Differential Antioxidant Enzyme Gene Expression and Functional Analysis of Pyridaben-Susceptible and -Resistant Strains of Tetranychus truncatus (Acari: Tetranychidae) under High Temperature Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mite Source
2.2. High Temperature Stress Treatment
2.3. Sequence and Phylogenetic Analyses of Antioxidant Enzyme Protein
2.4. RNA Extraction and cDNA Synthesis
2.5. Real-Time Quantitative PCR
2.6. RNA Interference
2.7. Statistical Analysis
3. Results
3.1. Bioinformatics Analysis of Antioxidant Enzyme Genes of T. truncatus
3.1.1. Identification of Antioxidant Enzyme Genes of T. truncatus
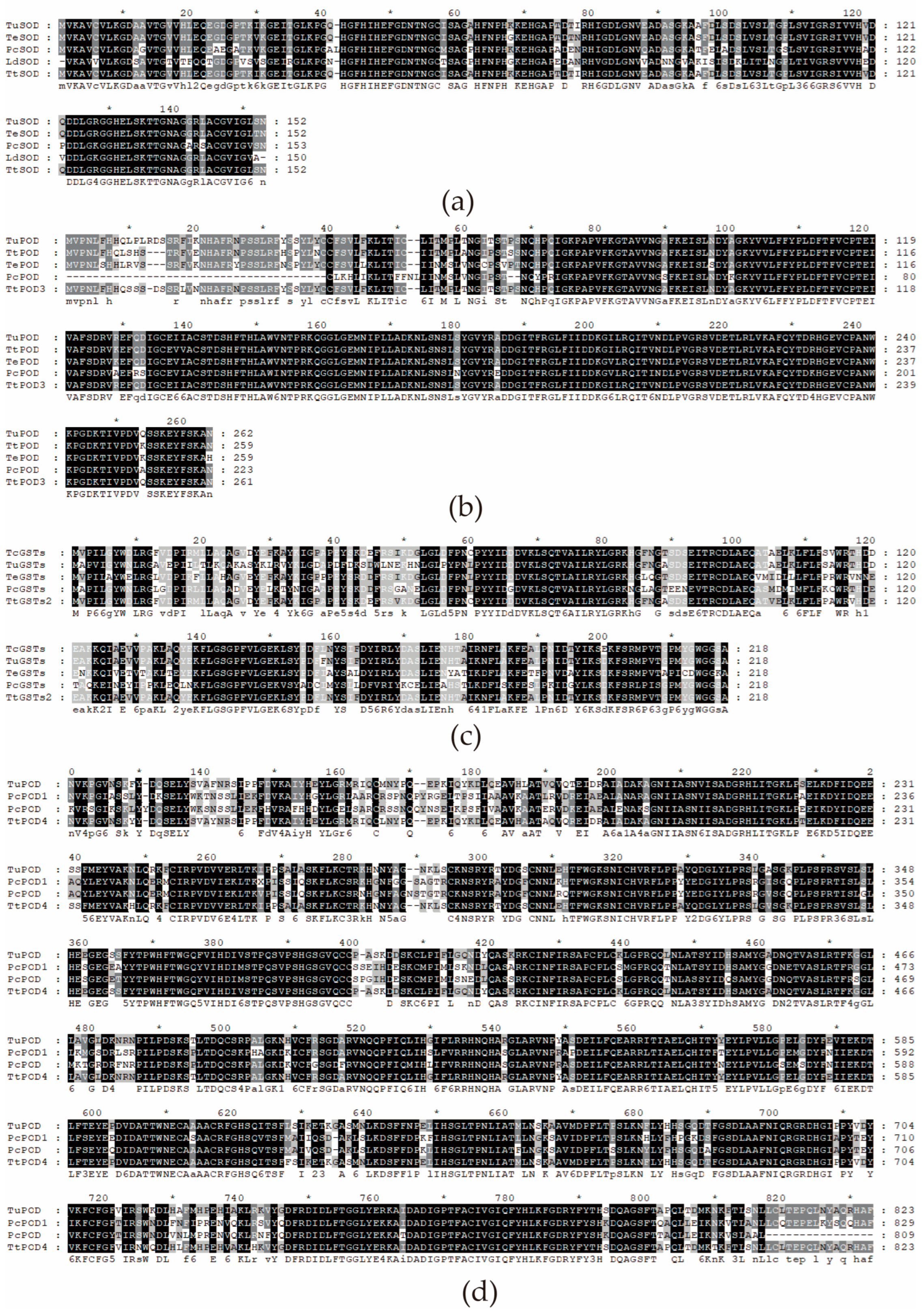
3.1.2. Domain Analysis and Sequence Alignment of Antioxidant Enzyme Protein
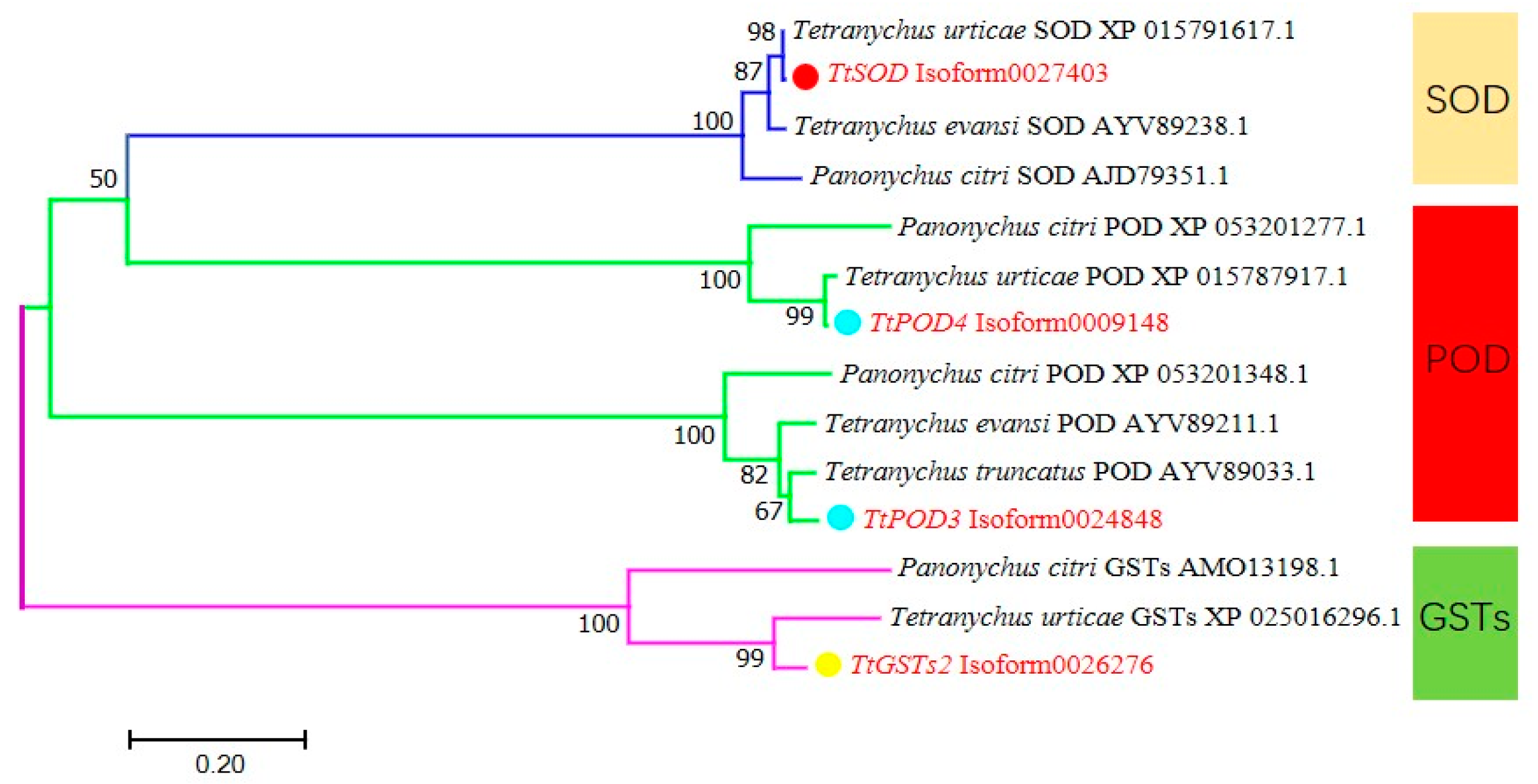
3.1.3. Phylogenetic Analysis of Antioxidant Enzyme
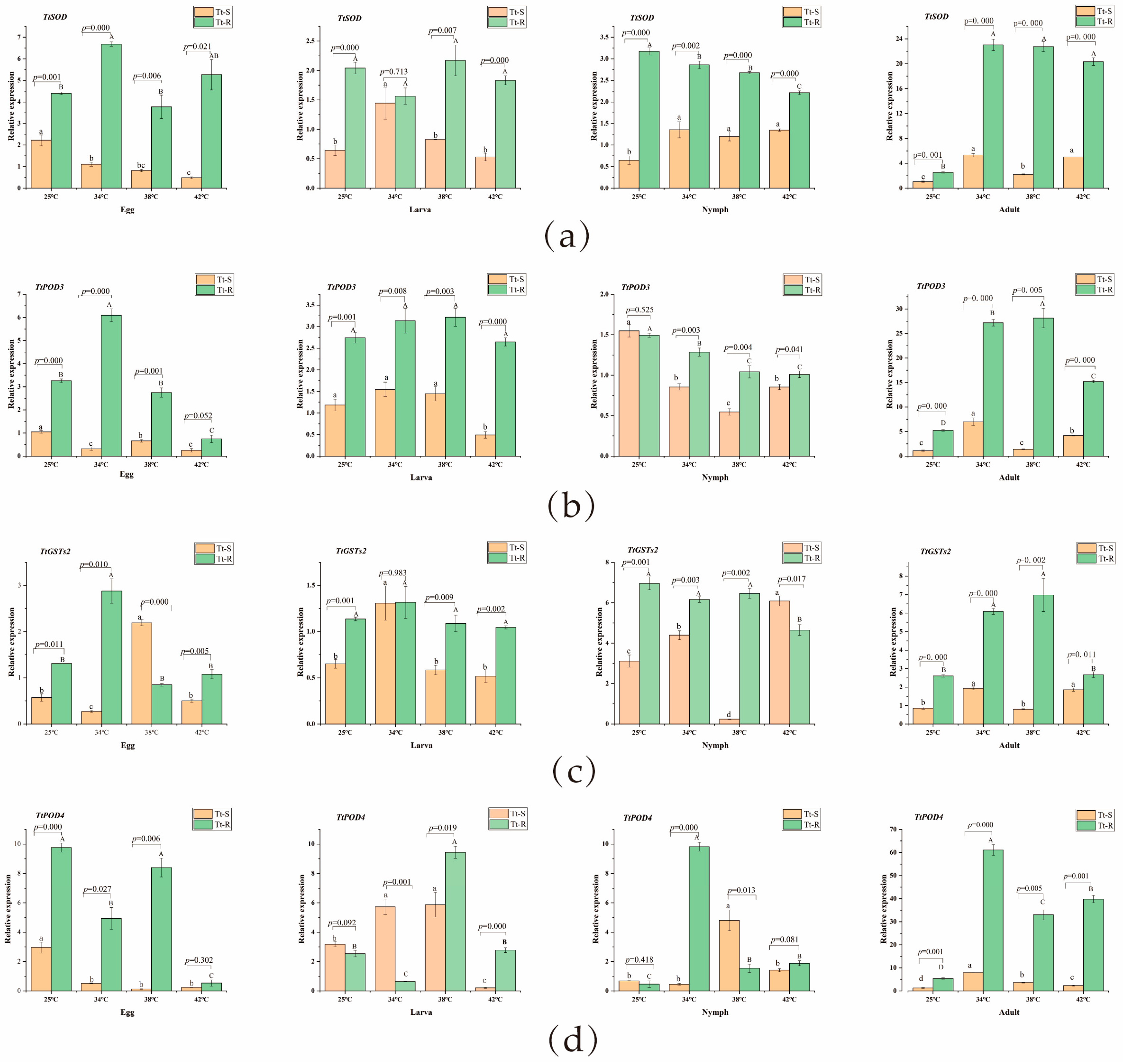
3.2. Expression Patterns of Antioxidant Enzyme Genes in T. truncatus at Different Temperatures
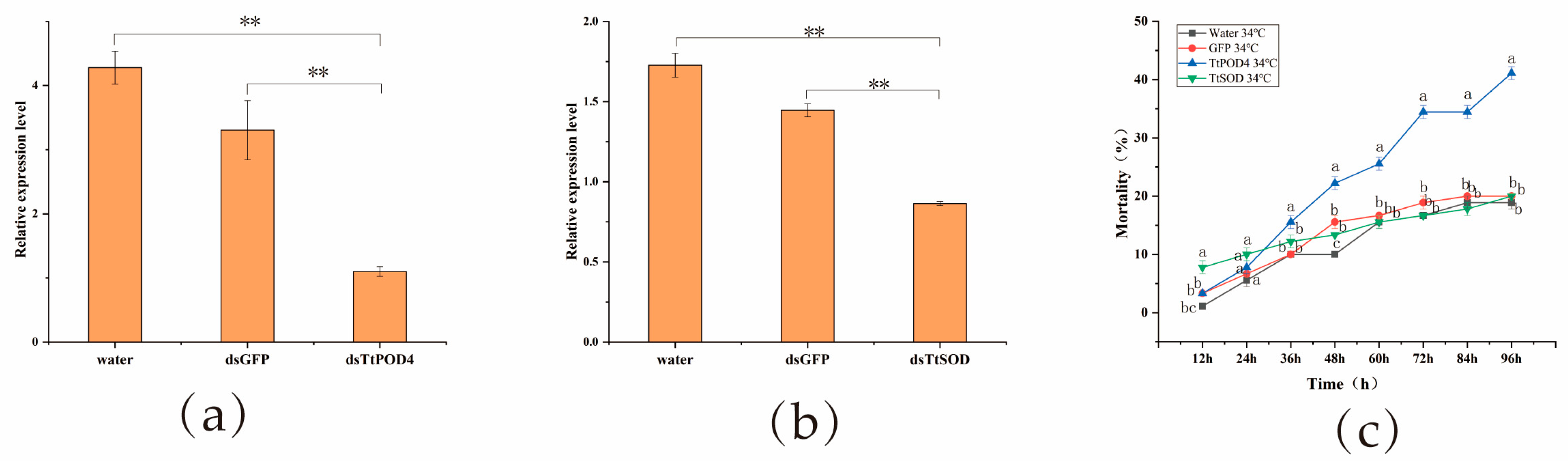
3.3. Effect of dsRNA Treatment on Mortality of T. truncatus under High Temperature Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jéssica, T.P.; Jacques, A.D.; João, V.S.; Steven, C.; Alison, B.D.; Merijn, R.K.; Juan, M.A. Rising temperatures favour defence-suppressing herbivores. J. Pest Sci. 2024, 4, 1–14. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Adhikari, S. Climate change and managing insect pests and beneficials in agricultural systems. Agron. J. 2023, 115, 2194–2215. [Google Scholar] [CrossRef]
- Mogilicherla, K.; Roy, A. Epigenetic regulations as drivers of insecticide resistance and resilience to climate change in arthropod pests. Front. Genet. 2023, 13, 1044980. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Park, Y.J. Differential transcriptome analysis reveals genes related to low and high temperature stress in the fall armyworm Spodoptera frugiperda. Front. Physiol. 2022, 12, 827077. