Simple Summary
Some trees produce a plastic-like product called resin, which when fossilized is called amber. In this study we comment on the current terminological difficulties regarding the description of fossil, non-fossil and so called “sub-fossil” resins. We furthermore report two long-haired lepidopteran caterpillars in resin from Brazil and Madagascar. It is likely they represent larvae of Erebidae (tussock moths and others) which typically have long hairs and spines. Long-haired caterpillars are exceptionally rare in amber with only one similar specimen to date, as most other caterpillars in resin are either “naked” or have a protective case. These new specimens also increase the known size range of caterpillars preserved in resin to up to 12 mm. We also emphasize the importance of images when describing and publishing caterpillars in resin, to allow broader morphological studies using all available specimens.
Abstract
Resin is a plastic-like product of trees. Older occurrences of such resin are referred to as amber and are considered fossil resin. Younger resins are termed copals. Even younger ones have been dubbed defaunation resins. Non-fossil resins remain in a terminological limbo, often referred to as “sub-fossils”. We report two lepidopteran caterpillars preserved in non-fossil resin: one from Madagascar, one from Brazil. Prominent hairs (=setae) and spines (=spine-like setae) of the specimens make it likely that they represent larvae of Erebidae (e.g., tussock moths and others). So far, most known caterpillars preserved in resins are either “naked” or bear protective cases; only few are armoured with spines or hairs. In particular, long-haired caterpillars such as the ones reported here are so far almost absent. Only one specimen with comparable setae has been reported from 15-million-year-old Dominican amber, but no significant details of this specimen are accessible. We briefly also review the record of caterpillars known from the Holocene, recognising that it is very sparse. The new specimens demonstrate that very hairy caterpillars can readily be preserved in resins in fine detail. Furthermore, the specimens increase the known size range of caterpillars preserved in resins, with one measuring more than 12 mm.
1. Introduction
Biodiversity has become a topic of interest for our society. In particular, the conservation of this increasingly endangered biodiversity has been recognised as an important task for humankind. To recognise changes in biodiversity, it is necessary to measure this diversity at different times and to compare them.
Short-term changes can be easily recognised by year-to-year surveys or by monitoring. Sources for longer-term comparisons can be older collections in museums, as many large-scale collections contain specimens collected in the field that have been conserved for many decades or even up a century. Even longer-term comparisons can involve fossils.
Furthermore, there is the conceptual difficulty in grasping the area between historical specimens and fossils. The term ”sub-fossil” has been used in many instances to address the remains of animals that are not (yet?) considered fossils. Cases from the literature include the following:
- (1)
- Sub-fossil bones. For example, Holocene (the current geological epoch, starting ca. 11,700 years ago [1]) skulls of lemurs have been reported by Albrecht et al. [2] and dated to about 8245–1000 years before the present (see discussion in Albrecht et al. [2] (their p. 10)). These specimens differ from fossils in their mineralogy.
- (2)
- Sub-fossil shells. For example, the shells of mussels described by Hjort & Funder [3]. These specimens, like the sub-fossil bones, are also from the Holocene, dating between 8500–5000 years before the present, and have different mineralogical properties than fossil shells.
- (3)
- Sub-fossil plant remains. These can also be potentially differentiated from fossils by mineralogy. Yet, in some types of preservation other than petrification, such as charcoal formations [4], mineralogy does not play a major role. Hence, in some cases, age seems to be an important factor for characterising a sub-fossil. Examples of plant remains considered sub-fossils range from 6660–530 years before the present [4] (their p. 70), or even 7100–130 years before the present [5] (their Table 1, p. 298).
- (4)
- Sub-fossil traces. For the interpretation of 9000–8000 years old mammalian tracks as sub-fossils, age was considered to be the main factor [6].
- (5)
- Cuticle remains (specifically from representatives of Euarthropoda). Many organisms have cuticles that withstand relatively long time periods. Sub-fossil cuticle remains are known from different animals, including, for example, cladoceran crustaceans (water fleas) [7,8,9] and larvae of non-biting midges (Chironomidae) [10]. In many cases, the age of such remains, usually retrieved from lake sediments, is unknown or not reported. However, in a study involving the cuticle remains of larvae of non-biting midges, sub-fossils dating from 320 years before the present down to 10,000 years before the present were reported [11] (their table 2 p. 3347).
Despite the euarthropodan cuticle chemically degrading over time [12], some cuticle remains can persist for a long time. Preserved remains of small crustaceans have been reported from relatively recent times, such as the Neolithic period (12,000–6500 years ago, [13], [14] (their figure 15.2)), or from as late as the Mesozoic and the later Palaeozoic (e.g., [15,16]), possibly even the early Palaeozoic period [17]. The idea that fluorescence could be used to differentiate between extant and fossil remains (as suggested by Braun [15]) seems not to be universally applicable (e.g., scorpions show fluorescence today and in Silurian fossils [18]).
Hence, in many of the examples in which the term “sub-fossil” has been applied, they differ regarding their mineralogy, chemistry, and age, although most are restricted to the Holocene. Yet, there still seem to exist different views.
Schwartz et al. [19] (their p. 6124) used “recently extinct” as equivalent to sub-fossil. This imposes a difficulty, because some species preserved as sub-fossils (or fossils) might not be extinct (for example, see the discussion of beetles preserved in Miocene amber by Hörnschemeyer et al. [20]). This challenge, together with the different criteria used to identify a sub-fossil, shows that there is no well-formulated concept of what a sub-fossil is.
Another type of preservation often related to the term “sub-fossil” is copal. Copal is a type of resin known to bear inclusions, especially of representatives of various groups of Euarthropoda, and mainly of its ingroup Insecta. The assumed age of copal seems to differ greatly from author to author (e.g., [21] (his p. 440), [22] (their pp. 21–22), [1] (their figure 1, p. 2)); the transition point also varies greatly as to from which age a resin is considered a fossil resin, then commonly called amber (e.g., [1,23], [1] (their figure 1, p. 2)). Recently, Solórzano-Kraemer et al. [1] introduced another subdivision of non-fossil resins into an older copal and a younger defaunation resin.
We hereby report two pieces of non-fossil resins, one from Madagascar and one from Brazil, although it is unclear if they are copal or defaunation resins (see below for details). Each one includes a caterpillar-type larva of a lepidopteran. Caterpillars, in general, are comparably rare in the fossil record [24,25]. We discuss why these finds are of scientific interest, especially in taphonomy, and give a brief overview of the known Holocene record of lepidopteran caterpillars.
2. Materials and Methods
2.1. Materials
Two new specimens in non-fossil resins are at the centre of this study: one from Madagascar, one from Brazil. The specimen from Madagascar is preserved in a stalactite-like piece of non-fossil resin; this indicates that it might have been collected directly from the tree (see [22]). Therefore, it likely represents defaunation resin. For the specimen from Brazil, the resin origin is less clear; the shape of the copal does not give a hint as to whether it has been collected from the tree, from the ground, or elsewhere.
Both pieces do not contain obvious plant remains that could have been used for radiocarbon analyses (as in Solórzano-Kraemer et al. [1]). To address the uncertainty of whether the pieces are copal or defaunation resins, we will neutrally refer to the pieces as non-fossil resins to acknowledge the possibility that the pieces are not defaunation resin but copal (sensu Solórzano-Kraemer et al. [1]), although this seems unlikely. The pieces of non-fossil resin also contain other inclusions (e.g., numerous flies in the piece from Madagascar), but the largest inclusion in each piece is a caterpillar.
The piece from Madagascar was legally purchased from a trader (https://www.thefossildude.com/, accessed on 15 May 2024). A deposition in Madagascar was not (yet) possible. Therefore, it is now part of the Palaeo-Evo-Devo Research Group Collection of Arthropods, Ludwig-Maximilians-Universität München (LMU Munich), Germany, under repository number PED 0893.
