Functional Morphology of Leg Mechanosensory Organs in Early Postembryonic Development in the Stick Insect (Sipyloidea chlorotica)
Abstract
Simple Summary
Abstract
1. Introduction

2. Materials and Methods
2.1. Insects
2.2. Neuroanatomical Staining by Axonal Tracing
2.3. Microscopy
2.4. Terminology of Leg Axis
2.5. Statistical Analysis
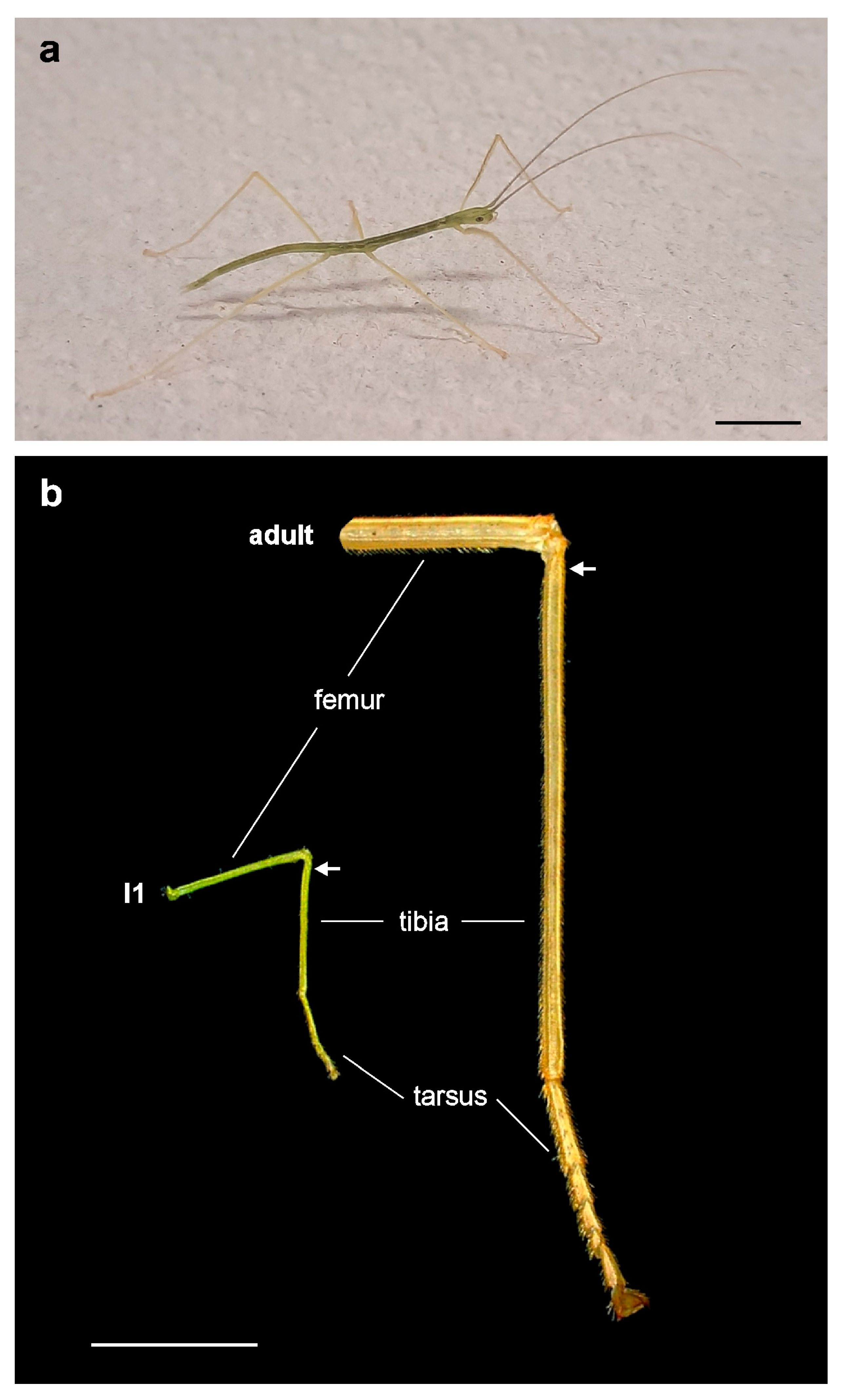
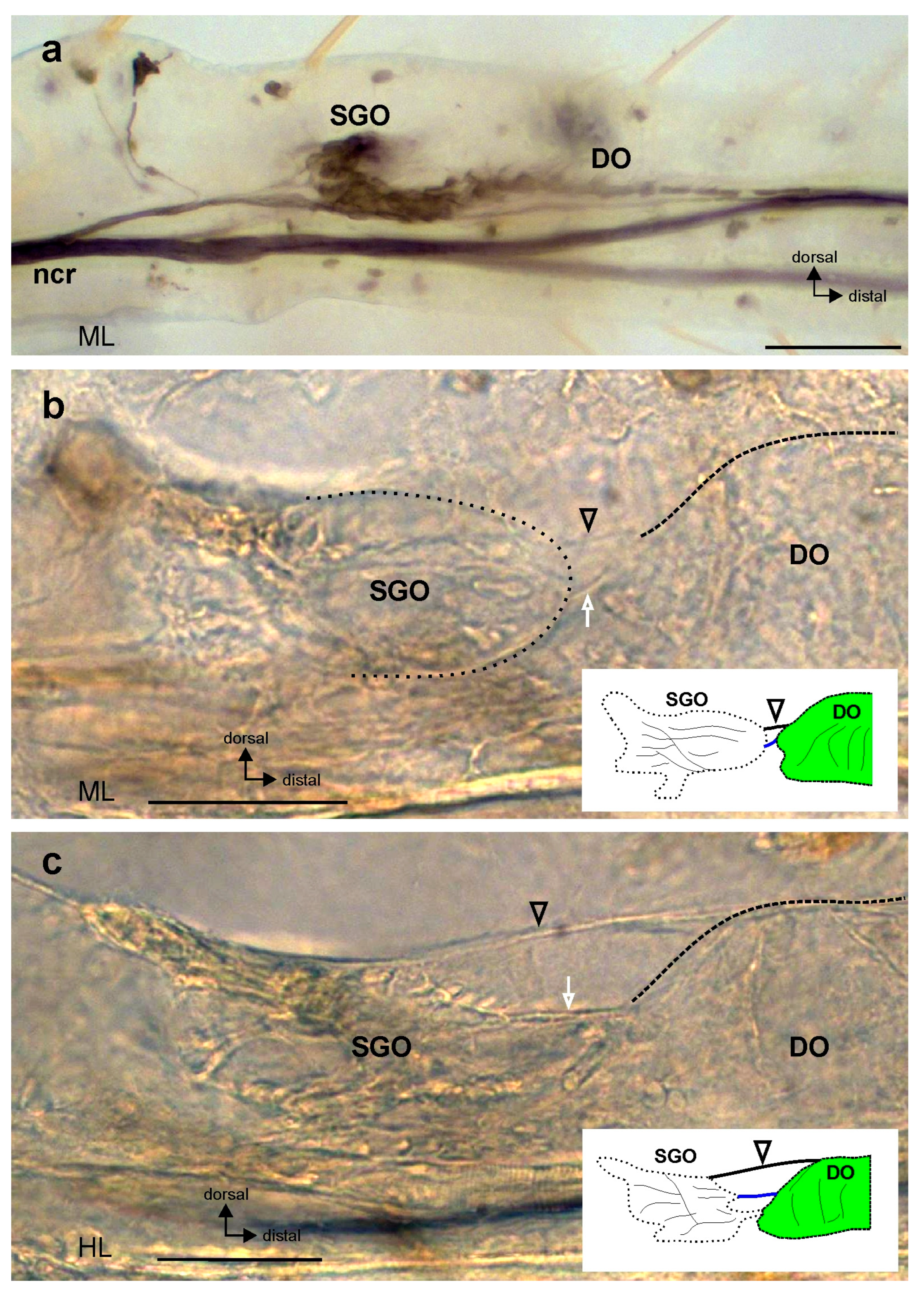
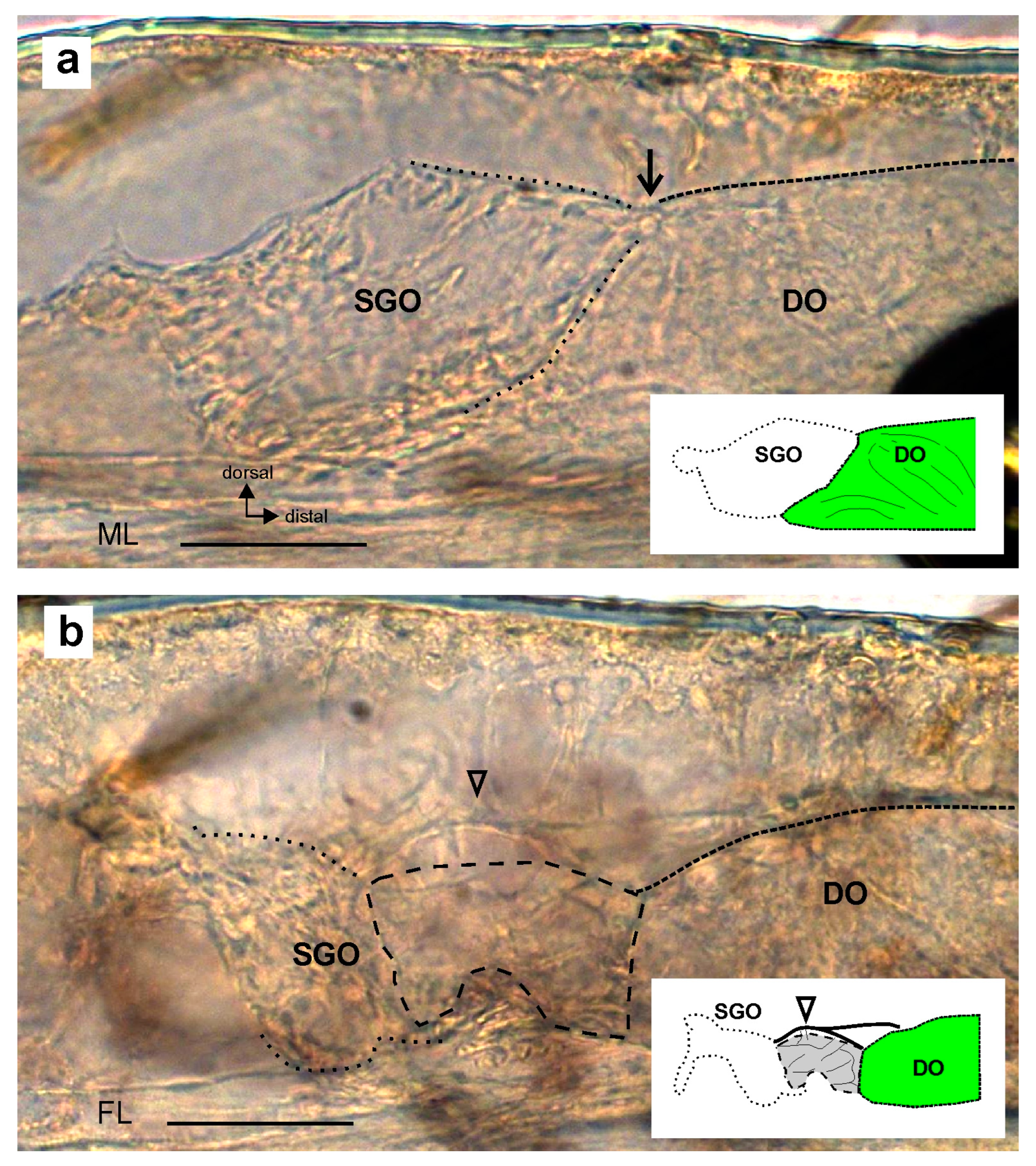
3. Results
4. Discussion
4.1. The Morphological Connection between Sensory Organs and the Postembryonic Development of the Subgenual Organ Complex in Stick Insects
4.2. Functional Implications of a Connection between Chordotonal Organs in Juvenile Instars
4.3. Absence of the Membrane between Sensory Organs
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, L.H.; Matheson, T. Chordotonal organs of insects. Adv. Insect Physiol. 1998, 27, 1–228. [Google Scholar] [CrossRef]
- Moulins, M. Ultrastructure of chordotonal organs. In Structure and Function of Proprioceptors in the Invertebrates; Mill, P.J., Ed.; Chapman and Hall: London, UK, 1976; pp. 387–426. [Google Scholar]
- Keil, T. Sensory cilia in arthropods. Arthropod Struct. Dev. 2012, 41, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Sane, S.P. Antennal mechanosensors and their evolutionary antecedents. Adv. Insect Physiol. 2015, 49, 59–99. [Google Scholar] [CrossRef]
- Füller, H.; Ernst, A. Die Ultrastruktur der femoralen Chordotonalorgane von Carausius morosus Br. Zool. Jahrb. Anat. 1973, 91, 574–601. [Google Scholar]