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Kim, R.O.; Rhee, J.S.; Won, E.J.; Lee, K.W.; Kang, C.M.; Lee, Y.M.; Lee, J.S. Ultraviolet B retards growth, induces oxidative stress, and modulates DNA repair-related gene and heat shock protein gene expression in the Monogonont rotifer, Brachionus sp. Aquat. Toxicol. 2011, 101, 529–539. [Google Scholar] [CrossRef]
- Ju, R.T.; Wei, H.P.; Wang, F.; Zhou, X.H.; Li, B. Anaerobic respiration and antioxidant responses of Corythucha ciliata (Say) adults to heat-induced oxidative stress under laboratory and field condition. Cell Stress Chaperones 2014, 19, 255–262. [Google Scholar] [CrossRef]
- Muhammad, S.W.; Asem, S.S.E.; Ali, A.Z.S.; Cheng, X.L.; Zhang, Q.Q.; Shi, Z.H. Lethal and sublethal effect of heat shock on Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). J. Therm. Biol. 2020, 92, 102679. [Google Scholar] [CrossRef]
- Jan, D.; Monika, K.M.; Mateusz, M.M.; Roma, D. Enzymatic defense response of apple aphid aphis pomi to increased temperature. Insects 2020, 11, 436. [Google Scholar] [CrossRef]
- Roma, D.; Jan, D.; Monika, K.M.; Mateusz, M.; Tomasz, D. Changes in antioxidative, oxidoreductive and detoxification enzymes during development of aphids and temperature increase. Antioxidants 2021, 10, 1181. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Z.X.; Kang, Z.W.; Li, H.; Liu, T.X.; Wang, D. Identification and characterization of antioxidant enzyme genes in parasitoid Aphelinus asychis (Hymenoptera: Aphelinidae) and expression profiling analysis under temperature stress. Insects 2022, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, A.; Inayat, R.; Tamkeen, A.; Haq, I.U.; Li, C.M.; Boamah, S.; Zhou, J.J.; Liu, C.Z. Antioxidant enzymes and heat-shock protein genes of green peach aphid (Myzus persicae) under short-time heat stress. Front. Physiol. 2021, 12, 805509. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, X.Q.; Xiao, P.A.; Tang, G.H.; Liu, S.H.; Lou, B.H.; Wang, J.J.; Jiang, H.B. Comparative transcriptome analysis reveals differentially expressed genes in the Asian citrus psyllid (Diaphorina citri) upon heat shock. Comp. Biochem. Phys. D 2019, 30, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Lu, Y.J.; Yang, K.; Huo, S.M.; Hong, X.Y. Co-infection of Wolbachia and Spiroplasma in spider mite Tetranychus truncatus increases male fitness. Insect Sci. 2020, 27, 921–937. [Google Scholar] [CrossRef]
- Sararit, P.; Auamcharoen, W. Biological activities of essential oils from Anethum graveolens L. and Allium sativum L. for controlling Tetranychus truncatus Ehara and Tetranychus urticae Koch. J. Biopestic. 2020, 13, 1–12. [Google Scholar]
- Jin, P.Y.; Tian, L.; Chen, L.; Hong, X.Y. Spider mites of agricultural importance in China, with focus on species composition during the last decade (2008–2017). Syst. Appl. Acarol. 2018, 23, 2087–2098. [Google Scholar] [CrossRef]
- Chen, L.; Yu, X.Y.; Xue, X.F.; Zhang, F.; Guo, L.X.; Zhang, H.M.; Hoffmann, A.A.; Hong, X.Y.; Sun, J.T. The genome sequence of a spider mite, Tetranychus truncatus, provides insights into interspecific host rangevariation and the genetic basis of adaptation to a low-qualityhost plant. Insect Sci. 2023, 30, 1208–1228. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef]
- Sander, D.R.; Emre, I.; Wannes, D.; Van Leeuwen, T. A review of the molecular mechanisms of acaricide resistance in mites and ticks. Insect Biochem. Mol. Biol. 2023, 159, 103981. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, H.M.; Cho, J.R.; Ahn, Y.J. Multiple resistance and biochemical mechanisms of pyridaben resistance in Tetranychus Urticae (Acari: Tetranychidae). J. Econ. Entomol. 2006, 99, 954–958. [Google Scholar] [CrossRef]
- Bajda, S.; Dermauw, W.; Panteleri, R.; Sugimoto, N.; Douris, V.; Tirry, L.; Osakabe, M.; Vontas, J.; Van Leeuwen, T. A mutation in the PSST homologue of complex I (NADH: Ubiquinone oxidoreductase) from Tetranychus urticae is associated with resistance to METI acaricides. Insect Biochem. Mol. Biol. 2017, 80, 79–90. [Google Scholar] [CrossRef]
