scieuzo, C.; Schmitt, E.; Russo, A.; Falabella, P. Lipids from insects in cosmetics and for personal care products. Insects 2021, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Scieuzo, C.; Salvia, R.; Pucciarelli, V.; Borrelli, L.; Addeo, N.F.; Bovera, F.; Laginestra, A.; Schmitt, E. Antimicrobial activity of lipids extracted from Hermetia illucens reared on different substrates. Appl. Microbiol. Biotechnol. 2024, 108, 167. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Parry, N.J.; Weldon, C.W. Nutritional content and bioconversion efficiency of Hermetia illucens (Diptera: Stratiomyidae): Harvest as larvae or prepupae? Austral Entomol. 2021, 60, 707–712. [Google Scholar] [CrossRef]
- Ites, S.; Smetana, S.; Toepfl, S.; Heinz, V. Modularity of insect production and processing as a path to efficient and sustainable food waste treatment. J. Clean. Prod. 2020, 248, 119248. [Google Scholar] [CrossRef]
- Van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Scieuzo, C.; Giglio, F.; Rinaldi, R.; Lekka, M.E.; Cozzolino, F.; Monaco, V.; Monti, M.; Salvia, R.; Falabella, P. In Vitro Evaluation of the Antibacterial Activity of the Peptide Fractions Extracted from the Hemolymph of Hermetia illucens (Diptera: Stratiomyidae). Insects 2023, 14, 464. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect chitin-based nanomaterials for innovative cosmetics and cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef] [PubMed]
- Cummins, V.C., Jr.; Rawles, S.D.; Thompson, K.R.; Velasquez, A.; Kobayashi, Y.; Hager, J.; Webster, C.D. Evaluation of black soldier fly (Hermetia illucens) larvae meal as partial or total replacement of marine fish meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2017, 473, 337–344. [Google Scholar] [CrossRef]
- Newton, G.L.; Booram, C.V.; Barker, R.W.; Hale, O.M. Dried Hermetia illucens larvae meal as a supplement for swine. J. Anim. Sci. 1977, 44, 395–400. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Zheng, L.; Wang, Y.; Zhang, J.; Yu, Z.; Zhang, Y. Potential biodiesel and biogas production from corncob by anaerobic fermentation and black soldier fly. Bioresour. Technol. 2015, 194, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Triunfo, M.; Guarnieri, A.; Ianniciello, D.; Coviello, L.; Vitti, A.; Nuzzaci, M.; Salvia, R.; Scieuzo, C.; Falabella, P. Hermetia illucens, an innovative and sustainable source of chitosan-based coating for postharvest preservation of strawberries. iScience 2023, 26, 108576. [Google Scholar] [CrossRef] [PubMed]
- Tafi, E.; Triunfo, M.; Guarnieri, A.; Ianniciello, D.; Salvia, R.; Scieuzo, C.; Ranieri, A.; Castagna, A.; Lepuri, S.; Hahn, T.; et al. Preliminary investigation on the effect of insect-based chitosan on preservation of coated fresh cherry tomatoes. Sci. Rep. 2023, 13, 7030. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Ianniciello, D.; Scieuzo, C.; Salvia, R.; Hahn, T.; Zibek, S.; Falabella, P. Usage of chitosan from Hermetia illucens as a preservative for fresh Prunus species fruits: A preliminary analysis. Chem. Biol. Technol. Agric. 2023, 10, 101. [Google Scholar] [CrossRef]
- Bortolini, S.; Macavei, L.I.; Saadoun, J.H.; Foca, G.; Ulrici, A.; Bernini, F.; Malferrari, D.; Setti, L.; Ronga, D.; Maistrello, L. Hermetia illucens (L.) larvae as chicken manure management tool for circular economy. J. Clean. Prod. 2020, 262, 121289. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Kelly, A.; Oldham, T.; Rogers, R.D. Agricultural uses of chitin polymers. Environ. Chem. Lett. 2020, 18, 53–60. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient quality and maturity status of frass fertilizer from nine edible insects. Sci. Rep. 2022, 12, 7182. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://eur-lex.europa.eu/eli/reg/2021/1925/oj (accessed on 4 April 2024).
- Poveda, J. Insect frass in the development of sustainable agriculture. A review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Wedwitschka, H.; Gallegos Ibanez, D.; Reyes Jáquez, D. Biogas Production from Residues of Industrial Insect Protein Production from Black Soldier Fly Larvae Hermetia illucens (L.): An Evaluation of Different Insect Frass Samples. Process 2023, 11, 362. [Google Scholar] [CrossRef]
- Available online: http://data.europa.eu/eli/reg/2017/893/oj (accessed on 4 April 2024).
- Basri, N.E.A.; Azman, N.A.; Ahmad, I.K.; Suja, F.; Jalil, N.A.A.; Amrul, N.F. Potential applications of frass derived from black soldier fly larvae treatment of food waste: A review. Foods 2022, 11, 2664. [Google Scholar] [CrossRef] [PubMed]
- Dortmans, B.; Diener, S.; Verstappen, B.; Zurbrügg, C. Black Soldier Fly Biowaste Processing—A Step-by-Step Guide Eawag; Swiss Federal Institute of Aquatic Science and Technology: Dübendorf, Switzerland, 2017; pp. 6–54. Available online: https://www.researchgate.net/publication/353923113_Black_Soldier_Fly_Biowaste_Processing_-_A_Step-by-Step_Guide_2nd_Edition (accessed on 28 August 2023).
