Amitraz Resistance in French Varroa Mite Populations—More Complex Than a Single-Nucleotide Polymorphism
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Organization of the Study
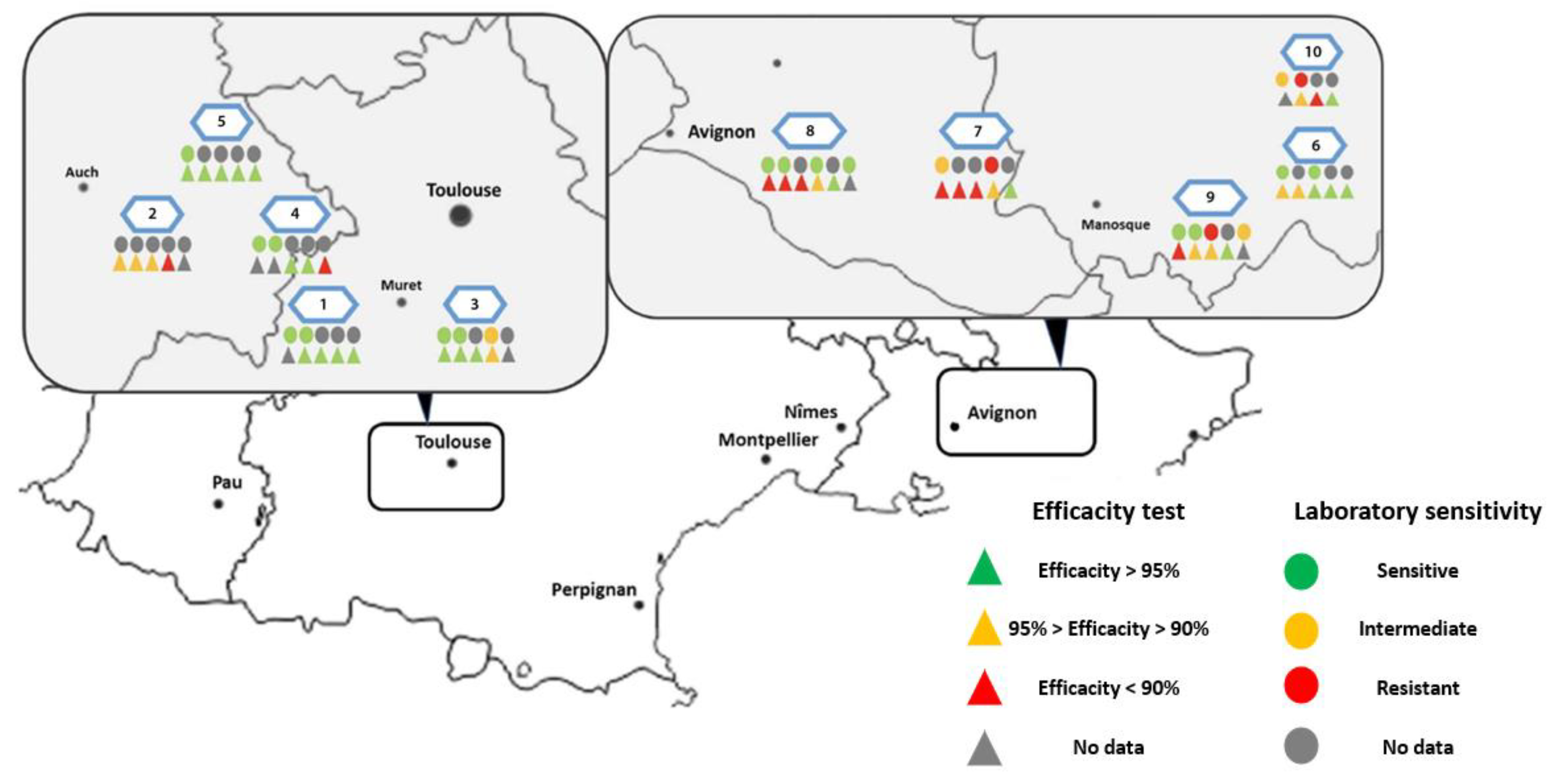
2.2. Apiary Locations and Samples’ Origin
3. Method for Laboratory Susceptibility Test
3.1. Principle of the Method
3.2. Preparation of Paraffin Capsules Coated with Amitraz
3.3. Varroa Sampling and Laboratory Exposure of Varroa Mites to Amitraz
- -
- Mobile mites: mites characterized by spontaneous movement or movement in response to external stimuli, such as brushing.
- -
- Paralyzed mites: mites exhibiting the ability to move one or more appendages but an inability to relocate.
- -
- Dead mites: mites showing no observable response following three consecutive stimuli.
3.4. Determination of Reference LC90 Concentration of Amitraz
- -
- -
- Recent introduction of new queens or merging with colonies exposed to amitraz within the previous 5 years was to be avoided.
- -
- Absence of prevalent diseases such as American or European foulbrood, or nosemosis, was obligatory.
- -
- Satisfactory nutritional provisions and the inclusion of renewed frames were prere-quisites.
- -
- Three replicates consisting of 10 Varroa mites per apiary were subjected to concentrations of 0, 0.5, 1, 2, 3, 5, 7.5, and 10 ppm.
- -
- Two replicates, each comprising 10 Varroa mites per apiary, were exposed to a concentration of 12.5 ppm.
- -
- A single replicate containing 10 Varroa mites per apiary was administered to concentrations of 15, 20, 50, and 100 ppm.
- -
- Three replicates were conducted at the following concentrations: 0, 1, 5, 7.5, 10, 12.5, 15, 20, 25, and 30 ppm.
- -
- A single replicate was administered to concentrations of 40, 50, and 100 ppm.
3.5. Laboratory Sensitivity of Varroa Mites to the LC90
3.6. Data Analysis
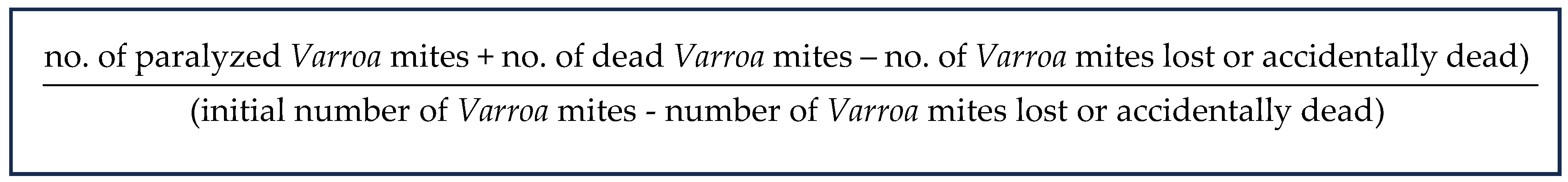
- -
- Sensitive population group: Varroa mites that achieved a percentage of effect at a LC90 between 75 and 100%.
- -
- Intermediate population group: Varroa mites that achieved a percentage of effect at a LC90 between 60 and 74%.
- -
- Resistant population group: Varroa mites that achieved a percentage of effect at a LC90 under 60%.