The specimen from Brazil was legally purchased on the trading platform ebay.com from the trader meteoritestuff. It was temporarily part of the Palaeo-Evo-Devo Research Group Collection of Arthropods, Ludwig-Maximilians-Universität München (LMU Munich), Germany, under repository number PED 2014. It is now deposited at the Coleção Entomológica “Mítia Heusi Silveira”, at the Universidade Federal de Santa Catarina (UFSC), Brazil, under repository code CEMHS-D0054.
2.2. Methods
The specimens were documented on a VHX-6000 digital microscope on a black background and a white background. Different types of illumination were used (cross-polarised coaxial illumination, unpolarised ring illumination). Each image was additionally recorded under different exposure times (HDR; [26,27]). The best-contrasted ones were chosen for the figures in this study. All images represent composite images, i.e., to overcome limitations in the field of view and depth of field, adjacent image stacks were documented and processed following standard procedures (e.g., [28] and references therein). Furthermore, the specimens were documented in all accessible directions.
2.3. Basic Approach
As pointed out in the introduction, there are only few reports of lepidopteran caterpillars as fossils. We will list the first figured occurrence of a specimen in the literature and also detail in which articles or books it has been re-figured. Similar approaches have been used in other studies [24].
3. Results
So far, there are only a few reports of lepidopteran caterpillars in the Holocene that can be considered sub-fossils:
- (1)
- Rosenkjaer [29] reported possible larval cases of caterpillars from Denmark. The specimens have been discussed again by Henriksen [30]. Both references were not available in the original version but are cited indirectly from Sohn et al. [31]. Apparently, there were no figures associated with the reports. Sohn et al. [31] (their p. 35) stated that they “may represent the larval cases”. Hence, this stands a possible case, yet clearly an indirect one, as larval cases are often not easy to interpret. There is no additional information on the preservation given besides that the apparently isolated cases (likely not in resin) were found in unconsolidated sediments.
- (2)
- Evers [32] reported lepidopteran specimens from resins that he referred to as copal. He depicted only winged adult forms. Yet, the author mentioned that he had seen three caterpillars preserved in these pieces of resin [32] (his p. 9).
- (3)
- Keble [33] reported two caterpillars that had been replaced by fungus (his p. 49), representing “mummies” of the original morphology (Figure 1A,B). The specimens were depicted in Gill [34] (his p. 88 pl. III) and, in more detail, in Simonsen et al. [35] (p. 14, Figure 7). These “mummies” were not encased in resin.
 Figure 1. Drawings of the two mummified caterpillars reported by Keble [33], with the anterior end facing left, modified after Simonsen et al. [35], deposited in the Museum Victoria, Melbourne, Australia. (A) Specimen P16153, lateral view. (B) Specimen P16154, lateral view.
Figure 1. Drawings of the two mummified caterpillars reported by Keble [33], with the anterior end facing left, modified after Simonsen et al. [35], deposited in the Museum Victoria, Melbourne, Australia. (A) Specimen P16153, lateral view. (B) Specimen P16154, lateral view. - (4)
- Lemdahl [36] reported isolated mandibles of caterpillars in sediments dated to between 9600–7900 years before the present [36] (his p. 307). No specimen was illustrated.
We additionally report two new specimens:
- (5)
- The first new specimen from the non-fossil resin from Madagascar (PED 0893; Figure 2 and Figure 3) is preserved in quite clear resin. It is not fully preserved; the right anterior region is not inside the resin and therefore missing. The preserved part of the body is longer than 12 mm.
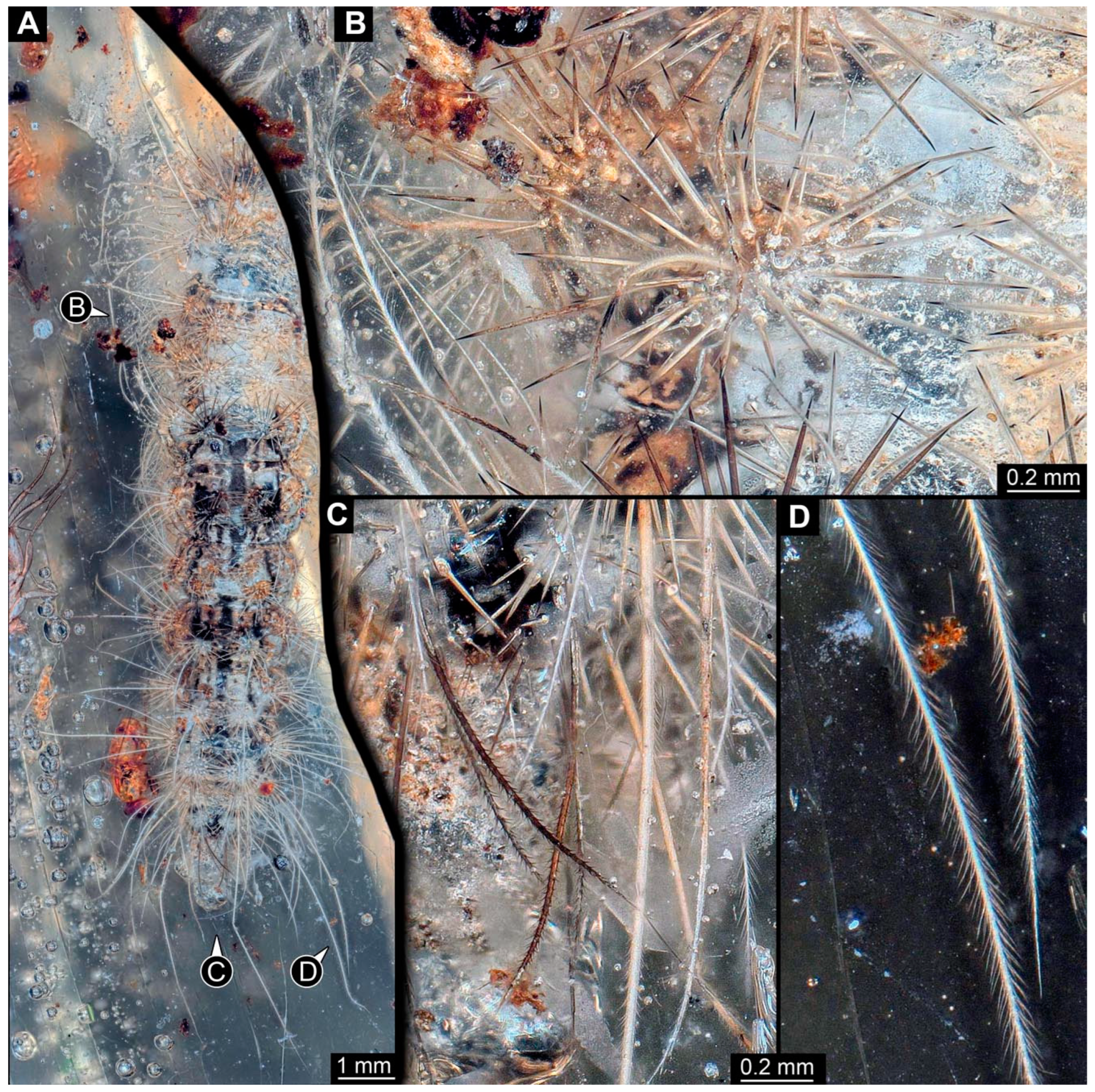
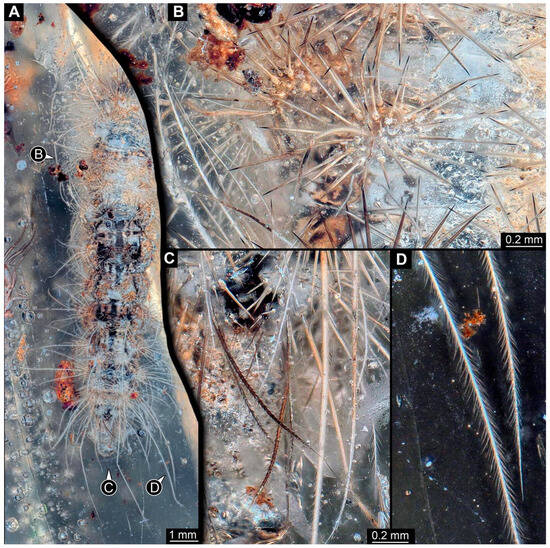 Figure 2. New specimen of a lepidopteran caterpillar in a non-fossil resin (PED 0893). (A) Specimen in dorsal view; the anterior part is not included in the amber. (B) Close-up of the larger humps on the abdomen with slightly fewer than 30 spine-like setae. (C) Close-up of the posterior body end; note the dark barbed setae. (D) Close-up of the long postero-lateral setae at the posterior body end; note the many small setules.