- Bräunig, P.; Hustert, R.; Pflüger, H.-J. Distribution and specific central projections of mechanoreceptors in the thorax and proximal leg joints of locusts. I. Morphology, location and innervation of internal proprioceptors of pro- and metathorax and their central projections. Cell Tissue Res. 1981, 216, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Doolan, J.M.; Young, D. The organization of the auditory organ of the bladder cicada, Cystosoma saundersii. Philos. Trans. R. Soc. Lond. B 1981, 291, 525–540. [Google Scholar] [CrossRef]
- Ghysen, A.; Dambly-Chaudière, C.; Aceves, E.; Jan, L.Y.; Jan, Y.N. Sensory neurons and peripheral pathways in Drosophila embryos. Roux Arch. Dev. Biol. 1986, 195, 281–289. [Google Scholar] [CrossRef]
- Yack, J.E.; Scudder, G.G.E.; Fullard, J.H. Evolution of the metathoracic tympanal ear and its mesothoracic homologue in the Macrolepidoptera (Insecta). Zoomorphology 1999, 119, 93–103. [Google Scholar] [CrossRef]
- van Staaden, M.J.; Rieser, M.; Orr, S.R.; Pabst, M.A.; Römer, H. Serial hearing organs in the atympanate grasshopper Bullacris membracioides (Orthoptera, Pneumoridae). J. Comp. Neurol. 2003, 465, 579–592. [Google Scholar] [CrossRef]
- Rossi Stacconi, M.V.; Romani, R. The Johnston’s organ of three homopteran species: A comparative ultrastructural study. Arthropod Struct. Dev. 2013, 42, 219–228. [Google Scholar] [CrossRef]
- Nishino, H.; Mukai, H.; Takanashi, T. Chordotonal organs in hemipteran insects: Unique peripheral structures but conserved central organization revealed by comparative neuroanatomy. Cell Tissue Res. 2016, 366, 549–572. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, T.; Uechi, N.; Tatsuta, H. Vibrations in hemipteran and coleopteran insects: Behaviors and application in pest management. Appl. Entomol. Zool. 2019, 54, 21–29. [Google Scholar] [CrossRef]