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Kalová, M.; Borkovcová, M. Voracious larvae Hermetia illucens and treatment of selected types of biodegradable waste. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 77–83. [Google Scholar] [CrossRef]
- Zhou, F.; Tomberlin, J.K.; Zheng, L.; Yu, Z.; Zhang, J. Developmental and waste reduction plasticity of three black soldier fly strains (Diptera: Stratiomyidae) raised on different livestock manures. J. Med. Entomol. 2013, 50, 1224–1230. [Google Scholar] [CrossRef]
- Cappellozza, S.; Leonardi, M.G.; Savoldelli, S.; Carminati, D.; Rizzolo, A.; Cortellino, G.; Tettamanti, G. A first attempt to produce proteins from insects by means of a circular economy. Animals 2019, 9, 278. [Google Scholar] [CrossRef]
- Van, J.C.F.; Tham, P.E.; Lim, H.R.; Khoo, K.S.; Chang, J.S.; Show, P.L. Integration of Internet-of-Things as sustainable smart farming technology for the rearing of black soldier fly to mitigate food waste. J. Taiwan Inst. Chem. Eng. 2022, 137, 104235. [Google Scholar] [CrossRef]
- Li, M.; Mao, C.; Li, X.; Jiang, L.; Zhang, W.; Li, M.; Liu, H.; Fang, Y.; Liu, S.; Yang, G.; et al. Edible Insects: A New Sustainable Nutritional Resource Worth Promoting. Foods 2023, 12, 4073. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 2020, 25, 100335. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Wangu, M.M.; Dubois, T.; Ekesi, S.; et al. Low-cost technology for recycling agro-industrial waste into nutrient-rich organic fertilizer using black soldier fly. Waste Manag. 2021, 119, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.P.; Dulaurent, A.M. Potential use of mealworm frass as a fertilizer: Impact on crop growth and soil properties. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Turan, V.; Fernandez-Delgado Juarez, M.; Oberegger, S.; Insam, H. Suitability of black soldier fly frass as soil amendment and implication for organic waste hygienization. Agronomy 2020, 10, 1578. [Google Scholar] [CrossRef]
- Menino, R.; Felizes, F.; Castelo-Branco, M.A.; Fareleira, P.; Moreira, O.; Nunes, R.; Murta, D. Agricultural value of Black Soldier Fly larvae frass as organic fertilizer on ryegrass. Heliyon 2021, 7, e05855. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Musyoka, M.W.; Ekesi, S.; et al. Exploring black soldier fly frass as novel fertilizer for improved growth, yield, and nitrogen use efficiency of maize under field conditions. Front. Plant Sci. 2020, 11, 574592. [Google Scholar] [CrossRef]
- Chavez, M.; Uchanski, M. Insect left-over substrate as plant fertiliser. J. Insects Food Feed 2021, 7, 683–694. [Google Scholar] [CrossRef]
- Fischer, H.; Romano, N. Fruit, vegetable, and starch mixtures on the nutritional quality of black soldier fly (Hermetia illucens) larvae and resulting frass. J. Insects Food Feed 2021, 7, 319–327. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, W.; Lu, X.; Zhu, F.; Liu, W.; Wang, X.; Lei, C. Bioconversion performance and life table of black soldier fly (Hermetia illucens) on fermented maize straw. J. Clean. Prod. 2019, 230, 974–980. [Google Scholar] [CrossRef]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Romano, N.; Islam, S. Productivity and elemental/chlorophyll composition of collard greens in an aquaponic system at different combinations of media and black soldier fly (Hermetia illucens) larvae frass supplementations. Aquac. Res. 2023, 2023, 3308537. [Google Scholar] [CrossRef]
- Lopes, I.G.; Lalander, C.; Vidotti, R.M.; Vinnerås, B. Reduction of bacteria in relation to feeding regimes when treating aquaculture waste in fly larvae composting. Front. Microbiol. 2020, 11, 1616. [Google Scholar] [CrossRef] [PubMed]
- Anyega, A.O.; Korir, N.K.; Beesigamukama, D.; Changeh, G.J.; Nkoba, K.; Subramanian, S.; van Loon, J.J.A.; Dicke, M.; Tanga, C.M. Black soldier fly-composted organic fertilizer enhances growth, yield, and nutrient quality of three key vegetable crops in Sub-Saharan Africa. Front. Plant Sci. 2021, 12, 680312. [Google Scholar] [CrossRef]