Figure 2. New specimen of a lepidopteran caterpillar in a non-fossil resin (PED 0893). (A) Specimen in dorsal view; the anterior part is not included in the amber. (B) Close-up of the larger humps on the abdomen with slightly fewer than 30 spine-like setae. (C) Close-up of the posterior body end; note the dark barbed setae. (D) Close-up of the long postero-lateral setae at the posterior body end; note the many small setules.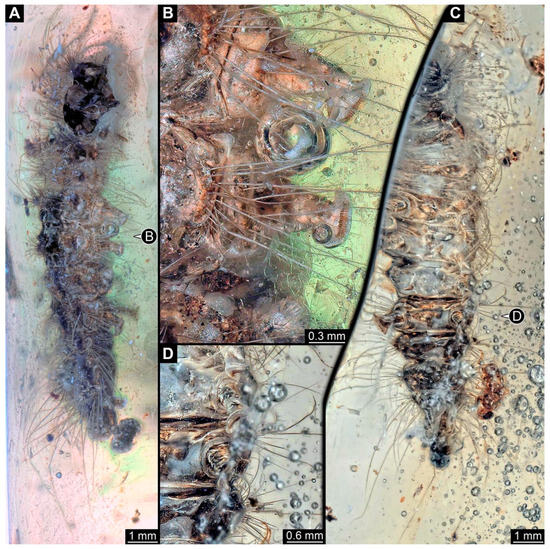 Figure 3. New specimen of a lepidopteran caterpillar in a non-fossil resin (PED 0893), continued. (A) Specimen in lateral view. (B) Close-up of the prolegs. (C) Specimen in ventral view; the anterior part is not included in the amber. (D) Close-up of the prolegs.
Figure 3. New specimen of a lepidopteran caterpillar in a non-fossil resin (PED 0893), continued. (A) Specimen in lateral view. (B) Close-up of the prolegs. (C) Specimen in ventral view; the anterior part is not included in the amber. (D) Close-up of the prolegs.
The specimen is elongated and tube-shaped, presumably with a head and trunk. The head seems largely missing. The trunk appears differentiated into the anterior thorax (three segments, only incompletely preserved) and posterior abdomen (nine segments + trunk end (possibly conjoined structure of segments 10 and 11)).
The visible thorax segments have four dorsal humps in a line. Each hump has about 20 spine-like setae (Figure 2A). There is a pair of smaller humps further posteriorly and medially. Each hump has about twelve spine-like setae (Figure 2A). The anterior eight abdomen segments have a slightly different armature: they have four larger and two smaller humps; the smaller humps are located antero-medially, the two larger humps antero-laterally, and the two larger humps postero-medially (Figure 2B). The larger humps have slightly fewer than 30 spine-like setae (Figure 2B). The larger humps have an additional single, very long softer seta (“hair”) with numerous setules (Figure 2D). The smaller humps have about ten spine-like setae. Abdomen segment 9 has a sub-similar armature. The postero-medial medial humps are without soft, setulae-bearing setae; instead, they have with slightly shorter, barbed setae (Figure 2C). The trunk end is elongated without prominent setae.
The habitus is less accessible in the lateral view. Each segment additionally bears a crescent-shaped elevation at the far lateral end. Each elevation has about ten short spine-like setae and about ten long softer setae with numerous setules (Figure 3A). Abdomen segments 3–6 each have a pair of appendages (“prolegs”). The appendages are organized into a proximally truncated and cone-shaped structure with few simple setae, distally widening to a trapezoid structure (“planta”) bearing about 20 hook-like spines (“crochets”) arranged into a single row (Figure 3B,D). The trunk end seems to bear a pair of appendages (“prolegs”); however, as the region is difficult to access, no details are visible. The habitus is hardly visible in the ventral view (Figure 3C).
- (6)
- The second specimen newly reported from a non-fossil resin from Brazil (CEMHS-D0054; Figure 4) is preserved in quite clear resin, but the piece has numerous cracks. The specimen is only visible in the dorsal view. The specimen seems fully preserved, but not many details are visible due to the brittle state of the resin (Figure 4A). The specimen is roughly 7.2 mm long. The head appears intact, but no details are visible. The trunk is differentiated into segments, but differentiation of the thorax and abdomen is difficult, since no ventral structures, especially legs, are available. The specimen has at least 13 major body units: the head, possibly 11 trunk segments, and the trunk end.
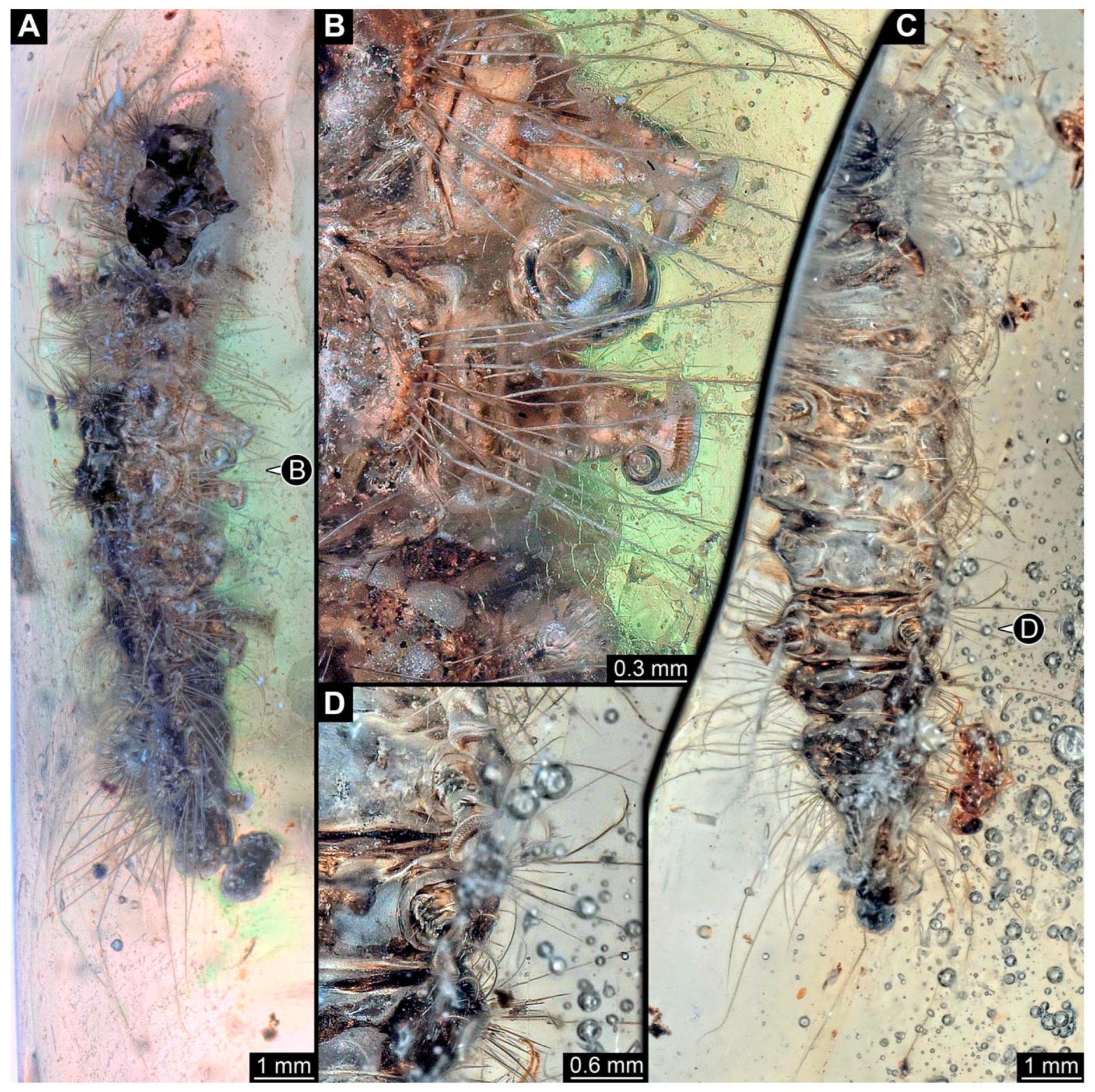
 Figure 4. New specimen of a lepidopteran caterpillar in a non-fossil resin (CEMHS-D0054). (A) Overview of the specimen in the dorsal view. (B) Close-up of the lateral hump with short spines. (C) Close-up of the lateral hump with long setae, including additional small spines. (D) Close-up of what is likely to be the head capsule and anterior part of the body devoid of the dark green substance present elsewhere. Abbreviations: hc? = possible head capsule; ls = long barbed setae; te? = possible trunk end.