4. Genotyping of the ORβ-2R-L Gene and Potential Links to Amitraz Resistance
4.1. Genomic DNA Extraction
4.2. Genotyping by Sanger Sequencing
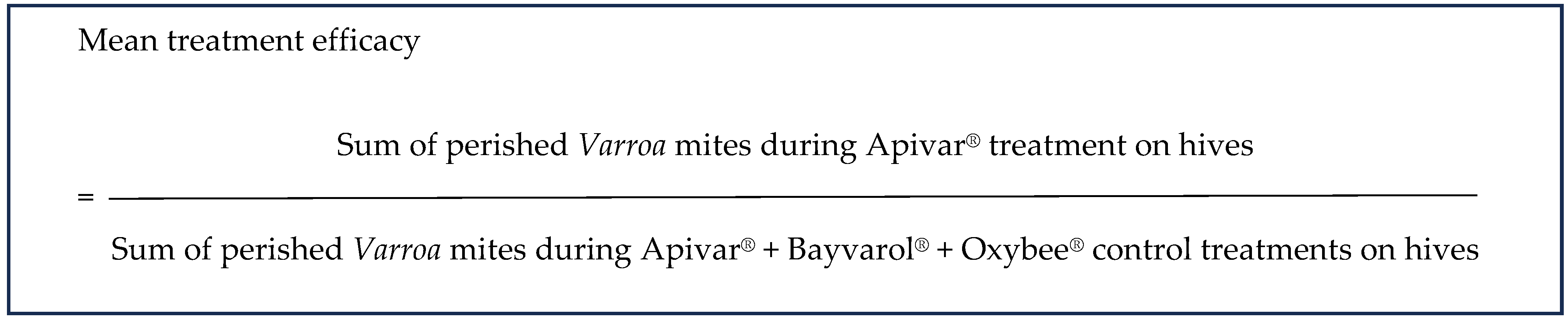
5. Treatment Efficacy of Apivar® in the Field
Treatment Efficacy of Apivar®—Protocol 2021 (PACA and Occitanie Region)
6. Results
6.1. Laboratory Sensitivity of Varroa Mites Exposed to Amitraz LC90 and Genotyping of Varroa Mites in 2020
6.1.1. Laboratory Sensitivity of Varroa Mites to the LC90 of Amitraz (2020)
6.1.2. Genotyping of Varroa Mites Previously Tested in the 2020 Laboratory Assay
6.2. Laboratory Sensitivity of Varroa Mites Exposed to Amitraz LC90, Treatment Efficacy of Apivar® in the Field, and Genotyping of Varroa Mites in 2021
6.2.1. Laboratory Sensitivity of Varroa Mites to the LC90 of Amitraz (2021)
6.2.2. Genotyping of Varroa Mites Previously Tested in the 2021 Laboratory Assay
6.2.3. Treatment Efficacy of Apivar® (2021) in the Occitanie and PACA Regions
7. Discussion
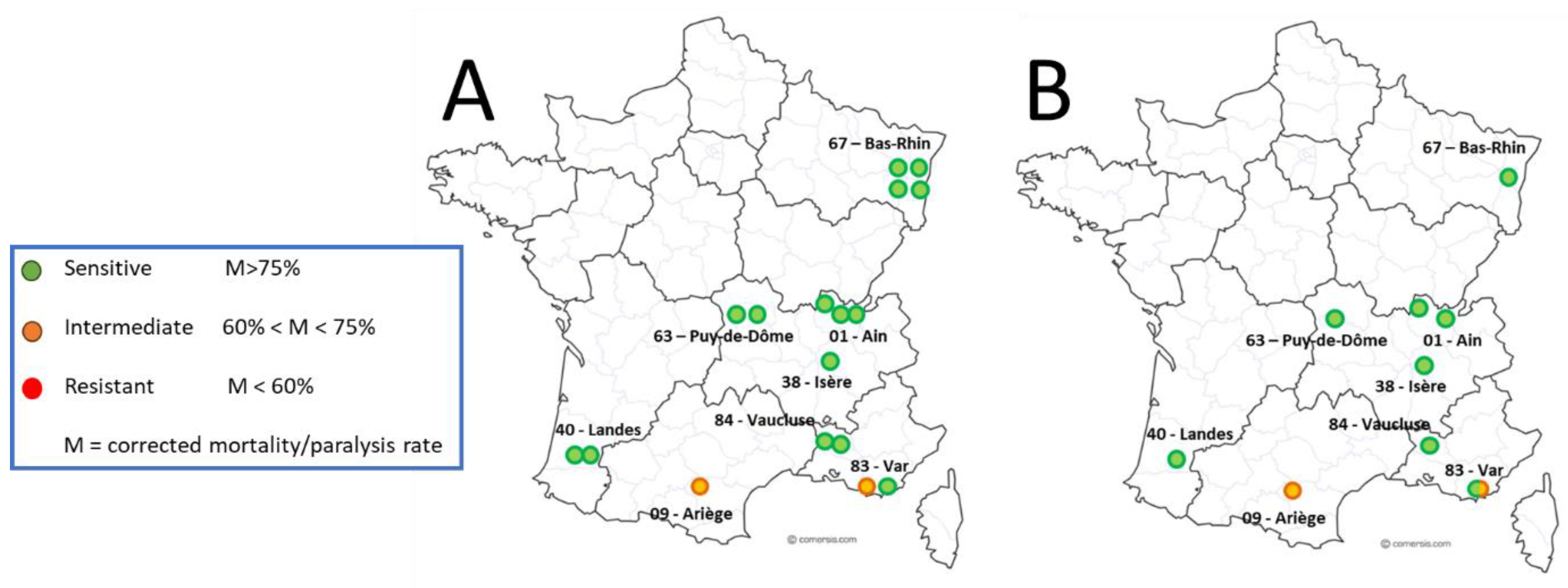
7.1. Geographic Distribution and Spread and Magnitude of Amitraz Resistance Detected in the Field in French Apiaries
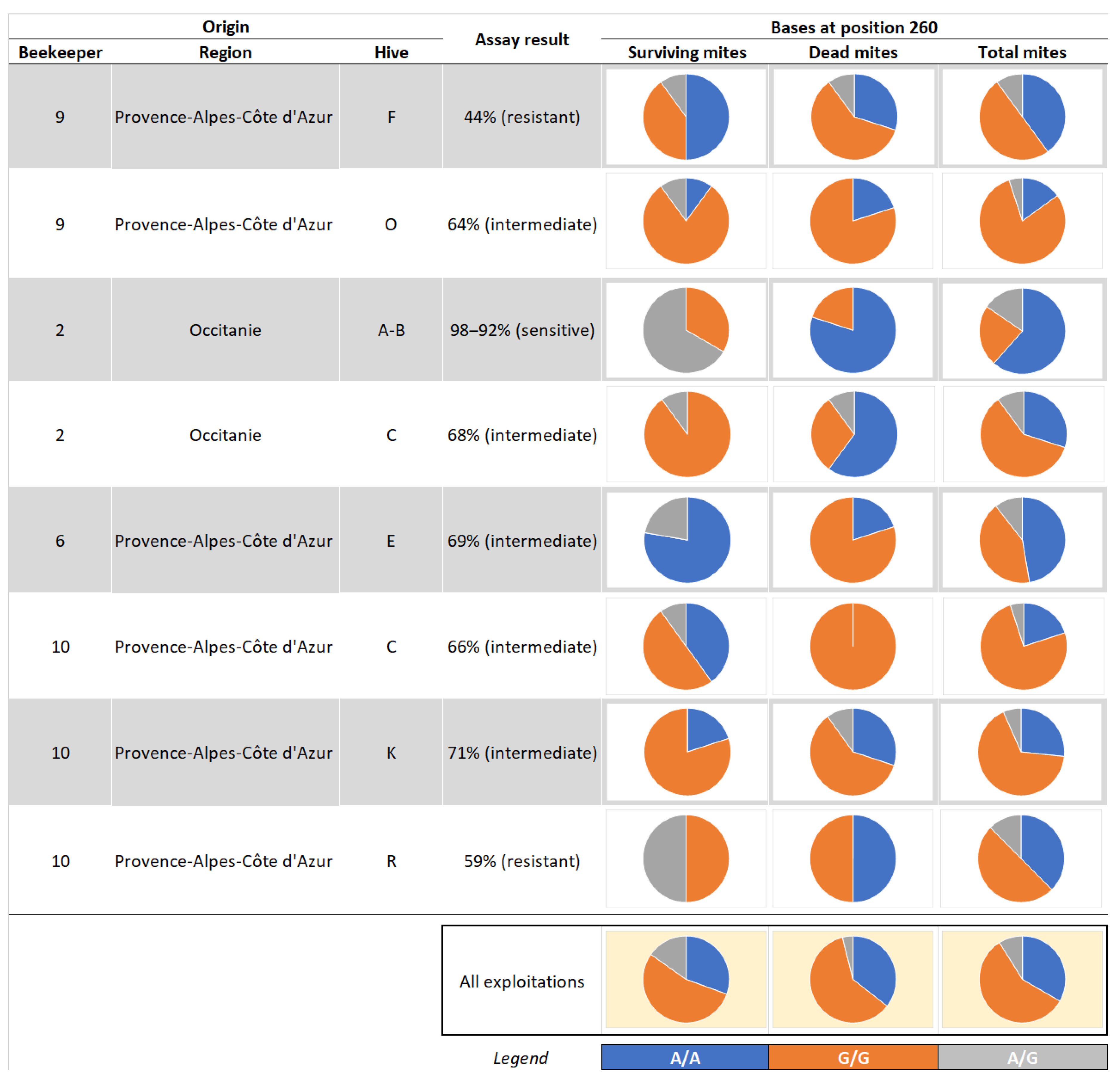
7.2. Genotyping of the ORβ-2R-L Gene and Potential Links to Amitraz Resistance
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Origin | Sensitivity Test | Genotyping Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % of Mites Dead 24 h after Exposure at the LC90 | Population Classification | Number of Surviving Mites Analyzed | Biomnigene Identifiant | Base at Position 260 | |||||
| Apiary | Town | Hive | A/A | G/G | A/G | ||||
| 11 | SAINT-AUBIN | A | 89 | sensitive | 2 | R1 | X | ||
| R2 | X | ||||||||
| 11 | SAINT-AUBIN | B | 78 | sensitive | 6 | R3 | X | ||
| R4 | X | ||||||||
| R5 | X | ||||||||
| R6 | X | ||||||||
| R7 | X | ||||||||
| R8 | X | ||||||||
| 12 | COUSSA | C | 73 | intermediary | 10 | R9 | X | ||
| R10 | X | ||||||||
| R11 | X | ||||||||
| R12 | X | ||||||||
| R13 | X | ||||||||
| R14 | X | ||||||||
| R15 | X | ||||||||
| R16 | X | ||||||||
| R17 | X | ||||||||
| R18 | X | ||||||||
| 13 | ENTRAIGUES | D | 95 | sensitive | 2 | R19 | X | ||
| R20 | X | ||||||||
| 13 | ENTRAIGUES | E | 98 | sensitive | 1 | R21 | X | ||
| 14 | LE-REVEST-LES-EAUX | F | 95 | sensitive | 2 | R22 | X | ||
| R23 | X | ||||||||
| 14 | LE-REVEST-LES-EAUX | G | 74 | intermediary | 1 | R24 | X | ||
| 15 | ST GEORGES D'ESPERANCHE | H | 77 | sensitive | 12 | R25 | X | ||
| R26 | X | ||||||||
| R27 | X | ||||||||
| R28 | X | ||||||||
| R29 | X | ||||||||
| R30 | X | ||||||||
| R31 | X | ||||||||
| R32 | X | ||||||||
| R33 | X | ||||||||
| R34 | X | ||||||||
| R35 | X | ||||||||
| R36 | X | ||||||||
| 16 | JASSERON | L | ND | ND | 4 | R37 | X | ||
| R38 | X | ||||||||
| R39 | X | ||||||||
| R40 | X | ||||||||
| 16 | JASSERON | K | 91 | sensitive | 6 | R41 | X | ||
| R42 | X | ||||||||
| R43 | X | ||||||||
| R44 | X | ||||||||
| R45 | X | ||||||||
| R46 | X | ||||||||
| 17 | VILLEREVERSURE | n° 2 | 78 | sensitive | 2 | R47 | X | ||
| R48 | X | ||||||||
| 17 | VILLEREVERSURE | n° 3 | 94 | sensitive | 2 | R49 | X | ||
| R50 | X | ||||||||
| 18 | ORLEAT | M | 97 | sensitive | 1 | R51 | X | ||
| 18 | ORLEAT | N | 98 | sensitive | 1 | R52 | X | ||
| 19 | MAISONSGOUTTE | Green | 96 | sensitive | 1 | R53 | X | ||
| N | 12 | 28 | 13 | ||||||
| % | 22.6 | 52.8 | 24.5 | ||||||
| Origin | Sensitivity Test | Genotyping Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % of Mites dead 24 h after Exposure at the LC90 | Population Classification | Number of Dead Mites Analyzed | Biomnigene Identifiant | Base at Position 260 | |||||
| Exploitation Name | Town | Hive | A/A | G/G | A/G | ||||
| 11 | SAINT-AUBIN | n° 1 | 89 | sensitive | 4 | S1 | X | ||
| S2 | X | ||||||||
| S3 | X | ||||||||
| S4 | X | ||||||||
| 11 | SAINT-AUBIN | n° 2 | 78 | sensitive | 6 | S5 | X | ||
| S6 | X | ||||||||
| S7 | X | ||||||||
| S8 | X | ||||||||
| S9 | X | ||||||||
| S10 | X | ||||||||
| 12 | COUSSA | n° 72 | 73 | intermediary | 10 | S11 | X | ||
| S12 | X | ||||||||
| S13 | X | ||||||||
| S14 | X | ||||||||
| S15 | X | ||||||||
| S16 | X | ||||||||
| S17 | X | ||||||||
| S18 | X | ||||||||
| S19 | X | ||||||||
| S20 | X | ||||||||
| 15 | ST GEORGES D'ESPERANCHE | n° 3 | 77 | sensitive | 12 | S21 | X | ||
| S22 | X | ||||||||
| S23 | X | ||||||||
| S24 | X | ||||||||
| S25 | X | ||||||||
| S26 | X | ||||||||
| S27 | X | ||||||||
| S28 | X | ||||||||
| S29 | X | ||||||||
| S30 | X | ||||||||
| S31 | X | ||||||||
| S32 | X | ||||||||
| 16 | JASSERON | n° 4 | ND | ND | 2 | S33 | X | ||
| S34 | X | ||||||||
| 16 | JASSERON | n° 6 | 91 | sensitive | 8 | S35 | X | ||
| S36 | X | ||||||||
| S37 | X | ||||||||
| S38 | X | ||||||||
| S39 | X | ||||||||
| S40 | X | ||||||||
| S41 | X | ||||||||
| S42 | X | ||||||||
| 17 | VILLEREVERSURE | n° 2 | 78 | sensitive | 4 | S43 | X | ||
| S44 | X | ||||||||
| S45 | X | ||||||||
| S46 | X | ||||||||