- Song, S.; Ee, A.W.L.; Tan, J.K.N.; Cheong, J.C.; Chiam, Z.; Arora, S.; Tan, H.T.W. Upcycling food waste using black soldier fly larvae: Effects of further composting on frass quality, fertilising effect and its global warming potential. J. Clean. Prod. 2021, 288, 125664. [Google Scholar] [CrossRef]
- Green, T.R.; Popa, R. Enhanced ammonia content in compost leachate processed by black soldier fly larvae. Appl. Biochem. Biotechnol. 2012, 166, 1381–1387. [Google Scholar] [CrossRef]
- Ma, J.; Lei, Y.; Rehman, K.U.; Yu, Z.; Zhang, J.; Li, W.; Li, Q.; Tomberlin, J.K.; Zheng, L. Dynamic effects of initial pH of substrate on biological growth and metamorphosis of black soldier fly (Diptera: Stratiomyidae). Environ. Entomol. 2018, 47, 159–165. [Google Scholar] [CrossRef]
- Meneguz, M.; Gasco, L.; Tomberlin, J.K. Impact of pH and feeding system on black soldier fly (Hermetia illucens, L.; Diptera: Stratiomyidae) larval development. PLoS ONE 2018, 13, e0202591. [Google Scholar] [CrossRef] [PubMed]
- Alidadi, H.; Hosseinzadeh, A.; Najafpoor, A.A.; Esmaili, H.; Zanganeh, J.; Takabi, M.D.; Piranloo, F.G. Waste recycling by vermicomposting: Maturity and quality assessment via dehydrogenase enzyme activity, lignin, water soluble carbon, nitrogen, phosphorous and other indicators. J. Environ. Manag. 2016, 182, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Parra Paz, A.S.; Carrejo, N.S.; Gómez Rodríguez, C.H. Effects of larval density and feeding rates on the bioconversion of vegetable waste using black soldier fly larvae Hermetia illucens (L.) (Diptera: Stratiomyidae). Waste Biomass Valorization 2015, 6, 1059–1065. [Google Scholar] [CrossRef]
- Gärttling, D.; Schulz, H. Compilation of black soldier fly frass analyses. J. Soil Sci. Plant Nutr. 2022, 22, 937–943. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Chiu, S.L.; Lo, I.M. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Diener, S.; Zurbrügg, C.; Gutiérrez, F.R.; Nguyen, D.H.; Morel, A.; Koottatep, T.; Tockner, K. Black soldier fly larvae for organic waste treatment-prospects and constraints. Proc. WasteSafe 2011, 2, 13–15. [Google Scholar]
- Jose, J.V. Physiological and molecular aspects of macronutrient uptake by higher plants. In Sustainable Plant Nutrition; Academic Press: Cambridge, MA, USA, 2023; pp. 1–21. [Google Scholar]
- Setti, L.; Francia, E.; Pulvirenti, A.; Gigliano, S.; Zaccardelli, M.; Pane, C.; Caradonia, F.; Bortolini, S.; Maistrello, L.; Ronga, D. Use of black soldier fly (Hermetia illucens (L.), Diptera: Stratiomyidae) larvae processing residue in peat-based growing media. Waste Manag. 2019, 95, 278–288. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Eljack, R.; Beck, B.H. Nutritional value of frass from black soldier fly larvae, Hermetia illucens, in a channel catfish, Ictalurus punctatus, diet. Aquac. Nutr. 2020, 26, 812–819. [Google Scholar] [CrossRef]
- Kawasaki, K.; Kawasaki, T.; Hirayasu, H.; Matsumoto, Y.; Fujitani, Y. Evaluation of fertilizer value of residues obtained after processing household organic waste with black soldier fly larvae (Hermetia illucens). Sustainability 2020, 12, 4920. [Google Scholar] [CrossRef]
- Watson, C.; Schlösser, C.; Vögerl, J.; Wichern, F. Excellent excrement? Frass impacts on a soil’s microbial community, processes and metal bioavailability. Appl. Soil Ecol. 2021, 168, 104110. [Google Scholar] [CrossRef]
- Chiam, Z.; Lee, J.T.E.; Tan, J.K.N.; Song, S.; Arora, S.; Tong, Y.W.; Tan, H.T.W. Evaluating the potential of okara-derived black soldier fly larval frass as a soil amendment. J. Environ. Manag. 2021, 286, 112163. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Awasthi, M.K.; Chen, H.; Duan, Y.; Awasthi, S.K.; Zhang, Z. Performance of black soldier fly larvae (Diptera: Stratiomyidae) for manure composting and production of cleaner compost. J. Environ. Manag. 2019, 251, 109593. [Google Scholar] [CrossRef] [PubMed]
- Green, T. A biochemical analysis of Black Soldier fly (Hermetia illucens) larval frass plant growth promoting activity. PLoS ONE 2023, 18, e0288913. [Google Scholar] [CrossRef] [PubMed]