Figure 4. New specimen of a lepidopteran caterpillar in a non-fossil resin (CEMHS-D0054). (A) Overview of the specimen in the dorsal view. (B) Close-up of the lateral hump with short spines. (C) Close-up of the lateral hump with long setae, including additional small spines. (D) Close-up of what is likely to be the head capsule and anterior part of the body devoid of the dark green substance present elsewhere. Abbreviations: hc? = possible head capsule; ls = long barbed setae; te? = possible trunk end.
The anterior part of the specimen seems damaged (Figure 4D). Most of the dorsal side is covered by a dark green substance (it is unclear if this is only an optical interaction of the specimen and the resin or if this is a true substance). The anterior body region lacks this substance. All intact and accessible segments have distinct dorsal humps. The details are obscured, but each segment consistently has at least four large humps: two lateral, two dorsal.
The head bears around 20 setae with both multiple hair-like setae and short spine-like setae (Figure 4A). On the trunk segments, each lateral hump has at least 20 spine-like setae (Figure 4B). The supposed last three abdomen segments (not including the trunk end) bear, in addition to the spines, at least four long setae with small spine-like protruding setules (barbed setae; Figure 4C). Dorsal humps have an uncertain number of spines; the spines appear short, similar to the ones on the anterior lateral humps (Figure 4B). The trunk end has at least twelve short spines and at least three hair-like setae.
4. Discussion
4.1. Systematic Affinity of the Specimens
Specimen PED 0893 can be easily recognised as a lepidopteran larva (otherwise known as caterpillar) based on the presence of crochet-bearing prolegs on abdomen segments 3–6. With the numerous spines arising from dorsal humps, the specimen likely represents a type of heavily armoured caterpillar. It is not always easy to determine such armoured caterpillars (e.g., [37]).
The arrangements of spine-bearing humps, like those on the new specimen, are only seen in the larvae of two ingroups of Erebidae (Noctuoidea), namely Arctiinae (e.g., [38] (their pp. 70, 71, 73–84), [39] (their figures 1 and 3)) and Lymantriinae (e.g., [38] (their pp. 173–175), [40] (their figure 1D), [41] (his figure 1)). The observed barbed setae would support such a systematic affinity (see [41], his figure 3). Species of both groups (Arctiinae and Lymantriinae) are found in Madagascar and Brazil today [42] (his figure 13, p. 134), [43] (their table 6.1, p. 82), [44,45]; therefore, it is difficult to rule out either group based on the location they were found in. Since the specimen is also not visible in full detail, it is unclear whether it is a representative of either Arctiinae or Lymantriinae based on the morphology of the specimen. Hence, we tentatively interpret the specimen as a representative of Erebidae but make no statements on any more specific systematic affinity.
The second new specimen (CEMHS-D0054) could not be identified as easily. The limited access precludes the ability to identify any appendages and only allows an estimation of the general body segmentation and orientation of the specimen. Due to a similar arrangement of spine-bearing humps as in the first new specimen (PED 0893), we also tentatively interpret the specimen to be a representative of Erebidae, with no statements on any more specific systematic affinity for the same reasons as stated above.
4.2. The Lack of an Upper Boundary for the Fossil Record
The list of caterpillars from the Holocene is rather short. It could be much longer, depending on how one interprets the Holocene. This discrepancy is linked with the overall problem of the upper boundary of the fossil record, as well as with certain (partly politically driven) ideas on the younger Earth history (e.g., if an Anthropocene exists; see discussions in [46,47,48]).
In the literature, there appears to be no consensus on what a sub-fossil is (see Introduction). Additionally, the term “sub-recent” is not a better one, especially as “recent” has become a less-accepted term for a geological period (and should, in fact, not be used; [49] (their p. 1001)). In other words, there is no clear criterion as to when the term “sub-fossil” should be applied, which leaves us to a certain degree in a terminological limbo.
Especially for resins from Madagascar and Brazil (and other places; see Delclòs et al. [22]), this terminological uncertainty complicates things. Indeed, Delclòs et al. [22] recently demonstrated that these resins are very young. Using radiocarbon dating, they concluded that most of the Madagascar resins are likely between 80 and 300 years old and therefore not palaeontologically relevant. In fact, quite a number of specimens stored in alcohol in entomological museum collections may be older than some of the specimens preserved in natural resins. Thus, this type of resin is a non-fossil resin.
Additionally, there is a possibility that some animals preserved in copal are representatives of extant species [19]; in this case, some authors would not apply the term sub-fossil as they restrict the term to “recently extinct” species [50]. This contradiction, in fact, depends on the general problem of species in time. It has been argued that specimens preserved in Miocene Dominican amber might represent extant species [20]. Yet, there is actually no real criterion for evaluating such a statement. The major generally accepted species concepts lack any aspect of time, and even the few that include it do not provide an applicable way of how to deal with this problem. This does, in fact, not only apply to fossils or sub-fossils, but also to specimens stored in alcohol for decades (or more than a century). For more details on this discussion, refer to Haug & Haug [50].
4.3. Why These Specimens Are Still Informative
After Delclòs et al. [22] recognised how young Madagascar copal (and other copals) in fact is, they argued that it is therefore not of palaeo-biological value (we partly disagree on this point, as discussed further below), but that it is still valuable for other approaches. For example, it may prove important for shorter-term comparisons of biodiversity, especially concerning anthropogenic declines in biodiversity (further elaborated in Solórzano-Kraemer et al. [1]). Yet, Delclòs et al. [22] (their p. 24) also pointed out that for such a use, it will be important to know the exact provenance of the copal, but this is often not available, including for the specimens reported here. Still, these specimens provide some significant information.