| 17 | VILLEREVERSURE | n° 3 | 94 | sensitive | 6 | S47 | X | ||
| S48 | X | ||||||||
| S49 | X | ||||||||
| S50 | X | ||||||||
| S51 | X | ||||||||
| S52 | X | ||||||||
| N | 13 | 37 | 2 | ||||||
| % | 25.0 | 71.2 | 3.8 | ||||||
| Origin | Assay Results | Number of Mites Analyzed | Genotyping Result | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Surviving Mites | Dead Mites | Identifiant | Bases at Position 260 | ||||||
| Beekeeper | Town | Hive | A/A | G/G | A/G | ||||
| 9 | MONTAGNAC-MONTPEZAT | F | 44% (resistant) | 10 | V3.1 | X | |||
| V3.2 | X | ||||||||
| V3.3 | X | ||||||||
| V3.4 | X | ||||||||
| V3.5 | X | ||||||||
| V3.6 | X | ||||||||
| V3.7 | X | ||||||||
| V3.8 | X | ||||||||
| V3.9 | X | ||||||||
| V3.10 | X | ||||||||
| 9 | MONTAGNAC-MONTPEZAT | F | 44% (resistant) | 10 | V3.11 | X | |||
| V3.12 | X | ||||||||
| V3.13 | X | ||||||||
| V3.14 | X | ||||||||
| V3.15 | X | ||||||||
| V3.16 | X | ||||||||
| V3.17 | X | ||||||||
| V3.18 | X | ||||||||
| V3.19 | X | ||||||||
| V3.20 | X | ||||||||
| 9 | MONTAGNAC-MONTPEZAT | O | 64% (intermediate) | 10 | V3.21 | X | |||
| V3.22 | X | ||||||||
| V3.23 | X | ||||||||
| V3.24 | X | ||||||||
| V3.25 | X | ||||||||
| V3.26 | X | ||||||||
| V3.27 | X | ||||||||
| V3.28 | X | ||||||||
| V3.29 | X | ||||||||
| V3.30 | X | ||||||||
| 9 | MONTAGNAC-MONTPEZAT | O | 64% (intermediate) | 10 | V3.31 | X | |||
| V3.32 | X | ||||||||
| V3.33 | X | ||||||||
| V3.34 | X | ||||||||
| V3.35 | X | ||||||||
| V3.36 | X | ||||||||
| V3.37 | X | ||||||||
| V3.38 | X | ||||||||
| V3.39 | X | ||||||||
| V3.40 | X | ||||||||
| 2 | CLERMONT-LE-FORT | A | 98-92% (sensitive) | 3 | V3.41 | X | |||
| V3.42 | X | ||||||||
| V3.43 | X | ||||||||
| 2 | CLERMONT-LE-FORT | A | 98-92% (sensitive) | 10 | V3.44 | X | |||
| V3.45 | X | ||||||||
| V3.46 | X | ||||||||
| V3.47 | X | ||||||||
| V3.48 | X | ||||||||
| V3.49 | X | ||||||||
| V3.50 | X | ||||||||
| V3.51 | X | ||||||||
| V3.52 | X | ||||||||
| V3.53 | X | ||||||||
| 2 | CLERMONT-LE-FORT | B | 68% (intermediate) | 10 | V3.54 | X | |||
| V3.55 | X | ||||||||
| V3.56 | X | ||||||||
| V3.57 | X | ||||||||
| V3.58 | X | ||||||||
| V3.59 | X | ||||||||
| V3.60 | X | ||||||||
| V3.61 | X | ||||||||
| V3.62 | X | ||||||||
| V3.63 | X | ||||||||
| 2 | CLERMONT-LE-FORT | B | 68% (intermediate) | 10 | V3.64 | X | |||
| V3.65 | X | ||||||||
| V3.66 | X | ||||||||
| V3.67 | X | ||||||||
| V3.68 | X | ||||||||
| V3.69 | X | ||||||||
| V3.70 | X | ||||||||
| V3.71 | X | ||||||||
| V3.72 | X | ||||||||
| V3.73 | X | ||||||||
| 6 | ENTRESSEN | E | 69% (intermediate) | 9 | V3.74 | X | |||
| V3.75 | X | ||||||||
| V3.76 | X | ||||||||
| V3.77 | X | ||||||||
| V3.78 | X | ||||||||
| V3.79 | X | ||||||||
| V3.80 | X | ||||||||
| V3.81 | X | ||||||||
| V3.82 | X | ||||||||
| 6 | ENTRESSEN | E | 69% (intermediate) | 10 | V3.83 | X | |||
| V3.84 | X | ||||||||
| V3.85 | X | ||||||||
| V3.86 | X | ||||||||
| V3.87 | X | ||||||||
| V3.88 | X | ||||||||
| V3.89 | X | ||||||||
| V3.90 | X | ||||||||
| V3.91 | X | ||||||||
| V3.92 | X | ||||||||
| 10 | VACHERE | C | 66% (intermediate) | 10 | V3.93 | X | |||
| V3.94 | X | ||||||||
| V3.95 | X | ||||||||
| V3.96 | X | ||||||||
| V3.97 | X | ||||||||
| V3.98 | X | ||||||||
| V3.99 | X | ||||||||
| V3.100 | X | ||||||||
| V3.101 | X | ||||||||
| V3.102 | X | ||||||||
| 10 | VACHERE | C | 66% (intermediate) | 10 | V3.103 | X | |||
| V3.104 | X | ||||||||
| V3.105 | X | ||||||||
| V3.106 | X | ||||||||
| V3.107 | X | ||||||||
| V3.108 | X | ||||||||
| V3.109 | X | ||||||||
| V3.110 | X | ||||||||
| V3.111 | X | ||||||||
| V3.112 | X | ||||||||
| 10 | VACHERE | K | 71% (intermediate) | 5 | V3.113 | X | |||
| V3.114 | X | ||||||||
| V3.115 | X | ||||||||
| V3.116 | X | ||||||||
| V3.117 | X | ||||||||
| 10 | VACHERE | K | 71% (intermediate) | 10 | V3.118 | X | |||
| V3.119 | X | ||||||||
| V3.120 | X | ||||||||
| V3.121 | X | ||||||||
| V3.122 | X | ||||||||
| V3.123 | X | ||||||||
| V3.124 | X | ||||||||
| V3.125 | X | ||||||||
| V3.126 | X | ||||||||
| V3.127 | X | ||||||||
| 10 | VACHERE | R | 59% (resistant) | 2 | V3.128 | X | |||
| V3.129 | X | ||||||||
| 10 | VACHERE | R | 59% (resistant) | 6 | V3.130 | X | |||
| V3.131 | X | ||||||||
| V3.132 | X | ||||||||
| V3.133 | X | ||||||||
| V3.134 | X | ||||||||
| V3.135 | X | ||||||||
| Survivors | 18 | 32 | 9 | ||||||
| Dead mites | 27 | 46 | 3 | ||||||
| Total mites | 45 | 78 | 12 | ||||||
| Origin | Assay Results | Number of Mites Analyzed | Genotyping Results | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surviving Mites | Dead Mites | Identifiant | Bases of Interest at 260 | Other Mutations Found (Mutated base(s)^wt base(s) (position)) | ||||||||||||||
| Beekeeper | Town | Hive | A/A | G/G | A/G | W^T (146) | R^G (235) | T^C (248) | R^G (262) | R^A (320) | AT^CA (344/345) | MW^CA (344-345) | Y^C (366) | Y^T (375) | ||||
| 9 | MONTAGNAC-MONTPEZAT | F | 44% (resistant) | 10 | V3.1 | X | X | |||||||||||
| V3.2 | X | |||||||||||||||||