- Arabzadeh, G.; Delisle-Houde, M.; Tweddell, R.J.; Deschamps, M.H.; Dorais, M.; Lebeuf, Y.; Derome, N.; Vandenberg, G. Diet composition influences growth performance, bioconversion of black soldier fly larvae: Agronomic value and in vitro biofungicidal activity of derived frass. Agronomy 2022, 12, 1765. [Google Scholar] [CrossRef]
- Mulu, D.; Yimer, F.; Opande, G. Characterizing quality of frass produced by black soldier fly (Hermetia illucens) larvae after treating water hyacinth and fruit wastes in various proportions. Biomass Convers. Biorefinery 2023, 13, 15185–15196. [Google Scholar] [CrossRef]
- Attiogbe, F.K.; Ayim, N.Y.K.; Martey, J. Effectiveness of black soldier fly larvae in composting mercury contaminated organic waste. Sci. Afr. 2019, 6, e00205. [Google Scholar] [CrossRef]
- Fuhrmann, A.; Wilde, B.; Conz, R.F.; Kantengwa, S.; Konlambigue, M.; Masengesho, B.; Kintche, K.; Kassa, K.; Musazura, W.; Späth, L.; et al. Residues from black soldier fly (Hermetia illucens) larvae rearing influence the plant-associated soil microbiome in the short term. Front. Microbiol. 2022, 13, 994091. [Google Scholar] [CrossRef] [PubMed]
- Banavar, A.; Amirkolaei, S.K.; Duscher, L.; Khairunisa, B.H.; Mukhopadhyay, B.; Schwarz, M.; Urick, S.; Ovissipour, R. Nutritional Evaluation of Black Soldier Fly Frass as an Ingredient in Florida Pompano (Trachinotus carolinus L.) Diets. Animals 2022, 12, 2407. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Fischer, H.; Powell, A.; Sinha, A.K.; Islam, S.; Deb, U.; Francis, S. Applications of black solider fly (Hermetia illucens) larvae frass on sweetpotato slip production, mineral content and benefit-cost analysis. Agronomy 2022, 12, 928. [Google Scholar] [CrossRef]
- Salomone, R.; Saija, G.; Mondello, G.; Giannetto, A.; Fasulo, S.; Savastano, D. Environmental impact of food waste bioconversion by insects: Application of Life Cycle Assessment to process using Hermetia illucens. J. Clean. Prod. 2017, 140, 890–905. [Google Scholar] [CrossRef]
- Labella, R.; Bochicchio, R.; Addesso, R.; Labella, D.; Franco, A.; Falabella, P.; Amato, M. Germination Behavior and Geographical Information System-Based Phenotyping of Root Hairs to Evaluate the Effects of Different Sources of Black Soldier Fly (Hermetia illucens) Larval Frass on Herbaceous Crops. Plants 2024, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Pas, C.; Brodeur, D.; Deschamps, M.H.; Lebeuf, Y.; Adjalle, K.; Barnabé, S.; Eeckhout, M.; Vandenberg, G.; Vaneeckhaute, C. Valorization of pretreated biogas digestate with black soldier fly (Hermetia illucens, L.; Diptera: Stratiomyidae) larvae. J. Environ. Manag. 2022, 319, 115529. [Google Scholar] [CrossRef] [PubMed]
- Seyedalmoosavi, M.M.; Mielenz, M.; Schleifer, K.; Görs, S.; Wolf, P.; Tränckner, J.; Hüther, L.; Dänicke, S.; Daş, G.; Metges, C.C. Upcycling of recycled minerals from sewage sludge through black soldier fly larvae (Hermetia illucens): Impact on growth and mineral accumulation. J. Environ. Manag. 2023, 344, 118695. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Pantha, P.; Dassanayake, M. Living with salt. Innovation 2020, 1, 100050. [Google Scholar] [CrossRef] [PubMed]
- de Souza Vandenberghe, L.P.; Garcia, L.M.B.; Rodrigues, C.; Camara, M.C.; de Melo Pereira, G.V.; de Oliveira, J.; Soccol, C.R. Potential applications of plant probiotic microorganisms in agriculture and forestry. AIMS Microbiol. 2017, 3, 629. [Google Scholar] [CrossRef] [PubMed]
- Reis, V.M.; Alves, B.J.; Hartmann, A.; James, E.K.; Zilli, J.E. Beneficial microorganisms in agriculture: The future of plant growth-promoting rhizobacteria. Plant Soil 2020, 451, 1–3. [Google Scholar] [CrossRef]
- Ma, Y. Seed coating with beneficial microorganisms for precision agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef]
- Mahawar, H.; Prasanna, R. Prospecting the interactions of nanoparticles with beneficial microorganisms for developing green technologies for agriculture. Environ. Nanotechnol. Monit. Manag. 2018, 10, 477–485. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, K.; Jun, X.; Bo, L. Role and functions of beneficial microorganisms in sustainable aquaculture. Bioresour. Technol. 2009, 100, 3780–3786. [Google Scholar] [CrossRef]