- Xue, W.; Lu, X.; Mavridis, K.; Vontas, J.; Jonckheere, W.; Van Leeuwen, T. The H92R substitution in PSST is a reliable diagnostic biomarker for predicting resistance to mitochondrial electron transport inhibitors of complex I in European populations of Tetranychus urticae. Pest Manag. Sci. 2022, 78, 3644–3653. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.J.; Zhang, Y.J.; Wang, S.Y.; Chen, J.C.; Cao, L.J.; Gong, Y.J.; Pang, B.S.; Hoffmann, A.A.; Wei, S.J. A high-throughput KASP assay provides insights into the evolution of multiple resistant mutations in populations of the two-spotted spider mite Tetranychus urticae across China. Pest Manag. Sci. 2023, 79, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Su, M.M.; Liang, P.; Li, S.; Chu, D. Effects of high temperature on insecticide tolerance in whitefly Bemisia tabaci (Gennadius) Q biotype. Pestic. Biochem. Physiol. 2018, 150, 97–104. [Google Scholar] [CrossRef]
- Ge, L.Q.; Huang, L.J.; Yang, G.Q.; Song, Q.S.; David, S.; Gurr, G.M.; Wu, J.C. Molecular basis for insecticide-enhanced thermos tolerance in the brown planthopper Nilaparvata lugens Stal (Hemiptera: Delphacidae). Mol. Ecol. 2013, 22, 5624–5634. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Zhang, Y.L.; Liu, X.D.; Guo, H.F. Does resistance to buprofezin improve heat and cold tolerance of Laodelphax striatellus (Hemiptera: Delphacidae)? Environ. Entomol. 2017, 46, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.H.; Qiao, W.Q.; Zhang, C.W.; Zhang, S.H.; Wang, J.Z.; Yu, C.L.; Wang, S.S.; Song, L.W. Stress response of Tetranychus truncatus to pyridaben and high temperatures. Chin. J. Appl. Entomol. 2021, 58, 1152–1158. [Google Scholar] [CrossRef]
- Song, L.W.; Fu, W.H.; Li, W.L.; Liu, L.; Wang, S.S. The influence of high-temperature frequency variation on the life-history traits of pyridaben sensitive and resistant strains of Tetranychus truncates. Exp. Appl. Acarol. 2024, 92, 109–122. [Google Scholar] [CrossRef]