| V3.3 | X | X | ||||||||||||||||
| V3.4 | X | X | ||||||||||||||||
| V3.5 | X | X | ||||||||||||||||
| V3.6 | X | |||||||||||||||||
| V3.7 | X | X | ||||||||||||||||
| V3.8 | X | X | X | |||||||||||||||
| V3.9 | X | |||||||||||||||||
| V3.10 | X | |||||||||||||||||
| 9 | MONTAGNAC-MONTPEZAT | F | 44% (resistant) | 10 | V3.11 | X | ||||||||||||
| V3.12 | X | |||||||||||||||||
| V3.13 | X | |||||||||||||||||
| V3.14 | X | |||||||||||||||||
| V3.15 | X | |||||||||||||||||
| V3.16 | X | |||||||||||||||||
| V3.17 | X | |||||||||||||||||
| V3.18 | X | |||||||||||||||||
| V3.19 | X | X | ||||||||||||||||
| V3.20 | X | |||||||||||||||||
| 9 | MONTAGNAC-MONTPEZAT | O | 64% (intermediate) | 10 | V3.21 | X | ||||||||||||
| V3.22 | X | |||||||||||||||||
| V3.23 | X | |||||||||||||||||
| V3.24 | X | |||||||||||||||||
| V3.25 | X | |||||||||||||||||
| V3.26 | X | |||||||||||||||||
| V3.27 | X | X | X | |||||||||||||||
| V3.28 | X | |||||||||||||||||
| V3.29 | X | |||||||||||||||||
| V3.30 | X | |||||||||||||||||
| 9 | MONTAGNAC-MONTPEZAT | O | 64% (intermediate) | 10 | V3.31 | X | ||||||||||||
| V3.32 | X | |||||||||||||||||
| V3.33 | X | |||||||||||||||||
| V3.34 | X | |||||||||||||||||
| V3.35 | X | |||||||||||||||||
| V3.36 | X | |||||||||||||||||
| V3.37 | X | |||||||||||||||||
| V3.38 | X | |||||||||||||||||
| V3.39 | X | |||||||||||||||||
| V3.40 | X | |||||||||||||||||
| Genotyping Results | ||||||||||||||||||
| Identifiant | Bases of interest at 260 | Other mutations found (mutated base(s)^wt base(s) (position)) | ||||||||||||||||
| A/A | G/G | A/G | W^T (146) | R^G (235) | T^C (248) | R^G (262) | R^A (320) | AT^CA (344/345) | MW^CA (344-345) | Y^C (366) | Y^T (375) | |||||||
| 2 | CLERMONT-LE-FORT | A | 98-92% (sensitive) | 3 | V3.41 | X | ||||||||||||
| V3.42 | X | X | X | |||||||||||||||
| V3.43 | X | |||||||||||||||||
| 2 | CLERMONT-LE-FORT | A | 98-92% (sensitive) | 10 | V3.44 | X | ||||||||||||
| V3.45 | X | |||||||||||||||||
| V3.46 | X | |||||||||||||||||
| V3.47 | X | |||||||||||||||||
| V3.48 | X | |||||||||||||||||
| V3.49 | X | |||||||||||||||||
| V3.50 | X | |||||||||||||||||
| V3.51 | X | |||||||||||||||||
| V3.52 | X | |||||||||||||||||
| V3.53 | X | |||||||||||||||||
| 2 | CLERMONT-LE-FORT | B | 68% (intermediate) | 10 | V3.54 | X | ||||||||||||
| V3.55 | X | |||||||||||||||||
| V3.56 | X | X | X | |||||||||||||||
| V3.57 | X | |||||||||||||||||
| V3.58 | X | |||||||||||||||||
| V3.59 | X | |||||||||||||||||
| V3.60 | X | |||||||||||||||||
| V3.61 | X | |||||||||||||||||
| V3.62 | X | |||||||||||||||||
| V3.63 | X | |||||||||||||||||
| 2 | CLERMONT-LE-FORT | B | 68% (intermediate) | 10 | V3.64 | X | ||||||||||||
| V3.65 | X | |||||||||||||||||
| V3.66 | X | |||||||||||||||||
| V3.67 | X | |||||||||||||||||
| V3.68 | X | |||||||||||||||||
| V3.69 | X | |||||||||||||||||
| V3.70 | X | |||||||||||||||||
| V3.71 | X | |||||||||||||||||
| V3.72 | X | |||||||||||||||||
| V3.73 | X | |||||||||||||||||
| 6 | ENTRESSEN | E | 69% (intermediate) | 9 | V3.74 | X | X | |||||||||||
| V3.75 | X | X | ||||||||||||||||
| V3.76 | X | X | ||||||||||||||||
| V3.77 | X | X | ||||||||||||||||
| V3.78 | X | X | ||||||||||||||||
| V3.79 | X | X | ||||||||||||||||
| V3.80 | X | X | ||||||||||||||||
| V3.81 | X | X | ||||||||||||||||
| V3.82 | X | X | ||||||||||||||||
| 6 | ENTRESSEN | E | 69% (intermediate) | 10 | V3.83 | X | ||||||||||||
| V3.84 | X | |||||||||||||||||
| V3.85 | X | |||||||||||||||||
| V3.86 | X | X | ||||||||||||||||
| V3.87 | X | |||||||||||||||||
| V3.88 | X | X | ||||||||||||||||
| V3.89 | X | |||||||||||||||||
| V3.90 | X | |||||||||||||||||
| V3.91 | X | |||||||||||||||||
| V3.92 | X | |||||||||||||||||
| Genotyping results | ||||||||||||||||||
| Identifiant | Bases of interest at 260 | Other mutations found (mutated base(s)^wt base(s) (position)) | ||||||||||||||||
| A/A | G/G | A/G | W^T (146) | R^G (235) | T^C (248) | R^G (262) | R^A (320) | AT^CA (344/345) | MW^CA (344-345) | Y^C (366) | Y^T (375) | |||||||
| 10 | VACHERE | C | 66% (intermediate) | 10 | V3.93 | X | X | |||||||||||
| V3.94 | X | |||||||||||||||||
| V3.95 | X | X | ||||||||||||||||
| V3.96 | X | |||||||||||||||||
| V3.97 | X | |||||||||||||||||
| V3.98 | X | X | ||||||||||||||||
| V3.99 | X | |||||||||||||||||
| V3.100 | X | |||||||||||||||||
| V3.101 | X | |||||||||||||||||
| V3.102 | X | |||||||||||||||||
| 10 | VACHERE | C | 66% (intermediate) | 10 | V3.103 | X | ||||||||||||
| V3.104 | X | |||||||||||||||||
| V3.105 | X | |||||||||||||||||
| V3.106 | X | |||||||||||||||||
| V3.107 | X | |||||||||||||||||
| V3.108 | X | |||||||||||||||||
| V3.109 | X | |||||||||||||||||
| V3.110 | X | |||||||||||||||||
| V3.111 | X | |||||||||||||||||
| V3.112 | X | |||||||||||||||||
| 10 | VACHERE | K | 71% (intermediate) | 5 | V3.113 | X | ||||||||||||
| V3.114 | X | |||||||||||||||||
| V3.115 | X | |||||||||||||||||
| V3.116 | X | |||||||||||||||||
| V3.117 | X | X | ||||||||||||||||