- Jang, S.; Kikuchi, Y. Impact of the insect gut microbiota on ecology, evolution, and industry. Curr. Opin. Insect Sci. 2020, 41, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hongoh, Y. Diversity and genomes of uncultured microbial symbionts in the termite gut. Biosci. Biotechnol. Biochem. 2010, 74, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Gorrens, E.; Van Moll, L.; Frooninckx, L.; De Smet, J.; Van Campenhout, L. Isolation and identification of dominant bacteria from black soldier fly larvae (Hermetia illucens) envisaging practical applications. Front. Microbiol. 2021, 12, 665546. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Spampinato, G.; Macavei, L.I.; Lugli, L.; Candeliere, F.; Rossi, M.; Maistrello, L.; Amaretti, A. Effect of rearing temperature on growth and microbiota composition of Hermetia illucens. Microorganisms 2020, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.; Yang, C.; Wang, S.; Hu, Q.; Yi, L.; Zhang, J.; Yu, Z.; Cai, M.; Yu, C. Characteristics and nutrient function of intestinal bacterial communities in black soldier fly (Hermetia illucens L.) larvae in livestock manure conversion. Microb. Biotechnol. 2021, 14, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.; Von Allmen, F.; Zurbrügg, C.; Zhang, J.; Mathys, A. Identification of bacteria in two food waste black soldier fly larvae rearing residues. Front. Microbiol. 2020, 11, 582867. [Google Scholar] [CrossRef] [PubMed]
- Lopes, I.G.; Yong, J.W.; Lalander, C. Frass derived from black soldier fly larvae treatment of biodegradable wastes. A critical review and future perspectives. Waste Manag. 2022, 142, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Wynants, E.; Frooninckx, L.; Crauwels, S.; Verreth, C.; De Smet, J.; Sandrock, C.; Wohlfahrt, J.; Van Schelt, J.; Depraetere, S.; Lievens, B.; et al. Assessing the microbiota of black soldier fly larvae (Hermetia illucens) reared on organic waste streams on four different locations at laboratory and large scale. Microb. Ecol. 2019, 77, 913–930. [Google Scholar] [CrossRef]
- Nurfikari, A. Prospects of Residual Streams from Insect Cultivation for Sustainable Crop Management. Ph.D. Dissertation, Wageningen University, Wageningen, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Nuku-Adeku, C.; Maquart, P.; Little, D.; Newton, R.; Murray, F. Integrating insect frass biofertilisers into sustainable peri-urban agro-food systems. J. Insects Food Feed 2020, 6, 315–322. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Ranasinghe, A.; Hosseini, P.S.; Laboan, B.; Sonneveld, E.; Pipan, M.; Oni, F.E.; Montemurro, F.; Höfte, M.; Sleutel, S.; et al. How do novel and conventional agri-food wastes, co-products and by-products improve soil functions and soil quality? Waste Manag. 2020, 113, 132–144. [Google Scholar] [CrossRef]
- Mason, B. Production of High Value Protein Feeds and Fertilizer from Pre-Consumer Vegetable Waste Utilizing a Novel Black Soldier Fly Larvae Conversion Process; Verschuren Center for Sustainability in Energy & Environment, Cape Breton University: Sydney, NS, Canada, 2016. [Google Scholar]
- Wang, X.; Wu, N.; Wu, X.; Geng, W.; Xu, X. Effect of insect feces (Hermetia illucens) on rice growth and heavy metal migration from polluted soil to rice plant. Environ. Sci. Pollut. Res. 2022, 29, 14695–14704. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.; Fitzpatrick, M.; Hodge, S. The Effects of Two Organic Soil Amendments, Biochar and Insect Frass Fertilizer, on Shoot Growth of Cereal Seedlings. Plants 2023, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Dzepe, D.; Mbenda, T.K.; Ngassa, G.; Mube, H.; Chia, S.Y.; Aoudou, Y.; Djouaka, R. Application of black soldier fly frass, Hermetia illucens (diptera: Stratiomyidae) as sustainable organic fertilizer for lettuce, lactuca sativa production. Open J. Appl. Sci. 2022, 12, 1632–1648. [Google Scholar] [CrossRef]
- Hodge, S.; Conway, J. The Effects of Insect Frass Fertilizer and Biochar on the Shoot Growth of Chicory and Plantain, Two Forage Herbs Commonly Used in Multispecies Swards. Agronomy 2022, 12, 2459. [Google Scholar] [CrossRef]