- Yu, C.L.; Li, W.L.; Zhang, J.; Song, L.W. Transcriptome analysis of response to heat stress of female adult mites of Tetranychus truncatus. Acta Agric. Boreali Occidenlalis Sin. 2023, 32, 1112–1119. [Google Scholar]
- Feng, H.Z.; Wang, L.; Liu, Y.H.; He, L.; Li, M.; Lu, W.C.; Xue, C.H. Molecular characterization and expression of a heat shock protein gene (HSP90) from the carmine spider mite, Tetranychus cinnabarinus (Boisduval). J. Insect Sci. 2010, 10, 112. [Google Scholar] [CrossRef]
- Augustine, N.; Selvapandian, U.; Venkatesan, T.; Srinivasa, N.; Rao, A.M.; Saraswathy, B.P.; Mohan, M. Evaluation of reference genes for expression studies in the broad mite, Polyphagotarsonemus latus (Acari: Tarsonemidae). Appl. Entomol. Zool. 2024, 59, 31–40. [Google Scholar] [CrossRef]
- Sun, W.; Jin, Y.; He, L.; Lu, W.C.; Li, M. Suitable reference gene selection for different strains and developmental stages of the carmine spider mite, Tetranychus cinnabarinus, using Quantitative Real-Time PCR. J. Insect Sci. 2010, 10, 208. [Google Scholar] [CrossRef]
- Muhammad, W.A.; Zhang, Z.Y.; Xia, S.; Zhang, H.Y. Biofunctional analysis of Vitellogenin and Vitellogenin receptor in citrus red mites, Panonychus citri by RNA interferenceand. Sci. Rep. 2017, 7, 16123. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Zhao, J.; Gao, Y.; Liu, Z. Target gene selection for RNAi-based biopesticides against the hawthorn spider mite, Amphitetranychus viennensis (Acari: Tetranychidae). Pest Manag. Sci. 2023, 79, 2482–2492. [Google Scholar] [CrossRef]
- Wang, T.; DeVogel, N. A revisit to two-way factorial ANOVA with mixed effects and interactions. Commun. Stat.-Theory Methods 2020, 49, 4618–4635. [Google Scholar] [CrossRef]