Lepidopterans (moths and butterflies, although the latter is basically a derived form of the former) have generally been considered to be rare as fossils [31]. This rarity also extends to lepidopteran larvae, the caterpillars. That leaves the question of what the reasons are for this apparent rarity (especially in caterpillars). Clearly, caterpillars are rather soft, and likely rapidly decaying organisms, but amber and younger resins should clearly be capable of preserving caterpillars. Indeed, there are examples of caterpillars in amber. Yet, the numbers are still low. Only a handful of caterpillars are known from Cretaceous ambers [24,31,51,52], with slightly more specimens from Miocene ambers [25,31]. In Eocene Baltic amber, the numbers are higher; over 100 specimens seem to be known (e.g., [31,53,54,55,56,57,58,59,60,61,62,63,64]). Even so, this number is still low compared with the known number of specimens of other groups, such as the larvae of flies (Diptera; see discussion in Amaral et al. [65] and references therein) or the comparatively species-poor group of lacewings (Neuroptera; [25,66,67,68]). These scarce records give the impression that caterpillars are not easily trapped in resin to then become preserved in amber.
Curiously, many caterpillars preserved in amber seem to be “naked” caterpillars, i.e., having no or few indistinct spines and/or setae (see discussion in [24]). None of the caterpillars in Cretaceous ambers have long spines; in fact, only a single one is armoured at all, although with rather short, robust, and conical spines ([24,51] and references therein).
When looking at other holometabolan larvae in amber, we can see comparable situations, at least to a certain degree. Other caterpillar-type larvae with processes on their back, for example, those of scorpionflies (Mecoptera), are also rather small, although so far, the sample size is restricted to two specimens [52,69]. A larger sample size is available for lacewings (already used for a comparison in [25]), although the ecology is different from caterpillars as most lacewing larvae are predatory. Numerous fossil lacewing larvae possess prominent processes on their back [70,71,72,73,74,75,76,77]. Yet, the larger larvae [78,79], reaching into the centimetre range, lack such processes. For beetles, likewise, relatively many larvae have been found in amber, and some of these also bear processes [59], yet the really large beetle larvae in amber [80], measuring several centimetres, lack such processes. It seems, therefore, that large larvae with prominent processes (spines, hairs, etc.) are especially rare in resins, the two specimens reported here therefore being exceptionally important.
Among Eocene (Baltic amber) caterpillars, most seem to be of the naked type (e.g., [54] (their figure 335, p. 138, their figure 337, p. 139) and [54] (their p. 195, pl. 78a–c, d); the latter specimen was re-figured in [56] (their figure 13.48) and in [62] (their figure 7); [57] (his figure 145, p. 85), [60] (his figures 2567 and 7255, p. 340), re-figure from [53]; [61] (their Abb. 11.45, p. 77), re-figure from [60] (his figure 2567), [63] (their figure 1)), or they are case-building ones, which also lack spines ([53] (his figure 388, p. 139), [54] (their pl. 79 a–h, p. 197), [55] (their figure 3), [58] (their figures 1–12), [61] (their figures 1364, 2541, 2563, and 7532, p. 340, their figures 1374, 1384, 2503, 2524, and 7622, p. 341).
There is one exception in Baltic amber. A specimen reported by Poinar & Vega [64] (their figure 1A, B) has relatively long venomous setae, but it differs significantly from the specimen reported here in having shorter and fewer spines.
When looking into the Miocene, we also see several specimens of the naked type (including Mexican and Dominican amber; [81] (his figure F-216, p. 134), [56] (their figure 13.58), [82] (their figure 23), [83] (their figure 12), [84] (their figures 4–6, p. 150)) and case-bearing ones ([81] (his figures F-227 and F-228, p. 136), [56] (their figure 13.35)). In Dominican amber, more strongly armoured specimens have been found. A specimen reported by DeVries & Poinar [85] (their figure 1) (re-figured in [86] (their figure 107, colour plates 107, pp. 101–102) and also refigured in Poinar [87] (his figure 48)) has short, stubby, and balloon-like setae at the head–trunk transition and softer-appearing setae on the trunk. A more heavily armoured specimen in Dominican amber was reported by Poinar & Hammond [88] (their figures 1–3) (re-figured in [86] (their figure 53B, p. 58) and Poinar et al. [83] (their figure 13)). The spines are not very long, but they are prominent, carrying secondary spinules. Both specimens are less prominently armoured than the ones reported here.
A specimen with longer spines from Miocene Dominican amber was depicted in Poinar & Poinar [86] (their Figure 51, colour pl. 51, p. 55). Unlike the specimens we present, there seem to be fewer spine-like setae, and they appear not to be arranged in groups.
The only specimen more comparable to the ones here reported is from Miocene Dominican amber and was described in Wu [81] (his F-221, p. 135). It carries numerous long and strong setae and was also interpreted as a caterpillar of the group Erebidae (originally Lymantriidae, now ≈ Lymantriinae). Unfortunately, the image is rather small, which does allow for a closer inspection. Finally, one specimen preserved in presumably extant East African copal [32] was reported to have long setae and was interpreted as a possible larva of Lymantriidae (see [31] (their p. 81)).
Overall, the fossil record provides the impression that caterpillars are rarely preserved in amber, that naked caterpillars are more easily preserved than armoured specimens, and that heavily armoured specimens are virtually absent. There is no reason to believe that strongly armoured caterpillars with many spines and setae cannot be caught in resin. It is possible that these impressions are a reflection of their original abundance or simply a collection bias. The specimens reported here are therefore an interesting piece for actual-palaeontological consideration, which enable the present to be studied to better understand the past.
Additionally, the newly reported specimens provide us with information about the size range of caterpillars that can be preserved in resins. Notably, the preserved part of the body of the first specimen is more than 12 mm long. It now stands as the longest caterpillar preserved in resin reported so far (see recent review in [51]).
4.4. Show More Non-Fossil Resins, Please!
Non-fossil resins do, therefore, provide relatively important information concerning preservation in resins (see also Delclòs et al. [22]). However, to effectively use non-fossil resins in this way, it would obviously be necessary to have a much larger number of these types with inclusions, and for all of these inclusions to also be depicted when they are published. In the case of larvae, there is the general habit that immature ones are less likely to be published than adults, as there seems to be still a focus on new species descriptions based on adults (see discussion in Baranov et al. [89,90]). For non-fossil resins, the situation seems even worse. Delclòs et al. [22] mentioned more than 100 species described based on specimens preserved in copal (some of these would now likely be interpreted as defaunation resin). For larvae in copal, we have no numbers at hand; there is at least one lacewing larva in Colombian copal (defaunation resin?; [91] (his figure 206, p. 55)), but that seems to be an extreme exception. This tradition can also be seen in the case of Evers [32], who mentioned three caterpillars in his material at hand but did not illustrate a single one.
If non-fossil resins should indeed be used in diversity studies (as suggested in [22]), it would be necessary to have a much larger number of these to be accessible with images. Larvae, such as caterpillars, cannot be easily used in taxonomy-based approaches (for which a simple report of occurrence may often be sufficient), but they can be used in morphology-based studies ([68] and references therein). For such an approach, depicted specimens are necessary. Finally, as a last statement, we would like to add a suggestion to the community: please present more specimens, including those in copal and defaunation resins, as well as immature specimens.
Author Contributions
Conceptualization, A.P.A. and J.T.H.; Methodology, C.H. and J.T.H.; Investigation, J.T.H., J.G., A.P.A. and C.H.; Resources, C.H. and J.T.H.; Writing—Original Draft Preparation, J.T.H.; Writing—Review and Editing, A.P.A., C.H., J.T.H. and J.G.; Visualization, J.G.; Supervision, J.T.H.; Project Administration, C.H.; Funding Acquisition, A.P.A., J.G. and J.T.H. All authors have read and agreed to the published version of the manuscript.