- Romano, N.; Datta, S.N.; Sinha, A.K.; Pande, G.S.J. Partially replacing synthetic fertilizer with black soldier fly (Hermetia illucens) larvae frass enhances kale (Brassica oleracea var. sabellica) production. Technol. Hortic. 2023, 3, 8. [Google Scholar]
- Agustiyani, D.; Agandi, R.; Arinafril; Nugroho, A.A.; Antonius, S. The effect of application of compost and frass from Black Soldier Fly Larvae (Hermetia illucens L.) on growth of Pakchoi (Brassica rapa L.). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 762, p. 012036. [Google Scholar]
- Radzikowska-Kujawska, D.; Sawinska, Z.; Grzanka, M.; Kowalczewski, P..; Sobiech, Ł.; Świtek, S.; Skrzypczak, G.; Drożdżyńska, A.; Ślachciński, M.; Nowicki, M. Hermetia illucens frass improves the physiological state of basil (Ocimum basilicum L.) and its nutritional value under drought. PLoS ONE 2023, 18, e0280037. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.A.; Paredes, C.; Moral, R.; Agulló, E.; Pérez-Murcia, M.D.; Abad, M. Composts from distillery wastes as peat substitutes for transplant production. Resour. Conserv. Recycl. 2008, 52, 792–799. [Google Scholar] [CrossRef]
- Ribeiro, H.M.; Romero, A.M.; Pereira, H.; Borges, P.; Cabral, F.; Vasconcelos, E. Evaluation of a compost obtained from forestry wastes and solid phase of pig slurry as a substrate for seedlings production. Bioresour. Technol. 2007, 98, 3294–3297. [Google Scholar] [CrossRef] [PubMed]
- Ronga, D.; Pane, C.; Zaccardelli, M.; Pecchioni, N. Use of spent coffee ground compost in peat-based growing media for the production of basil and tomato potting plants. Commun. Soil Sci. Plant Anal. 2016, 47, 356–368. [Google Scholar] [CrossRef]
- Ronga, D.; Pellati, F.; Brighenti, V.; Laudicella, K.; Laviano, L.; Fedailaine, M.; Benvenuti, S.; Pecchioni, N.; Francia, E. Testing the influence of digestate from biogas on growth and volatile compounds of basil (Ocimum basilicum L.) and peppermint (Mentha × Piperita L.) Hydroponics. J. Appl. Res. Med. Aromat. Plants 2018, 11, 18–26. [Google Scholar] [CrossRef]
- Bernal-Vicente, A.; Ros, M.; Tittarelli, F.; Intrigliolo, F.; Pascual, J.A. Citrus compost and its water extract for cultivation of melon plants in greenhouse nurseries. Evaluation of nutriactive and biocontrol effects. Bioresour. Technol. 2008, 99, 8722–8728. [Google Scholar] [CrossRef] [PubMed]
| Physical Parameters | |
|---|---|
| Dry matter (DM) | 69.6% |
| Organic matter (OM) | 86.2% DM |
| pH | 7.46 |
| C:N ratio | 14.7 |
| Electrical conductivity (EC) | 4.0 mS cm−1 |
| Macronutrients | |
| Total nitrogen (Nt) | 32.2 g kg−1 DM |
| Ammonium nitrogen (NH4+-N) | 5.6 g kg−1 DM |
| Phosphorus (P) | 12.4 g kg−1 DM |
| Potassium (K) | 29.3 g kg−1 DM |
| Micronutrients | |
| Magnesium (Mg) | 4.7 g kg−1 DM |
| Sodium (Na) | 5.7 g kg−1 DM |
| Calcium (Ca) | 8.8 g kg−1 DM |
| Sulfur (S) | 6.3 g kg−1 DM |
| Copper (Cu) | 43.8 mg kg−1 DM |
| Boron (B) | 34.5 mg kg−1 DM |
| Zinc (Zn) | 136.3 mg kg−1 DM |
| Iron (Fe) | 1808.4 mg kg−1 DM |
| Manganese (Mn) | 79.5 mg kg−1 DM |
| Feeding Substrate | Macronutrient (%) | References | |||
|---|---|---|---|---|---|
| C | N | P | K | ||
| Gainesville diet * | 35.2 | 3.8 | 5.2 | 4.1 | [72] |
| Distiller grains * | – | 3.4 | 0.8 | 1.1 | [73] |
| Brewery spent grain **** | 38.6 | 3.6 | 0.5 | 0.3 | [61] |
| Okara and wheat bran *** | 37.1 | 4.8 | 1.0 | 0.9 | [62] |
| Okara and wheat bran ****** | 30.6 | 3.2 | 0.8 | 0.5 | [62] |
| Household waste * | 35.8 | 2.2 | 0.5 | 0.7 | [74] |
| Wheat bran * | 35.7 | 2.8 | 1.4 | 2.3 | [75] |
| Brewery spent grain **** | 35.2 | 2.1 | 1.2 | 0.2 | [54] |
| Fresh okara * | 37.1 | 5.1 | 0.3 | 1.9 | [76] |
| Chicken manure * | 23.6 | 2.3 | 1.1 | 1.8 | [77] |
| Pig manure * | 26.8 | 2.4 | 2.1 | 1.0 | [77] |
| Chicken feed * | 47.9 | 2.6 | – | – | [52] |
| Grass cuttings * | 44.3 | 2.4 | – | – | [52] |
| Fruits and vegetables * | 48.8 | 1.8 | – | – | [52] |
| Cow manure * | 27.7 | 1.9 | 1.0 | 0.2 | [77] |
| Vegetables * | 38.7 | 2.8 | 1.5 | 3.3 | [53] |
| Kitchen waste * | - | 3.3 | 3.1 | 4.5 | [78] |