| 10 | VACHERE | K | 71% (intermediate) | 10 | V3.118 | X | ||||||||||||
| V3.119 | X | |||||||||||||||||
| V3.120 | X | |||||||||||||||||
| V3.121 | X | |||||||||||||||||
| V3.122 | X | |||||||||||||||||
| V3.123 | X | |||||||||||||||||
| V3.124 | X | |||||||||||||||||
| V3.125 | X | |||||||||||||||||
| V3.126 | X | |||||||||||||||||
| V3.127 | X | |||||||||||||||||
| 10 | VACHERE | R | 59% (resistant) | 2 | V3.128 | X | ||||||||||||
| V3.129 | X | X | ||||||||||||||||
| 10 | VACHERE | R | 59% (resistant) | 6 | V3.130 | X | ||||||||||||
| V3.131 | X | |||||||||||||||||
| V3.132 | X | |||||||||||||||||
| V3.133 | X | |||||||||||||||||
| V3.134 | X | |||||||||||||||||
| V3.135 | X | |||||||||||||||||
References
- Mondet, F.; Parejo, M.; Meixner, M.D.; Costa, C.; Kryger, P.; Andonov, S.; Servin, B.; Basso, B.; Bieńkowska, M.; Bigio, G.; et al. Evaluation of suppressed mite reproduction (SMR) reveals potential for Varroa resistance in European honey bees (Apis mellifera L.). Insects 2020, 11, 595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruckner, S.; Wilson, M.; Aurell, D.; Rennich, K.; Vanengelsdorp, D.; Steinhauer, N.; Williams, G.R. A national survey of managed honey bee colony losses in the USA: Results from the Bee Informed Partnership for 2017–2018, 2018–2019, and 2019–2020. J. Apic. Res. 2023, 62, 429–443. [Google Scholar] [CrossRef]
- Stahlmann-Brown, P.; Hall, R.J.; Pragert, H.; Robertson, T. Varroa appears to drive persistent increases in New Zealand colony losses. Insects 2022, 13, 589. [Google Scholar] [CrossRef]
- Mutinelli, F.; Pinto, A.; Barzon, L.; Toson, M. Some considerations about winter colony losses in Italy according to the Coloss questionnaire. Insects 2022, 13, 1059. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodney, T.R.; Ida, C.; Renata, S.L.; Shelley, H.; Rob, C.; Pierre, G.; Marta, G.M.; Stephen, P.; Leonard, F.; Amro, Z. Land use changes associated with declining honey bee health across temperate North America. Environ. Res. Lett. 2023, 18, 064042. [Google Scholar] [CrossRef]
- Smoliński, S.; Langowska, A.; Glazaczow, A. Raised seasonal temperatures reinforce autumn Varroa destructor infestation in honey bee colonies. Sci. Rep. 2021, 11, 22256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jack, C.J.; de Bem Oliveira, I.; Kimmel, C.B.; Ellis, J.D. Seasonal differences in Varroa destructor population growth in western honey bee (Apis mellifera) colonies. Front. Ecol. Evol. 2023, 11, 1102457. [Google Scholar] [CrossRef]
- Stahlmann-Brown, P.; Robertson, T. Report on the 2023 New Zealand Colony Loss Survey; MPI Technical Paper No: 2024/01; Ministry for Primary Industries: Wellington, New Zealand, March 2021; ISBN 978-1-991087-53-9. [Google Scholar]
- Posada-Florez, F.; Lamas, Z.S.; Hawthorne, D.J.; Ryabov, E.V. Pupal cannibalism by worker honey bees contributes to the spread of deformed wing virus. Sci. Rep. 2021, 11, 8989. [Google Scholar] [CrossRef]
- Mitton, G.A.; Meroi Arcerito, F.; Cooley, H.; Fernández de Landa, G.; Eguaras, M.J.; Ruffinengo, S.R.; Maggi, M.D. More than sixty years living with Varroa destructor: A review of acaricide resistance. Int. J. Pest Manag. 2022, 1–18. [Google Scholar] [CrossRef]
- Marco, L.; Colombo, M.; Spreafico, M. Ineffectiveness of Apistan® treatment against the mite Varroa jacobsoni Oud in several districts of Lombardy (Italy). Apidologie 1995, 26, 67–72. [Google Scholar] [CrossRef]
- Johnson, R.; Henry, P.; May, R.B. Synergistic interactions between in-hive miticides in Apis mellifera. J. Econ. Entomol. 2009, 102, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Patti, E.; Frank, E.; James, B.; Gary, E.; William, W. Detection of resistance in US Varroa jacobsoni Oud. (Mesostigmata: Varroidae) to the acaricide fluvalinate. Apidologie 1999, 30, 13–17. [Google Scholar] [CrossRef]
- Jérôme, T. Monitoring Varroa jacobsoni resistance to pyrethroids in western Europe. Apidologie 1998, 29, 537–546. [Google Scholar]
- Thompson, H.M.; Brown, M.A.; Ball, R.F.; Bew, M.H. First report of Varroa destructor resistance to pyrethroids in the UK. Apidologie 2002, 33, 357–366. [Google Scholar] [CrossRef]
- Mitton, G.A.; Quintana, S.; Giménez Martínez, P.; Mendoza, Y.; Ramallo, G.; Brasesco, C.; Villalba, A.; Eguaras, M.J.; Maggi, M.D.; Ruffinengo, S.R. First record of resistance to flumethrin in a Varroa population from Uruguay. J. Apic. Res. 2016, 55, 422–427. [Google Scholar] [CrossRef]
- González-Cabrera, J.; Davies, T.G.; Field, L.M.; Kennedy, P.J.; Williamson, M.S. An amino acid substitution (L925V) associated with resistance to pyrethroids in Varroa destructor. PLoS ONE 2013, 8, e82941. [Google Scholar] [CrossRef] [PubMed]