- Hummel, J.; Schöneich, S.; Kössl, M.; Scherberich, J.; Hedwig, B.; Prinz, S.; Nowotny, M. Gating of acoustic transducer channels is shaped by biomechanical filter processes. J. Neurosci. 2016, 36, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Prahlad, A.; Spalthoff, C.; Kong, D.; Großhans, J.; Göpfert, M.C.; Schmidt, C.F. Mechanical properties of a Drosophila larval chordotonal organ. Biophys. J. 2017, 113, 2796–2804. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, U. Actin filaments: The main components of the scolopale in insect sensilla. Cell Tissue Res. 1990, 261, 85–96. [Google Scholar] [CrossRef]
- Yack, J.E. The structure and function of auditory chordotonal organs in insects. Microsc. Res. Techniq. 2004, 63, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Strauß, J.; Stritih-Peljhan, N. Vibration detection in arthropods: Signal transfer, biomechanics and sensory adaptations. Arthropod Struct. Dev. 2022, 68, 101167. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.T.; Göpfert, M.C. Hearing in Drosophila. Curr. Opin. Neurobiol. 2015, 34, 79–85. [Google Scholar] [CrossRef]
- Dürr, V.; Berendes, V.; Strube-Bloss, M. Sensorimotor ecology of the insect antenna: Active sampling by a multimodal sensory organ. Adv. Insect Physiol. 2022, 63, 1–105. [Google Scholar] [CrossRef]
- Strauß, J.; Moritz, L.; Rühr, P.T. The subgenual organ complex in stick insects: Functional morphology and mechanical coupling in a complex mechanosensory organ. Front. Ecol. Evol. 2021, 9, 632493. [Google Scholar] [CrossRef]
- Schnorbus, H. Die subgenualen Sinnesorgane von Periplaneta americana: Histologie und Vibrationsschwellen. Z. Vergl. Physiol. 1971, 71, 14–48. [Google Scholar] [CrossRef]