| Gainesville diet * | 50.8 | 2.0 | 1.9 | 3.7 | [79] |
| Fruit/vegetable/bakery/brewery * | 51.4 | 2.7 | 1.3 | 2.9 | [79] |
| Ratios of water hyacinth, fruit waste, and manure (65:25:10) * | 23.5 | 0.8 | 0.4 | 2.6 | [80] |
| Ratios of water hyacinth, fruit waste, and manure (50:40:10) * | 24.8 | 1.4 | 0.4 | 2.5 | [80] |
| Ratios of water hyacinth, fruit waste, and manure (35:55:10) * | 25.3 | 1.6 | 0.3 | 2.2 | [80] |
| Ratios of water hyacinth, fruit waste, and manure (15:75:10) * | 27.0 | 1.7 | 0.3 | 1.7 | [80] |
| Ratios of water hyacinth, fruit waste, and manure (10:80:10) * | 29.1 | 1.9 | 0.2 | 0.2 | [80] |
| Food waste, chicken feces, and sawdust (3:2:1 ratio) * | - | 1.7 | 1.1 | 2.1 | [81] |
| Maize straw * | - | 0.6 | 2.5 | 2.1 | [57] |
| Spent malted barley grain * | 40.1 | 3.2 | 0.6 | 0.3 | [82] |
| Hemp waste * | - | 2.0 | 0.7 | 1.5 | [83] |
| Mix grains, fruits, and vegetables ***** | - | - | 1.4 | 1.8 | [84] |
| Food waste * | - | 1.5 | 0.9 | 1.1 | [85] |
| Gainesville diet ** | 51.5 | 1.3 | 0.9 | 1.9 | [86] |
| Gainesville diet *** | 51.1 | 1.9 | 0.9 | 1.9 | [86] |
| 43% sheep whey + 57% seeds ** | 54.3 | 1.6 | 0.5 | 1.1 | [86] |
| 43% sheep whey + 57% seeds *** | 54.3 | 1.9 | 0.5 | 1.2 | [86] |
| Gainesville diet * | 24.0 | 1.5 | 1.2 | 2.1 | [87] |
| Gainesville diet * | - | - | 0.9 | 2.9 | [88] |
| Feeding Substrate | Micronutrients | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Ca | Mg | Na | Fe | Cu | Mn | Zn | ||
| (g kg−1) | (mg kg−1) | |||||||
| Gainesville diet * | 45 | 8.0 | 3.0 | 600 | 46.1 | − | 140 | [72] |
| Distillers grains * | 13 | 3.0 | 5.0 | 125 | 15 | 45 | 90 | [73] |
| Brewery spent grain **** | 9.7 | 1.0 | – | 310 | 25 | 109 | 182 | [61] |
| Okara and wheat bran *** | 1.3 | 0.1 | – | 26 | 2.2 | 4.2 | 0.1 | [62] |
| Okara and wheat bran ****** | 0.8 | 0.2 | – | 26 | 0.7 | 2.3 | 0.1 | [62] |
| Household waste * | 10 | 0.9 | 0.8 | 240 | 10 | 10 | 10 | [74] |
| Wheat bran * | – | 0.3 | – | 15 | 8.9 | 19.4 | 15 | [75] |
| Brewery spent grain **** | 0.2 | 0.2 | – | – | – | – | – | [54] |
| Fresh okara * | 16.8 | 10.5 | – | 3.7 | 0.9 | 0.2 | 1.7 | [76] |
| Vegetables * | 15 | 7.0 | 0.3 | 896 | 19 | 149 | 137 | [53] |
| Fruit/vegetable/bakery/brewery * | 0.4 | 4.2 | 9.7 | 150.5 | 9.0 | 17.0 | 57.3 | [79] |
| Ratios of water hyacinth, fruit waste, and manure (65:25:10) * | 1.4 | 1.9 | - | 537 | 42.9 | 55.7 | 78.2 | [80] |
| Ratios of water hyacinth, fruit waste, and manure (50:40:10) * | 1.4 | 1.5 | - | 534.3 | 36.6 | 33.7 | 68.3 | [80] |
| Ratios of water hyacinth, fruit waste, and manure (35:55:10) * | 0.9 | 1.1 | - | 415.7 | 34.5 | 32.0 | 60.2 | [80] |
| Ratios of water hyacinth, fruit waste, and manure (15:75:10) * | 0.6 | 0.9 | - | 95.0 | 32.6 | 30.9 | 43.3 | [80] |
| Ratios of water hyacinth, fruit waste, and manure (10:80:10) * | 0.3 | 0.7 | - | 290.3 | 22.7 | 21.5 | 38.0 | [80] |
| Spent malted barley grain * | 6.4 | 2.2 | 0.2 | 4100 | 12.8 | 100 | 100 | [82] |
| Hemp waste * | 19.2 | 4.6 | 7.3 | 1111 | 26.1 | 163.9 | 187.5 | [83] |
| Mix grains, fruits, and vegetables ***** | 18.1 | 3.8 | 6.2 | - | 44 | 76 | 372 | [84] |
| Food waste * | - | - | - | - | 57 | - | 206 | [85] |
| Gainesville diet ** | 2.1 | 6.8 | 0.9 | - | - | - | 89.3 | [86] |
| Gainesville diet *** | 4.4 | 7.2 | 0.9 | - | - | - | 93.2 | [86] |
| 43% sheep whey + 57% seeds ** | 1.1 | 3.7 | 1.9 | - | - | - | 50.1 | [86] |
| 43% sheep whey + 57% seeds *** | 1.3 | 3.8 | 2.0 | - | - | - | 55.2 | [86] |
| Gainesville diet * | 5.0 | 5.0 | - | - | - | - | - | [87] |
| Gainesville diet * | 8.9 | 5.2 | 0.9 | 507 | 9.8 | 66.0 | 58.2 | [88] |
| Larval Growth Substrate | Optimal Frass Dosage | Plant Species | Experiment Duration | Effect of Frass Doses | Reference |
|---|---|---|---|---|---|
| Fruit/vegetable pulp + poultry litter * | 4% by volume | Hordeum vulgare | 44 days | Increased shoot length, increased biomass, and increased photosynthetic activity | [107] |
| Spent grain **** | 2.5 t/ha | Zea mays | 125 days | Increased plant height and chlorophyll content | [54] |