- González-Cabrera, J.; Rodríguez-Vargas, S.; Davies, T.G.; Field, L.M.; Schmehl, D.; Ellis, J.D.; Krieger, K.; Williamson, M.S. Novel mutations in the voltage-gated sodium channel of pyrethroid-resistant Varroa destructor populations from the southeastern USA. PLoS ONE 2016, 11, e0155332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Millán-Leiva, A.; Marín, Ó.; Christmon, K.; vanEngelsdorp, D.; González-Cabrera, J. Mutations associated with pyrethroid resistance in Varroa mite, a parasite of honey bees, are widespread across the United States. Pest. Manag. Sci. 2021, 77, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Alissandrakis, E.; Ilias, A.; Tsagkarakou, A. Pyrethroid target site resistance in Greek populations of the honey bee parasite Varroa destructor (Acari: Varroidae). J. Apic. Res. 2017, 56, 625–630. [Google Scholar] [CrossRef]
- Esengül, E.; Nafiye, K.; Mustafa, R.; Emre, I. Geographical distribution of pyrethroid resistance mutations in Varroa destructor across Türkiye and a European overview. Exp. Appl. Acarol. 2024, 92, 1–13. [Google Scholar] [CrossRef]
- Yarsan, E.; Yilmaz, F.; Sevin, S.; Akdeniz, G.; Celebi, B.; Hasan Ozturk, S.; Nurhan Ayikol, S.; Karatas, U.; Hasan, E.; Fidan, N.; et al. Investigation of resistance against to flumethrin using against Varroa destructor in Türkiye. Vet. Res. Commun. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Brodschneider, R.; Schlagbauer, J.; Arakelyan, I.; Ballis, A.; Brus, J.; Brusbardis, V.; Cadahía, L.; Charrière, J.D.; Chlebo, R.; Coffey, M.F.; et al. Spatial clusters of Varroa destructor control strategies in Europe. J. Pest. Sci. 2023, 96, 759–783. [Google Scholar] [CrossRef]
- Piotr, S.; Piotr, S.; Krystyna, P. The amitraz strips efficacy in control of Varroa destructor after many years application of amitraz in apiaries. J. Apic. Sci. 2013, 57, 107–121. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Hernández-Rodríguez, C.S.; González-Cabrera, J. Assessing the resistance to acaricides in Varroa destructor from several Spanish locations. Parasitol Res. 2020, 119, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Gabrielle, A.; Benjamin, P.; Précillia, C.; Christelle, S. Inventory of Varroa destructor susceptibility to amitraz and tau-fluvalinate in France. Exp. Appl. Acarol. 2020, 82, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, F. Detection of amitraz resistance and reduced treatment efficacy in the Varroa Mite, Varroa destructor, within commercial beekeeping operations. PLoS ONE 2020, 15, e0227264. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, C.S.; Moreno-Martí, S.; Almecija, G.; Christmon, K.; Johnson, J.D.; Ventelon, M.; vanEngelsdorp, D.; Cook, S.C.; González-Cabrera, J. Resistance to amitraz in the parasitic honey bee mite Varroa destructor is associated with mutations in the β-adrenergic-like octopamine receptor. J. Pest. Sci. 2022, 95, 1179–1195. [Google Scholar] [CrossRef]
- Pettis, J.S.; Shimanuki, H.; Feldlaufer, M.F. Detection of Varroa resistant to fluvalinate. Am. Bee J. 1998, 138, 538–541. [Google Scholar]
- Almecija, G.; Poirot, B. Résistances de Varroa destructor aux Acaricides: Conséquences sur L’efficacité des Traitements. Application au Tau-Fluvalinate et à L’amitraze. Doctoral Thesis, University of Tours, Tours, France, 12 October 2021. [Google Scholar]
- Morfin, N.; Rawn, D.; Petukhova, T.; Kozak, P.; Eccles, L.; Chaput, J.; Pasma, T.; Guzman-Novoa, E. Surveillance of synthetic acaricide efficacy against Varroa destructor in Ontario, Canada. Can. Entomol. 2022, 154, 1. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D. Standard methods for Varroa research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Milani, N. The resistance of Varroa jacobsoni Oud. To pyrethroids—A laboratory assay. Apidologie 1995, 26, 415–429. [Google Scholar] [CrossRef]
- Sandon, Y.; Viry, A. Lutte contre le Varroa—Etude de sensibilité/résistance à l’amitraze chez Varroa destructor. La Santé de l’Abeille 2017, 277, 47–56. [Google Scholar]
- Giles, D.P.; Rothwell, D.N. The sub-lethal activity of amidines on insects and acarids. Pestic. Sci. 1983, 14, 303–312. [Google Scholar] [CrossRef]
- Evans, P.D.; Gee, J.D. Action of formamidine pesticides on octopamine receptors. Nature 1980, 287, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Milani, N.; Vedova, G. Decline in the proportion of mites resistant to fluvalinate in a population of Varroa destructor not treated with pyrethroids. Apidologie 2002, 33, 417–422. [Google Scholar] [CrossRef]
- Elzen, P.; Westervelt, D. A scientific note on reversion of fluvalinate resistance to a degree of susceptibility in Varroa destructor. Apidologie 2004, 35, 519–520. [Google Scholar] [CrossRef]
- Maisonnasse, A.; Hernandez, J.; Le Quintec, C.; Cousin, M.; Beri, C.; Kretzschamat, A. Evaluation de la structure des colonies d’abeilles, création et utilisation de la méthode ColEval (Colony Evaluation). Innov. Agron. 2016, 53, 27–37. [Google Scholar] [CrossRef]
- Avelin, C. Observatoire de la production de miel et de gelée royale. Programme Apicole Européen. FranceAgriMer. Edition Juillet. 2022. Available online: https://www.apipro-ffap.fr/Observatoire-de-la-production-de-miel-et-gelee-royale-2022 (accessed on 20 May 2024).