- Field, L.H.; Pflüger, H.-J. The femoral chordotonal organ: A bifunctional orthopteran (Locusta migratoria) sense organ? Comp. Biochem. Physiol. A 1989, 93, 729–743. [Google Scholar] [CrossRef]
- Lin, Y.; Rössler, W.; Kalmring, K. Complex tibial organs in fore-, mid-, and hindlegs of the bushcricket Gampsocleis gratiosa (Tettigoniidae): Comparison of morphology of the organs. J. Morphol. 1994, 221, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Strauß, J.; Lomas, K.; Field, L.H. The complex tibial organ of the New Zealand ground weta: Sensory adaptations for vibrational signal detection. Sci. Rep. 2017, 7, 2031. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.R. Re-evaluation of the absolute threshold and response mode of the most sensitive known “vibration” detector, the cockroach’s subgenual organ: A cochlea-like displacement threshold and a direct response to sound. J. Neurobiol. 1994, 25, 1167–1185. [Google Scholar] [CrossRef] [PubMed]
- Strauß, J.; Stritih-Peljhan, N.; Nishino, H. Vibration receptor organs in the insect leg: Neuroanatomical diversity and functional principles. Curr. Opin. Insect Sci. 2024, 61, 101153. [Google Scholar] [CrossRef] [PubMed]
- Strauß, J.; Lakes-Harlan, R. Sensory neuroanatomy of stick insects highlights the evolutionary diversity of the orthopteroid subgenual organ complex. J. Comp. Neurol. 2013, 521, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Strauß, J. Morphological coupling of the distal organ in the Peruvian walking stick (Oreophoetes peruana): Structural and functional aspects. Invertebr. Biol. 2022, 141, e12387. [Google Scholar] [CrossRef]
- Strauß, J. A membrane between chordotonal organs in the subgenual organ complex of the stick insect Peruphasma schultei. Bull. Insectol. 2024, 77, 113–118. [Google Scholar]