- Myung, I.A.; Cheol, Y.C. Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: Effects on hemolymph and biochemical parameters. Comp. Biochem. Phys. B 2009, 155, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.B.; Felton, G.W. Antioxidant systems in insect. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar]
- Qin, J.; Lu, M.X.; Zheng, Y.T.; Du, Y.Z. Molecular cloning, characterization, and functional analysis of catalase in Frankliniella occidentalis (Thysanoptera: Thripidae). Ann. Entomol. Soc. Am. 2017, 110, 212–220. [Google Scholar] [CrossRef]
- Yang, L.H.; Huang, H.; Wang, J.J. Antioxidant responses of citrus red mite, Panonychus citri (McGregor) (Acari Tetranychidae), exposed to thermal stress. J. Insect Physiol. 2010, 56, 1871–1876. [Google Scholar] [CrossRef]
- Xu, R.P.; Yu, C.W.; Mao, L.Y.; Jiang, M.C.; Gao, L.Y.; Li, M.; Liu, J.S. Antioxidant defense mechanisms and fatty acid catabolism in Red-billed Leiothrix (Leiothrix lutea) exposed to high temperatures. Avian Res. 2022, 13, 94–101. [Google Scholar] [CrossRef]
- Nie, P.C.; Yang, R.L.; Zhou, J.J.; Dewer, Y.; Shang, S.Q. Elucidating the effect of temperature stress on the protein content, total antioxidant capacity, and antioxidant enzyme activities in Tetranychus urticae (Acari: Tetranychidae). Insects 2023, 14, 429. [Google Scholar] [CrossRef]
- Lu, F.P.; Chen, Q.; Wu, C.L.; Lu, H.; Liang, X. Influence of extreme high temperature exposure on protective enzymes and Hsp70 gene expression of Mononychellus mcgregori (Acari: Tetranychidae). Chin. J. Trop. Crops 2017, 38, 919–925. [Google Scholar]
- Tian, C.B.; Zhang, G.H.; Li, Y.Y.; Liu, H. Identification of two putative phospholipid hydroperoxide glutathione peroxidase genes and the induction of three environmental stresses in Neoseiulus barkeri (Acari: Phytoseiidae) (Article). J. Asia-Pac. Entomol. 2017, 20, 261–267. [Google Scholar] [CrossRef]