Funding
Joshua Gauweiler is funded by the State Graduate Funding Scheme of Mecklenburg–Western Pomerania through the University of Greifswald. André P. Amaral receives support from the Graduate School Life Science Munich (LSM) and from the German Academic Exchange Service (DAAD) through a PhD scholarship. Joachim T. Haug is supported by the German Research Foundation (DFG HA 6300/6-1) and by the Volkswagen Foundation with a Lichtenberg Professorship.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Acknowledgments
We are grateful to Fenja I. Haug and Gideon T. Haug, both of Neuried, for help with the drawings. We thank Steffen Harzsch, Greifswald, and Marie K. Hörnig, Rostock for continued support. All people providing free software are thanked for donating their time.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Solórzano-Kraemer, M.M.; Delclòs, X.; Engel, M.S.; Peñalver, E. A revised definition for copal and its significance for palaeontological and Anthropocene biodiversity-loss studies. Sci. Rep. 2020, 10, 19904. [Google Scholar] [CrossRef]
- Albrecht, G.H.; Jenkins, P.D.; Godfrey, L.R. Ecogeographic size variation among the living and subfossil prosimians of Madagascar. Am. J. Primatol. 1990, 22, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Hjort, C.; Funder, S. The subfossil occurrence of Mytilus edulis L. in central East Greenland. Boreas 1974, 3, 23–33. [Google Scholar] [CrossRef]
- Molloy, B.P.; Burrows, C.J.; Cox, J.E.; Johnston, J.A.; Wardle, P. Distribution of subfossil forest remains, eastern South Island, New Zealand. N. Z. J. Bot. 1963, 1, 68–77. [Google Scholar] [CrossRef]
- Eronen, M.; Huttunen, P. Radiocarbon-dated subfossil pines from Finnish Lapland. Geogr. Ann. Ser. A Phys. Geogr. 1987, 69, 297–304. [Google Scholar] [CrossRef]
- Allen, J.R. Subfossil mammalian tracks (Flandrian) in the Severn Estuary, SW Britain: Mechanics of formation, preservation and distribution. Philos. Trans. R. Soc. B Biol. Sci. 1997, 352, 481–518. [Google Scholar] [CrossRef]
- Szeroczyfiska, K.; Sarmaja-Korjonen, K. Atlas of Subfossil Cladocera from Central and Northern Europe; Friends of the Lower Vistula Society: Świecie, Poland, 2007. [Google Scholar]
- Berta, C.; Tóthmérész, B.; Wojewódka, M.; Augustyniuk, O.; Korponai, J.; Bertalan-Balázs, B.; Nagy, A.S.; Grigorsky, I.; Gyulai, I. Community response of Cladocera to trophic stress by biomanipulation in a shallow oxbow lake. Water 2019, 11, 929. [Google Scholar] [CrossRef]
- Wojewódka, M.; Sinev, A.Y.; Zawisza, E. A guide to the identification of subfossil non-chydorid Cladocera (Crustacea: Branchiopoda) from lake sediments of Central America and the Yucatan Peninsula, Mexico: Part I. J. Paleolimnol. 2020, 63, 269–282. [Google Scholar] [CrossRef]
- Rieradevall, M.; Brooks, S.J. An identification guide to subfossil Tanypodinae larvae (Insecta: Diptera: Chrironomidae) based on cephalic setation. J. Paleolimnol. 2001, 25, 81–99. [Google Scholar] [CrossRef]
- Axford, Y.; Miller, G.H.; Geirsdóttir, Á.; Langdon, P.G. Holocene temperature history of northern Iceland inferred from subfossil midges. Quat. Sci. Rev. 2007, 26, 3344–3358. [Google Scholar] [CrossRef]
- Briggs, D.E.G. Molecular taphonomy of animal and plant cuticles: Selective preservation and diagenesis. Philos. Trans. R. Soc. B Biol. Sci. 1999, 354, 7–17. [Google Scholar] [CrossRef]
- Harding, J.P. A rare estuarine copepod crustacean, Enhydrosoma garienis, found in the Holocene of Kent. Nature 1956, 178, 1127–1128. [Google Scholar] [CrossRef]
- Haug, C.; Haug, J.T.; Fayers, S.R.; Trewin, N.H.; Castellani, C.; Waloszek, D.; Maas, A. Exceptionally preserved nauplius larvae from the Devonian Windyfield chert, Rhynie, Aberdeenshire, Scotland. Palaeont. Electr. 2012, 15, 24A. [Google Scholar] [CrossRef] [PubMed]
- Braun, A. Vorkommen, Untersuchungsmethoden und Bedeutung tierischer Cuticulae in kohligen Sedimentgesteinen des Devons und Karbons. Palaeontogr. Abt. A 1997, 245, 83–156. [Google Scholar] [CrossRef]
- Selden, P.A.; Huys, R.; Stephenson, M.H.; Heward, A.P.; Taylor, P.N. Crustaceans from bitumen clast in Carboniferous glacial diamictite extend fossil record of copepods. Nat. Commun. 2010, 1, 50. [Google Scholar] [CrossRef] [PubMed]
- Harvey, T.H.; Vélez, M.I.; Butterfield, N.J. Exceptionally preserved crustaceans from western Canada reveal a cryptic Cambrian radiation. Proc. Natl. Acad. Sci. USA 2012, 109, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Haug, C.; Wagner, P.; Haug, J.T. The evolutionary history of body organisation in the lineage towards modern scorpions. Bull. Geosci. 2019, 94, 389–408. [Google Scholar] [CrossRef]
- Schwartz, G.T.; Samonds, K.E.; Godfrey, L.R.; Jungers, W.L.; Simons, E.L. Dental microstructure and life history in subfossil Malagasy lemurs. Proc. Natl. Acad. Sci. USA 2002, 99, 6124–6129. [Google Scholar] [CrossRef] [PubMed]
- Hörnschemeyer, T.; Wedmann, S.; Poinar, G. How long can insect species exist? Evidence from extant and fossil Micromalthus beetles (Insecta: Coleoptera). Zool. J. Linn. Soc. 2010, 158, 300–311. [Google Scholar] [CrossRef]
- Penney, D. Sub/fossil resin research in the 21st century: Trends and perspectives. PalZ 2016, 90, 425–447. [Google Scholar] [CrossRef]
- Delclòs, X.; Peñalver, E.; Ranaivosoa, V.; Solórzano-Kraemer, M.M. Unravelling the mystery of “Madagascar copal”: Age, origin and preservation of a Recent resin. PLoS ONE 2020, 15, e0232623. [Google Scholar] [CrossRef]
- Vávra, N. Amber, fossil resins, and copal–Contributions to the terminology of fossil plant resins. Denisia 2009, 26, 213–222. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, C. A 100 million-year-old armoured caterpillar supports the early diversification of moths and butterflies. Gondwana Res. 2021, 93, 101–105. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, C.; Wang, Y.; Baranov, V.A. The fossil record of lepidopteran caterpillars in Dominican and Mexican amber. Lethaia 2022, 55, 1–14. [Google Scholar] [CrossRef]
- Haug, C.; Shannon, K.R.; Nyborg, T.; Vega, F.J. Isolated mantis shrimp dactyli from the Pliocene of North Carolina and their bearing on the history of Stomatopoda. Bol. Soc. Geol. Mex. 2013, 65, 273–284. [Google Scholar] [CrossRef]
- Haug, J.T.; Müller, P.; Haug, C. The ride of the parasite: A 100-million-year old mantis lacewing larva captured while mounting its spider host. Zool. Lett. 2018, 4, 31. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, C. Beetle larvae with unusually large terminal ends and a fossil that beats them all (Scraptiidae, Coleoptera). PeerJ 2019, 7, e7871. [Google Scholar] [CrossRef]
- Rosenkjaer, H.N. Fra det Underjordiske København. Geologiske og Historiske Undersøgelser; Det Schønbergske Forlag: København, Denmark, 1906. [Google Scholar]
- Henriksen, K.L. Undersøgelser over Danmark-Skånes kvartaere Insektfauna. Vidensk. Meddel. Natuirist. Foren. 1933, 96, 77–355. [Google Scholar]
- Sohn, J.C.; Labandeira, C.C.; Davis, D.R.; Mitter, C. An annotated catalog of fossil and subfossil Lepidoptera (Insecta: Holometabola) of the world. Zootaxa 2012, 3286, 1–132. [Google Scholar] [CrossRef]
- Evers, J. Copal-Schmetterlinge. In Entomologisches Jahrbuch; Verlag von Frankenstein & Wagner: Frankfurt, Germany, 1907; pp. 129–132. [Google Scholar]
- Keble, R.A. Notes on Australian Quaternary climates and migration. Mem. Mus. Vic. 1947, 15, 28–81. [Google Scholar] [CrossRef]
- Gill, E.D. Fossil insects, centipede and spider. Vic. Nat. 1955, 72, 87–92. [Google Scholar]
- Simonsen, T.J.; Wagner, D.L.; Heikkilä, M. Ghosts from the past: A review of fossil Hepialoidea (Lepidoptera). PeerJ 2019, 7, e7982. [Google Scholar] [CrossRef] [PubMed]
- Lemdahl, G. Late Glacial and Early Holocene insect assemblages from sites at different altitudes in the Swiss Alps—Implications on climate and environment. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 159, 293–312. [Google Scholar] [CrossRef]
- Dixon, W.N.; Foltz, J.L. Caterpillars That Are Not the Gypsy Moth Caterpillar; Some Forest Lepidoptera in Florida (Lepidoptera: Arctiidae, Lasiocampidae, Lymantriidae), Entomology Circular; Florida Department of Agriculture & Consumer Services, Division of Plant Industry: Gainesville, FL, USA, 1991; Volume 270, p. 2.