- Sehnal, F. Growth and life cycle. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Postembryonic Development; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 2, pp. 1–86. [Google Scholar]
- Carlberg, U. Phasmida: A biological review. Zool. Anz. 1986, 216, 1–18. [Google Scholar]
- Cocroft, R.B.; Gogala, M.; Hill, P.S.M.; Wessel, A. (Eds.) Studying Vibrational Communication; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Virant-Doberlet, M.; Stritih-Peljhan, N.; Žunič-Kosi, A.; Polajnar, J. Functional diversity of vibrational signaling systems in insects. Annu. Rev. Entomol. 2023, 68, 191–210. [Google Scholar] [CrossRef]
- Devetak, D.; Pabst, M.A. Structure of the subgenual organ in the green lacewing, Chrysoperla carnea. Tissue Cell 1994, 26, 249–257. [Google Scholar] [CrossRef]
- Jägers-Röhr, E. Untersuchungen zur Morphologie und Entwicklung der Scolopidialorgane bei der Stabschrecke Carausius morosus Br. Biol. Zentralbl. 1968, 87, 393–409. [Google Scholar]
- Kalmring, K.; Sickmann, T.; Jatho, M.; Rössler, W.; Hoffmann, E.; Unrast, C.; Bangert, M.; Nebeling, B. The auditory-vibratory sensory system in buscrickets (Tettigoniidae, Ensifera, Orthoptera) I Comparison of morphology, development and physiology. In Environmental Signal Processing and Adaptation; Heldmaier, G., Werner, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 167–207. [Google Scholar]
- Strauß, J.; Moritz, L.; Rühr, P.T. Supplementary data for The subgenual organ complex in stick insects: Functional morphology and mechanical coupling in a complex mechanosensory organ. 2021. Available online: https://zenodo.org/records/3856676 (accessed on 1 February 2024).