- Guideline on Veterinary Medicinal Products Controlling Varroa Destructor Parasitosis in Bees EMA/CVMP/EWP/459883/2008-Rev.1. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-veterinary-medicinal-products-controlling-varroa-destructor-parasitosis-bees-revision-1_en.pdf (accessed on 20 May 2024).
- Rapport D’étude Evaluation de L’efficacité du Traitement Apivar® en Lien Avec la Sensibilité des Populations de Varroas à L’amitraze. Microsoft Word—Rapport_EfficacitéApivar2021_v04082022 2021. Available online: https://www.adaoccitanie.org (accessed on 20 May 2024).
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, 96–119. [Google Scholar] [CrossRef] [PubMed]
- Nurit, E.; Alexander, M. Varroa mite evolution: A neglected aspect of worldwide bee collapses? (2020). Curr. Opin. Insect Sci. 2020, 39, 21–26. [Google Scholar]
- Oldroyd, B.P. Coevolution while you wait: Varroa jacobsoni, a new parasite of western honeybees. Trends Ecol. Evol. 1999, 14, 312–315. [Google Scholar] [CrossRef]
- Ayan, A.; Aldemır, O.S.; Selamoglu, Z. Analysis of COI gene region of Varroa destructor in honey bees (Apis mellifera) in province of Siirt. Turkish J. Vet. Res. 2017, 1, 20–23. [Google Scholar]
- Hajializadeh, Z.; Asadi, M.; Kavousi, H. First report of Varroa genotype in western Asia based on genotype identification of Iranian Varroa destructor populations (Mesostigmata: Varroidae) using RAPD marker. Syst. Appl. Acarol. 2018, 23, 199–205. [Google Scholar] [CrossRef]
- Ogihara, M.H.; Yoshiyama, M.; Morimoto, N.; Kimura, K. Dominant honeybee colony infestation by Varroa destructor (Acari: Varroidae) K haplotype in Japan. Appl. Entomol. Zool. 2020, 55, 189–197. [Google Scholar] [CrossRef]
- Navajas, M.; Anderson, D.L.; De Guzman, L.I.; Huang, Z.Y.; Clement, J.; Zhou, T.; Le Conte, Y. New Asian types of Varroa destructor: A potential new threat for world apiculture. Apidologie 2010, 41, 181–193. [Google Scholar] [CrossRef]
- Techer, M.A.; Roberts, J.M.K.; Cartwright, R.A.; Mikheyev, A.S. The first steps toward a global pandemic: Reconstructing the demographic history of parasite host switches in its native range. Mol. Ecol. 2021, 31, 1358–1374. [Google Scholar] [CrossRef]
- Gajic, B.; Radulovic, Z.; Stevanovic, J.; Kulisic, Z.; Vucicevic, M.; Simeunovic, P.; Stanimirovic, Z. Variability of the honey bee mite Varroa destructor in Serbia, based on mtDNA analysis. Exp. Appl. Acarol. 2013, 61, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Gajić, B.; Stevanović, J.; Radulović, Ž.; Kulišić, Z.; Vejnović, B.; Glavinić, U.; Stanimirović, Z. Haplotype identification and detection of mitochondrial DNA heteroplasmy in Varroa destructor mites using ARMS and PCR-RFLP methods. Exp. Appl. Acarol. 2016, 70, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Gajić, B.; Muñoz, I.; De la Rúa, P.; Stevanović, J.; Lakić, N.; Kulišić, Z.; Stanimirović, Z. Coexistence of genetically different Varroa destructor in Apis mellifera colonies. Exp. Appl. Acarol. 2019, 78, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Dynes, T.L.; De Roode, J.C.; Lyons, J.I.; Berry, J.A.; Delaplane, K.S.; Brosi, B.J. Fine scale population genetic structure of Varroa destructor, an ectoparasitic mite of the honey bee (Apis mellifera). Apidologie 2017, 48, 93–101. [Google Scholar] [CrossRef]
- Beaurepaire, A.L.; Moro, A.; Mondet, F.; Le Conte, Y.; Neumann, P.; Locke, B. Population genetics of ectoparasitic mites suggest arms race with honeybee hosts. Sci. Rep. 2019, 9, 11355. [Google Scholar] [CrossRef]
- Ascunce, M.S.; Toups, M.A.; Kassu, G.; Fane, J.; Scholl, K.; Reed, D.L. Nuclear genetic diversity in human lice (Pediculus humanus) reveals continental differences and high inbreeding among worldwide populations. PLoS ONE 2013, 8, e57619. [Google Scholar] [CrossRef] [PubMed]
- Nozais, J.P. The origin and dispersion of human parasitic diseases in the old world (Africa, Europe and Madagascar). Mem. Inst. Oswaldo Cruz. 2003, 98 (Suppl. I), 13–19. [Google Scholar] [CrossRef] [PubMed]
- Dietemann, V.; Beaurepaire, A.; Page, P.; Yañez, O.; Buawangpong, N.; Chantawannakul, P.; Neumann, P. Population genetics of ectoparasitic mites Varroa spp. in Eastern and Western honey bees. Parasitology 2019, 146, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Techer, M.A.; Rane, R.V.; Grau, M.L.; Roberts, J.M.K.; Sullivan, S.T.; Liachko, I.; Childers, A.K.; Evans, J.D.; Mikheyev, A.S. Divergent evolutionary trajectories following speciation in two ectoparasitic honey bee mites. Commun. Biol. 2019, 2, 357. [Google Scholar] [CrossRef]


| Origin | Dead or Paralyzed Mites Rate in Percentage (%) at Day 0 + 24 h | Classification | |||
|---|---|---|---|---|---|
| Apiary | Hive | LC90 (n) | Negative Control (n) | Corrected LC90 (n) | Varroa population |
| 1 | A | 90% (60) | 13% (31) | 0.89 | sensitive |
| 1 | B | 78% (60) | 3% (30) | 0.78 | sensitive |
| 3 | C | 74% (50) | 3% (30) | 0.73 | intermediate |
| 4 | D | 95% (60) | 2% (43) | 0.95 | sensitive |
| 4 | E | 98% (60) | 3% (40) | 0.98 | sensitive |
| 5 | F | 95% (59) | 3% (38) | 0.95 | sensitive |
| 5 | G | 79% (14) | 17% (6) | 0.74 | intermediate |
| 6 | H | 77% (56) | 0% (30) | 0.77 | sensitive |
| 7 | I | 100% (13) | 0% (10) | 1 | sensitive |