- Huang, X.S.; Chen, H.P.; Yu, H.H.; Yan, Y.F.; Liao, Z.P.; Huang, Q.R. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for hypoxic preconditioning-mediated delayed cardioprotection. Mol. Cell. Biochem. 2014, 385, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.L.; Bai, F.F.; Zhang, B.X.; Fang, J.G. Synthesis of dithiolethiones and identification of potential neuroprotective agents via activation of Nrf2-driven antioxidant enzymes. J. Agric. Food Chem. 2020, 68, 2214–2231. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.T.; Liang, X.; Wu, C.L.; Chen, Q.; Chen, Q. Function of red spider mite (Tetranychus cinnabarinus) transcription factor TcNrf2 on regulating the transcription of antioxidant enzyme genes. Chin. J. Trop. Crops 2019, 40, 1163–1170. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chang, Y.W.; Bai, J.; Zhang, X.X.; Junaid, I.; Lu, M.X.; Hu, J.; Du, Y.Z. High temperature stress induces expression of CYP450 genes and contributes to insecticide tolerance in Liriomyza trifolii. Pestic. Biochem. Physiol 2021, 174, 104826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Wu, Z.L.; Wang, K.F.; Liu, Q.; Zhuang, H.M.; Wu, G. Trade-off between thermal tolerance and insecticide resistance in Plutella xylostella. Ecol. Evol. 2015, 5, 515–530. [Google Scholar] [CrossRef]
- Yang, B.J.; Liu, M.L.; Zhang, Y.X.; Liu, Z.W. Effects of temperature on fitness costs in chlorpyrifos-resistant brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Sci. 2018, 25, 409–417. [Google Scholar] [CrossRef]
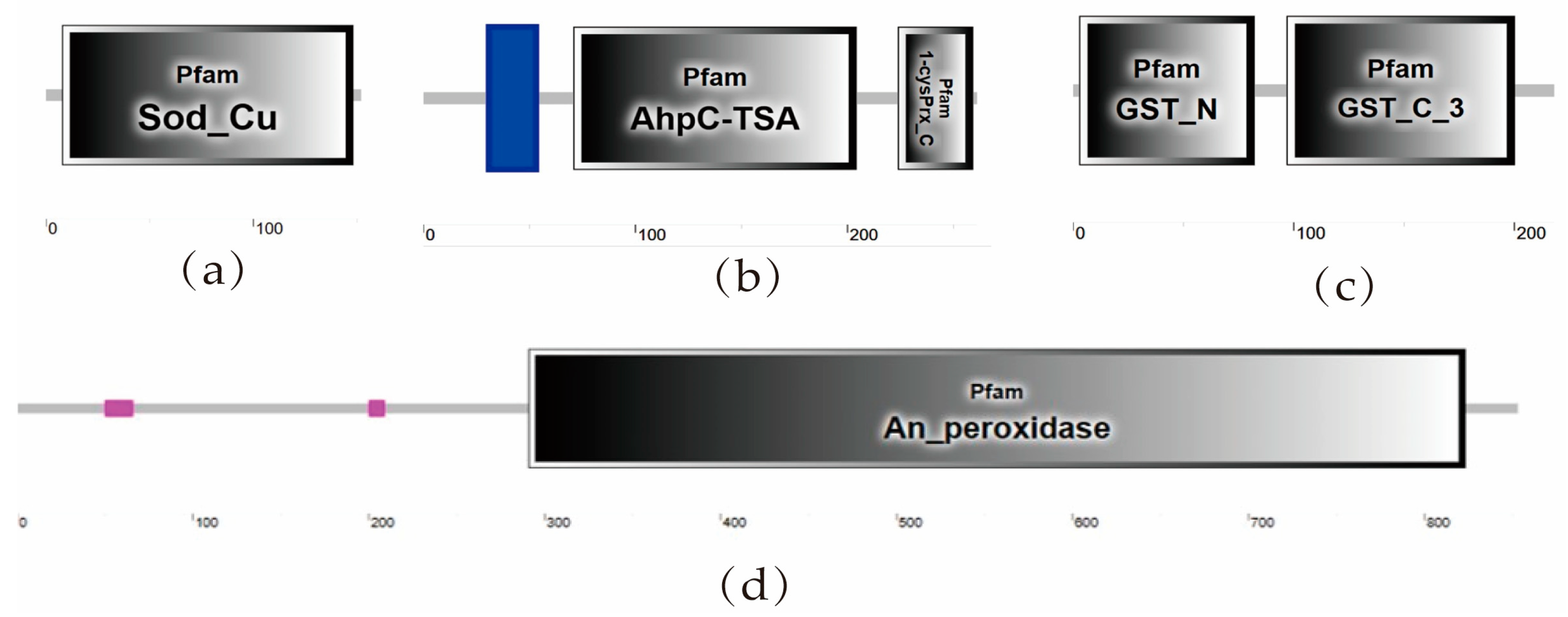
| Genes | Gene ID | Ref Seq mRNA | ORF | aa | Molecular Weight (kDa) | Theoretical pI | Protein Subcellular Localization Prediction |
|---|---|---|---|---|---|---|---|
| TtSOD | Isoform0027403 | XP_015791617.1 | 459 | 152 | 15,559.40 | 6.02 | Nucleus |
| TtPOD3 | Isoform0024848 | AYV89033.1 | 786 | 261 | 29,208.33 | 7.61 | Cytoplasm |
| TtGSTs2 | Isoform0026276 | XP_025016296.1 | 657 | 218 | 24,737.32 | 5.30 | Cytoplasm |
| TtPOD4 | Isoform0009148 | XP_015787917.1 | 2562 | 853 | 96,085.96 | 8.19 | Cytoplasm |
| Gene Names | Species | Accession Number | Similarity (%) | Identity (%) |
|---|---|---|---|---|