| Chicken manure * | 4% by weight first year | Oryza sativa | About 180 days | Increased productive yield | [108] |
| Chicken manure * | 8% by weight second year | Oryza sativa | About 180 days | Increased productive yield | [108] |
| Spent grain * | 7.1 g for plot | Avena sativa cv. Apollon | About 42–49 days | Increased shoot growth | [109] |
| Spent grain * | 6.3 g for plot | Triticum spelta | About 42–49 days | Increased shoot growth | [109] |
| Spent grain * | 6.8 g for plot | Triticosecale cv. Trisem | About 42–49 days | Increased shoot growth | [109] |
| Spent grain * | 7.8 g for plot | Hordeum vulgare cv. Westminster | About 42–49 days | Increased shoot growth | [109] |
| Spent grain * | 7.8 g for plot | Hordeum vulgare cv. Quadriga | About 42–49 days | Increased shoot growth | [109] |
| Onion and potato waste * | 100% of nitrogen demand | Lolium multiflorum | About 210 days | Increased production | [53] |
| Gainesville diet ** | frass extracts 25% | Hordeum vulgare | until the first leaf was produced. | Increased root dry weight and chlorophyll content | [86] |
| Gainesville diet *** | frass extracts 25% | Hordeum vulgare | until the first leaf was produced. | Increased root dry weight and chlorophyll content | [86] |
| 43% sheep whey + 57% seeds ** | frass extracts 25% | Hordeum vulgare | until the first leaf was produced. | Increased root dry weight and chlorophyll content | [86] |
| 43% sheep whey + 57% seeds *** | frass extracts 25% | Hordeum vulgare | until the first leaf was produced. | Increased root dry weight and chlorophyll content | [86] |
| Larval Growth Substrate | Optimal Frass Dosage | Plant Species | Experiment Duration | Effect of Frass Doses | Reference |
|---|---|---|---|---|---|
| Asteraceae | |||||
| Okara * | 10% by volume | Lactuca sativa | 45 days | Development similar to control | [76] |
| Gainesville Diet * | 10–20% by volume | Lactuca sativa | 30 days | Increased plant growth (height, stem diameter, leaf number) | [72] |
| Household waste * | 30 t/ha | Lactuca sativa | 42 days | Increased productive yield | [110] |
| Spent grain * | 4 g per pot | Cichorium intybus L. | 49 days | Increased in DM shoot | [111] |
| Brassicaceae | |||||
| Household food waste ** | 0.37 kg/m3 | Brassica oleracea var. sabellica | 35 days | Increased plant weight and height | [112] |
| Organic waste * | 15% of the weight | Brassica rapa L. | 35 days | Increased fresh plant weight | [113] |
| Plantaginaceae | |||||
| Spent grain * | 4 g per pot | Plantago lanceolata L. | 49 days | Increased in DM shoot | [111] |
| Labiate | |||||
| Unspecified * | 10 g/L | Ocimum basilicum | 46 days | Increased fresh weight and photosynthetic activity | [114] |
| Gainesville Diet * | 10–20% by volume | Ocimum basilicum | 30 days | Increased plant growth (height, stem diameter, leaf number) | [72] |
| Solanaceae | |||||
| Gainesville Diet * | 10–20% by volume | Solanum lycopersicum | 30 days | Increased plant growth (height, stem diameter, leaf number) | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomonaco, G.; Franco, A.; De Smet, J.; Scieuzo, C.; Salvia, R.; Falabella, P. Larval Frass of Hermetia illucens as Organic Fertilizer: Composition and Beneficial Effects on Different Crops. Insects 2024, 15, 293. https://doi.org/10.3390/insects15040293
Lomonaco G, Franco A, De Smet J, Scieuzo C, Salvia R, Falabella P. Larval Frass of Hermetia illucens as Organic Fertilizer: Composition and Beneficial Effects on Different Crops. Insects. 2024; 15(4):293. https://doi.org/10.3390/insects15040293
Chicago/Turabian StyleLomonaco, Giovanni, Antonio Franco, Jeroen De Smet, Carmen Scieuzo, Rosanna Salvia, and Patrizia Falabella. 2024. "Larval Frass of Hermetia illucens as Organic Fertilizer: Composition and Beneficial Effects on Different Crops" Insects 15, no. 4: 293. https://doi.org/10.3390/insects15040293
APA StyleLomonaco, G., Franco, A., De Smet, J., Scieuzo, C., Salvia, R., & Falabella, P. (2024). Larval Frass of Hermetia illucens as Organic Fertilizer: Composition and Beneficial Effects on Different Crops. Insects, 15(4), 293. https://doi.org/10.3390/insects15040293