| 7 | J | 94% (32) | 0% (10) | 0.94 | sensitive |
| 8 | K | 91% (90) | 0% (38) | 0.91 | sensitive |
| 9 | L | 100% (20) | 0% (10) | 1 | sensitive |
| 9 | M | 96% (25) | 0% (10) | 0.96 | sensitive |
| 9 | N | 100% (15) | 0% (7) | 1 | sensitive |
| 9 | O | 96% (46) | 0% (20) | 0.96 | |
| 10 | P | 100% (21) | 38% (8) | non valid (Centre-Val de Loire región) | |
| 11 | Q | 98% (42) | 7% (15) | 0.97 | sensitive |
| 11 | R | 98% (49) | 0% (20) | 0.98 | sensitive |
| Mites Sampled after LC-90 Exposure Towards Amitraz | N (%) | ||
|---|---|---|---|
| Base at Position 260 in the ORβ-2R-L | |||
| A/A | G/G | A/G | |
| Perished mites | 13 (25%) | 37 (71.2%) | 2 (3.8%) |
| Surviving mites | 12 (22.6%) | 28 (52.8%) | 13 (24.5%) |
| Total mites | 25 (23.8%) | 65 (61.9%) | 15 (14.3%) |
| Origin | Dead or Paralyzed Mites Rate in percentage (%) at Day 0 + 24h | Classification | ||||
|---|---|---|---|---|---|---|
| Region | Beekeeper | Colony | LC90 (n) | Negative Control (n) | Corrected LC90 (n) | Varroa population |
| Occitanie | 1 | 1.1 | 93% (42) | 0% (10) | 0.93 | sensitive |
| 1.5 | 87% (46) | 0% (10) | 0.87 | sensitive | ||
| 2 | 2.3 | 92% (60) | 40% (40) | non-valid | ||
| 2.4 | 93% (56) | 40% (30) | non-valid | |||
| 3 | 3.1 | 73% (70) | 15% (20) | 0.68 | intermediate | |
| 3.2 | 98% (59) | 0% (30) | 0.98 | sensitive | ||
| 3.3 | 94% (49) | 23% (22) | 0.92 | sensitive | ||
| 4 | 4.5 | 81% (16) | 0% (6) | 0.81 | sensitive | |
| 4.6 | 85% (176) | 0% (60) | 0.85 | sensitive | ||
| 5 | 5.1 | 96% (28) | 25% (8) | 0.95 | sensitive | |
| Total (Occitanie) | 87% (± 10) | |||||
| PACA | 6 | 6.3 | 91% (22) | 0% (8) | 0.91 | sensitive |
| 6.5 | 100% (20) | 10% (10) | 1 | sensitive | ||
| 7 | 7.1.3 | 66% (70) | 0% (24) | 0.66 | intermediate | |
| 7.1.11 | 71% (35) | 0% (10) | 0.71 | intermediate | ||
| 7.1.18 | 60% (49) | 0% (19) | 0.60 | intermediate | ||
| 7.2.3 | 64% (14) | 13% (8) | 0.59 | resistant | ||
| 7.2.5 | 40% (77) | 6% (50) | 0.36 | resistant | ||
| 8 | 8.2 | 75% (20) | 0% (3) | 0.75 | sensitive | |
| 8.3 | 83% (81) | 0% (49) | 0.83 | sensitive | ||
| 8.6 | 89% (80) | 3% (39) | 0.88 | sensitive | ||
| 8.8 | 86% (80) | 2% (50) | 0.86 | sensitive | ||
| 9 | 9.15 | 64% (80) | 0% (50) | 0.64 | intermediate | |
| 9.6 | 44% (80) | 0% (50) | 0.44 | resistant | ||
| 9.1 | 79% (14) | 0% (4) | 0.79 | sensitive | ||
| 9.3 | 65% (16) | 0% (7) | 0.75 | sensitive | ||
| 10 | 10.5 | 69% (29) | 0% (8) | 0.69 | intermediate | |
| 10.7 | 48% (59) | 0% (40) | 0.48 | resistant | ||
| Total (PACA) | 70% (± 17) | |||||
| Total (Global) | 76% (± 17) | |||||
| Origin | Assay Result | Survivors Mites Available | Dead Mites Available | Proposed Genotyping | Total No of Genotyped Mites | ||
|---|---|---|---|---|---|---|---|
| Beekeeper | Region | Hive | |||||
| 9 | Provence-Alpes-Côte d'Azur | F | 44% (resistant) | 39 | 40 | 10 vs. 10 | 20 |
| 9 | Provence-Alpes-Côte d'Azur | O | 64% (intermediate) | 24 | 45 | 10 vs.10 | 20 |
| 2 | Occitanie | A | 98% (sensitive) | 1 | 9 | 3 vs. 10 | 13 |
| 2 | Occitanie | B | 92% (sensitive) | 2 | 17 | ||
| 2 | Occitanie | C | 68% (intermediate) | 11 | 39 | 10 vs. 10 | 20 |
| 6 | Provence-Alpes-Côte d'Azur | E | 69% (intermediate) | 9 | 20 | 9 vs. 10 | 19 |
| 10 | Provence-Alpes-Côte d'Azur | C | 66% (intermediate) | 22 | 38 | 10 vs. 10 | 20 |
| 10 | Provence-Alpes-Côte d'Azur | K | 71% (intermediate) | 5 | 15 | 5 vs. 10 | 15 |
| 10 | Provence-Alpes-Côte d'Azur | R | 59% (resistant) | 2 | 6 | 2 vs. 6 | 8 |
| Mites Sampled after LC-90 Exposure towards Amitraz | N (%) | ||
|---|---|---|---|
| Bases at Position 260 in the ORβ-2R-L | |||
| A/A | G/G | A/G | |
| Surviving mites | 18 (30.5%) | 32 (54.2%) | 9 (15.3%) |
| Dead mites | 27 (35.5%) | 46 (60.5%) | 3 (3.9%) |
| Total mites | 45 (33.3%) | 78 (57.8%) | 12 (8.9%) |
| Occitanie (n = 20) | PACA (n = 21) | Global | |
|---|---|---|---|
| Efficacy ± SD (%) | 95.5% ± 2.7 | 90.5% ± 7.8 | 93% ± 6.4 |
| Colonies >95% efficacy (n) | 14 | 7 | 21 |
| Colonies [90–95%] efficacy (n) | 4 | 7 | 11 |
| Colonies <90% efficacy (n) | 2 | 7 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsky, U.; Rognon, B.; Douablin, A.; Viry, A.; Rodríguez Ramos, M.A.; Hammaidi, A. Amitraz Resistance in French Varroa Mite Populations—More Complex Than a Single-Nucleotide Polymorphism. Insects 2024, 15, 390. https://doi.org/10.3390/insects15060390
Marsky U, Rognon B, Douablin A, Viry A, Rodríguez Ramos MA, Hammaidi A. Amitraz Resistance in French Varroa Mite Populations—More Complex Than a Single-Nucleotide Polymorphism. Insects. 2024; 15(6):390. https://doi.org/10.3390/insects15060390
Chicago/Turabian StyleMarsky, Ulrike, Bénédicte Rognon, Alexandre Douablin, Alain Viry, Miguel Angel Rodríguez Ramos, and Abderrahim Hammaidi. 2024. "Amitraz Resistance in French Varroa Mite Populations—More Complex Than a Single-Nucleotide Polymorphism" Insects 15, no. 6: 390. https://doi.org/10.3390/insects15060390
APA StyleMarsky, U., Rognon, B., Douablin, A., Viry, A., Rodríguez Ramos, M. A., & Hammaidi, A. (2024). Amitraz Resistance in French Varroa Mite Populations—More Complex Than a Single-Nucleotide Polymorphism. Insects, 15(6), 390. https://doi.org/10.3390/insects15060390