| TtSOD | Tetranychus urticae | XP_015791617.1 | 100% | 97.33% |
| Tetranychus evansi | AYV89238.1 | 100% | 96.71% | |
| Panonychus citri | AJD79351.1 | 100% | 91.45% | |
| TtPOD3 | Tetranychus urticae | AYV89033.1 | 100% | 92.72% |
| Tetranychus evansi | AYV89211.1 | 100% | 90.04% | |
| Panonychus citri | XP_053201348.1 | 85% | 86.22% | |
| TtPOD4 | Tetranychus urticae | XP_015787917.1 | 100% | 96.95% |
| Panonychus citri | XP_053201277.1 | 99% | 65.52% | |
| TtGSTs2 | Tetranychus urticae | XP_025016296.1 | 100% | 82.57% |
| Panonychus citri | AMO13198.1 | 100% | 67.43% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Yu, C.; Li, W.; Liu, L.; Sun, Q.; Liu, H.; Wang, S. Differential Antioxidant Enzyme Gene Expression and Functional Analysis of Pyridaben-Susceptible and -Resistant Strains of Tetranychus truncatus (Acari: Tetranychidae) under High Temperature Stress. Insects 2024, 15, 381. https://doi.org/10.3390/insects15060381
Song L, Yu C, Li W, Liu L, Sun Q, Liu H, Wang S. Differential Antioxidant Enzyme Gene Expression and Functional Analysis of Pyridaben-Susceptible and -Resistant Strains of Tetranychus truncatus (Acari: Tetranychidae) under High Temperature Stress. Insects. 2024; 15(6):381. https://doi.org/10.3390/insects15060381
Chicago/Turabian StyleSong, Liwen, Cailan Yu, Wenliang Li, Lei Liu, Qinzhe Sun, Huan Liu, and Senshan Wang. 2024. "Differential Antioxidant Enzyme Gene Expression and Functional Analysis of Pyridaben-Susceptible and -Resistant Strains of Tetranychus truncatus (Acari: Tetranychidae) under High Temperature Stress" Insects 15, no. 6: 381. https://doi.org/10.3390/insects15060381
APA StyleSong, L., Yu, C., Li, W., Liu, L., Sun, Q., Liu, H., & Wang, S. (2024). Differential Antioxidant Enzyme Gene Expression and Functional Analysis of Pyridaben-Susceptible and -Resistant Strains of Tetranychus truncatus (Acari: Tetranychidae) under High Temperature Stress. Insects, 15(6), 381. https://doi.org/10.3390/insects15060381