- Miller, J.C.; Hammond, P.C. Lepidoptera of the Pacific Northwest: Caterpillars and Adults; Forest Health Technology Enterprise Team, US Department of Agriculture, Forest Service: Morganton, WV, USA, 2003. [CrossRef]
- Rab Green, S.B.; Gentry, G.L.; Greeney, H.F.; Dyer, L.A. Ecology, natural history, and larval descriptions of Arctiinae (Lepidoptera: Noctuoidea: Erebidae) from a cloud forest in the Eastern Andes of Ecuador. Ann. Entomol. Soc. Am. 2011, 104, 1135–1148. [Google Scholar] [CrossRef]
- Deml, R.; Dettner, K. Chemical defence of emperor moths and tussock moths (Lepidoptera: Saturniidae, Lymantriidae). Entomol. Gen. 1997, 21, 225–251. [Google Scholar] [CrossRef]
- Hossler, E.W. Caterpillars and moths: Part I. Dermatologic manifestations of encounters with Lepidoptera. J. Am. Acad. Dermatol. 2010, 62, 1–10. [Google Scholar] [CrossRef]
- Krüger, M. Composition and origin of the Lepidoptera faunas of southern Africa, Madagascar and Réunion (Insecta: Lepidoptera). Ann. Transvaal Mus. 2007, 44, 123–178. [Google Scholar]
- Barsics, F.; Razafimanantsoa, T.M.; Minet, J.; Haubruge, É.; Verheggen, F. Nocturnal moth inventory in Malagasy tapia woods, with focus on silk-producing species. In Les Vers à Soie Malgaches. Enjeux Écologiques et Socio-Économiques, 1st ed.; Verheggen, F., Bogaert, J., Haubruge, E., Eds.; Les Presses Agronomiques de Gembloux: Gembloux, Belgium, 2013; pp. 77–89. [Google Scholar]
- Wiorek, M.; Malik, K.; Lees, D.; Przybyłowicz, Ł. Malagasy Polka Dot Moths (Noctuoidea: Erebidae: Arctiinae: Syntomini) of Ambohitantely-endemism in the most important relict of Central Plateau rainforest in Madagascar. PeerJ 2021, 9, e11688. [Google Scholar] [CrossRef]
- Meneses, A.; Bevilaqua, M.; Hamada, N.; Querino, R. The aquatic habit and host plants of Parades klagesi (Rothschild) (Lepidoptera, Erebidae, Arctiinae) in Brazil. Rev. Bras. Entomol. 2013, 57, 350–352. [Google Scholar] [CrossRef]
- Veland, S.; Lynch, A.H. Scaling the Anthropocene: How the stories we tell matter. Geoforum 2016, 72, 1–5. [Google Scholar] [CrossRef]
- Waters, C.N.; Zalasiewicz, J.; Summerhayes, C.; Barnosky, A.D.; Poirier, C.; Gałuszka, A.; Cearreta, A.; Edgeworth, M.; Ellis, E.C.; Ellis, M.; et al. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 2016, 351, 6269. [Google Scholar] [CrossRef]
- Malhi, Y. The concept of the Anthropocene. Annu. Rev. Environ. Resour. 2017, 42, 77–104. [Google Scholar] [CrossRef]
- Pillans, B.; Gibbard, P. Chapter 30—The quaternary period. In The Geologic Time Scale 2012; Gradstein, F.M., Ogg, J.G., Schmitz, M.D., Ogg, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 979–1010. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, C. Species, populations and morphotypes through time—Challenges and possible concepts. BSGF Earth Sci. Bull. 2017, 188, 20. [Google Scholar] [CrossRef]
- Gauweiler, J.; Haug, C.; Müller, P.; Haug, J.T. Lepidopteran caterpillars in the Cretaceous: Were they a good food source for early birds? Palaeodiversity 2022, 15, 45–59. [Google Scholar] [CrossRef]
- Haug, C.; Haug, J.T.; Haug, G.T.; Müller, P.; Zippel, A.; Kiesmüller, C.; Gauweiler, J.; Hörnig, M.K. Fossils in Myanmar amber demonstrate the diversity of anti-predator strategies of Cretaceous holometabolan insect larvae. iScience 2024, 27, 108621. [Google Scholar] [CrossRef] [PubMed]
- Janzen, J.W. Arthropods in Baltic Amber; Ampyx-Verlag: Halle, Germany, 2002. [Google Scholar]
- Weitschat, W.; Wichard, W. Atlas of Plants and Animals in Baltic Amber; Pfeil Verlag: München, Germany, 2002. [Google Scholar]
- Perkovsky, E.E.; Zosimovich, V.Y.; Vlaskin, A.P. A Rovno amber fauna: A preliminary report. Acta Zool. Cracov. 2003, 46, 423–430. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, MA, USA, 2005. [Google Scholar] [CrossRef]
- Ross, A. Amber: The Natural Time Capsule; The Natural History Museum: London, UK, 2009. [Google Scholar]
- Sobczyk, T.; Kobbert, M.J. Die Psychidae des baltischen Bernsteins. Nota Lepidopterol. 2009, 32, 13–22. [Google Scholar]
- Weitschat, W.; Berning, B.; Podenas, S. Jäger, Gejagte, Parasiten und Blinde Passagiere–Momentaufnahmen aus dem Bernsteinwald. Denisia 2009, 26, 243–256. [Google Scholar]
- Gröhn, C. Einschlüsse im Baltischen Bernstein; Wachholtz Verlag-Murmann Publishers: Kiel, Germany, 2015. [Google Scholar]
- Gröhn, C.; Hornemann, M.; Joger, U.; Koch, A.; Kosma, R.; Krüger, F.J.; Vahldisk, B.-W.; Wilde, V.; Zellmer, H. Zeitkapsel Bernstein—Lebewesen Vergangener Welten; Verlag Dr. Friedrich Pfeil: München, Germany, 2015. [Google Scholar]
- Heikkilä, M.; Simonsen, T.J.; Solis, M.A. Reassessment of known fossil Pyraloidea (Lepidoptera) with descriptions of the oldest fossil pyraloid and a crambid larva in Baltic amber. Zootaxa 2018, 4483, 101–127. [Google Scholar] [CrossRef]
- Fischer, T.C.; Michalski, A.; Hausmann, A. Geometrid caterpillar in Eocene Baltic amber (Lepidoptera, Geometridae). Sci. Rep. 2019, 9, 17201. [Google Scholar] [CrossRef]
- Poinar, G.; Vega, F.E. Poisonous setae on a Baltic amber caterpillar. Arthropod Struct. Dev. 2019, 51, 37–40. [Google Scholar] [CrossRef]
- Amaral, A.P.; Gombos, D.; Haug, G.T.; Haug, C.; Gauweiler, J.; Hörnig, M.K.; Haug, J.T. Expanding the fossil record of soldier fly larvae—An important component of the Cretaceous amber forest. Diversity 2023, 15, 247. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Engel, M.S.; Delclòs, X.; Penalver, E. Straight-jawed lacewing larvae (Neuroptera) from Lower Cretaceous Spanish amber, with an account on the known amber diversity of neuropterid immatures. Cretac. Res. 2020, 106, 104200. [Google Scholar] [CrossRef]
- Haug, C.; Haug, G.T.; Baranov, V.A.; Solórzano-Kraemer, M.M.; Haug, J.T. An owlfly larva preserved in Mexican amber and the Miocene record of lacewing larvae. Bol. Soc. Geol. Mex. 2021, 73, A271220. [Google Scholar] [CrossRef]
- Haug, C.; Braig, F.; Haug, J.T. Quantitative analysis of lacewing larvae over more than 100 million years reveals a complex pattern of loss of morphological diversity. Sci. Rep. 2023, 13, 6127. [Google Scholar] [CrossRef]
- Szpila, K.; van de Kamp, T.; Sontag, E.; Krzemiński, W.; Kopeć, K.; Soszyńska, A. The hidden world of fossil larvae: Description and morphological insights of an immature scorpionfly (Mecoptera: Panorpidae) from the Baltic amber. Zool. J. Linn. Soc. 2024, zlae009. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Delclòs, X.; Peñalver, E.; Speranza, M.; Wierzchos, J.; Ascaso, C.; Engel, M.S. Early evolution and ecology of camouflage in insects. Proc. Natl. Acad. Sci. USA 2012, 109, 21414–21419. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Delclos, X.; Penalver, E.; Engel, M.S. A defensive behavior and plant-insect interaction in Early Cretaceous amber–the case of the immature lacewing Hallucinochrysa diogenesi. Arthropod Struct. Dev. 2016, 45, 133–139. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Peñalver, E.; Azar, D.; Engel, M.S. A soil-carrying lacewing larva in Early Cretaceous Lebanese amber. Sci. Rep. 2018, 8, 16663. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Fuente, R.; Engel, M.S.; Azar, D.; Peñalver, E. The hatching mechanism of 130-million-year-old insects: An association of neonates, egg shells and egg bursters in Lebanese amber. Palaeontology 2019, 62, 547–559. [Google Scholar] [CrossRef]
- Wang, B.; Xia, F.; Engel, M.S.; Perrichot, V.; Shi, G.; Zhang, H.; Chen, J.; Jarzembowski, E.A.; Wappler, T.; Rust, J. Debris-carrying camouflage among diverse lineages of Cretaceous insects. Sci. Adv. 2016, 2, e1501918. [Google Scholar] [CrossRef] [PubMed]
- Badano, D.; Engel, M.S.; Basso, A.; Wang, B.; Cerretti, P. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nat. Commun. 2018, 9, 3257. [Google Scholar] [CrossRef] [PubMed]
- Haug, J.T.; Linhart, S.; Haug, G.T.; Gröhn, C.; Hoffeins, C.; Hoffeins, H.-W.; Müller, P.; Weiterschan, T.; Wunderlich, J.; Haug, C. The diversity of aphidlion-like larvae over the last 130 million years. Insects 2022, 13, 336. [Google Scholar] [CrossRef] [PubMed]
- Haug, J.T.; Tun, K.L.; Haug, G.T.; Than, K.N.; Haug, C.; Hörnig, M.K. A hatching aphidlion-like lacewing larva in 100 million years old Kachin amber. Insect Sci. 2023, 30, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Haug, G.T.; Haug, C.; Pazinato, P.G.; Braig, F.; Perrichot, V.; Gröhn, C.; Müller, P.; Haug, J.T. The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time. Palaeont. Electr. 2020, 23, a39. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Liu, H.; Jarzembowski, E.A. High morphological disparity of neuropteran larvae during the Cretaceous revealed by a new large species. Geol. Mag. 2022, 159, 954–962. [Google Scholar] [CrossRef]
- Haug, C.; Baranov, V.A.; Hörnig, M.K.; Gauweiler, J.; Hammel, J.U.; Perkovsky, E.E.; Haug, J.T. 35 million-year-old solid-wood-borer beetle larvae support the idea of stressed Eocene amber forests. Palaeobiodivers. Palaeoenviron. 2023, 103, 521–530. [Google Scholar] [CrossRef]
- Wu, R.J.C. Secrets of a Lost World, Dominican Amber and Its Inclusions; Self Published: Santo Domingo, Dominican Republic, 1996. [Google Scholar]
- Poinar Jr, G.; Poinar, R. Fossil evidence of insect pathogens. J. Invert. Pathol. 2005, 89, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Poinar Jr, G.; Kevan, P.G.; Jackes, B.R. Fossil species in Boehmerieae (Urticaceae) in Dominican and Mexican amber: A new genus (Ekrixanthera) and two new species with anemophilous pollination by explosive pollen release, and possible lepidopteran herbivory. Botany 2016, 94, 599–606. [Google Scholar] [CrossRef]
- Solis, M.A.; Léger, T.; Neumann, C. First pyraloid (Insecta, Lepidoptera) caterpillar from Dominican amber. Nota Lepidopterol. 2023, 46, 145–154. [Google Scholar] [CrossRef]
- DeVries, P.J.; Poinar, G.O. Ancient butterfly-ant symbiosis: Direct evidence from Dominican amber. Philos. Trans. R. Soc. B Biol. Sci. 1997, 264, 1137–1140. [Google Scholar] [CrossRef]
- Poinar, G.O.; Poinar, R. The Amber Forest: A Reconstruction of a Vanished World; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar] [CrossRef]
- Poinar Jr, G. Palaeoecological perspectives in Dominican amber. Ann. Soc. Entomol. Fr. 2010, 46, 23–52. [Google Scholar] [CrossRef]
- Poinar, G.; Hammond, P.C. A larval brush-footed butterfly (Lepidoptera: Nymphalidae) in Dominican amber, with a summary of fossil Nymphalidae. Insect Syst. Evol. 1998, 29, 275–279. [Google Scholar] [CrossRef]
- Baranov, V.; Hoffeins, C.; Hoffeins, H.W.; Haug, J.T. More than dead males: Reconstructing the ontogenetic series of terrestrial non-biting midges from the Eocene amber forest. Bull. Geosci. 2019, 94, 187–199. [Google Scholar] [CrossRef]
- Baranov, V.A.; Schädel, M.; Haug, J.T. Fly palaeo-evo-devo: Immature stages of bibionomorphan dipterans in Baltic and Bitterfeld amber. PeerJ 2019, 7, e7843. [Google Scholar] [CrossRef]
- Kobbert, M.J. Wunderwelt Bernstein: Faszinierende Fossilien in 3-D; WBG: Darmstadt, Germany, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).