- Brock, P.D.; Büscher, T.H.; Baker, E. Sipyloidea chlorotica (Audinet-Serville, 1838). Phasmida Species File Online. 2024. Available online: https://phasmida.speciesfile.org/otus/855973/overview (accessed on 5 May 2024).
- Strauß, J. Early postembryogenic development of the subgenual organ complex in the stick insect Sipyloidea sipylus. Arthropod Struct. Dev. 2020, 56, 100933. [Google Scholar] [CrossRef]
- Strauß, J. Neuronal innervation of the subgenual organ complex and tibial campaniform sensilla in the stick insect midleg. Insects 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Strauß, J.; Lakes-Harlan, R. Vibrational sensitivity of the subgenual organ complex in female Sipyloidea sipylus stick insects in different experimental paradigms of stimulus direction, leg attachment, and ablation of a connective tibial sense organ. Comp. Biochem. Physiol. A 2017, 203, 100–108. [Google Scholar] [CrossRef]
- Carlberg, U. Postembryonic ontogeny in Sipyloidea sipylus (Westwood) (Insecta: Phasmidae). Zool. Jahrbücher Anat. 1987, 115, 273–279. [Google Scholar]
- Pitman, R.M.; Tweedle, C.D.; Cohen, M.J. Branching of central neurons: Intracellular cobalt injection for light and electron microscopy. Science 1972, 176, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, F. Beiträge zur Anatomie der Muskulatur und der peripheren Nerven von Carausius (Dixippus) morosus. Zool. Jahrb. Anat. Ontog. Tiere 1940, 66, 63–128. [Google Scholar]
- Godden, D.H. The motor innervation of the leg musculature and motor output during thanatosis in the stick insect, Carausius morosus Br. J. Comp. Physiol. 1972, 80, 201–225. [Google Scholar] [CrossRef]
- Bässler, U. Neural Basis of Elementary Behavior in Stick Insects; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Weidler, D.J.; Diecke, F.P.J. The role of cations in conduction in the central nervous system of the herbivorous insect Carausius morosus. Z. Vergl. Physiol. 1969, 64, 372–399. [Google Scholar] [CrossRef]
- Bässler, U. Sense organs in the femur of the stick insect and their relevance to the control of position of the femur-tibia-joint. J. Comp. Physiol. 1977, 121, 99–113. [Google Scholar] [CrossRef]
- Ball, E.E.; Field, L.H. Structure of the auditory system of the weta Hemideina crassidens (Blanchard, 1851) (Orthoptera, Ensifera, Gryllacridoidea, Stenopelmatidae). 1. Morphology and histology. Cell Tissue Res. 1981, 217, 321–344. [Google Scholar] [CrossRef] [PubMed]
- French, A.S. Transduction mechanisms of mechanosensilla. Annu. Rev. Entomol. 1988, 33, 39–58. [Google Scholar] [CrossRef]
- Kilpinen, O.; Storm, J. Biophysics of the subgenual organ of the honeybee, Apis mellifera. J. Comp. Physiol. A 1997, 181, 309–318. [Google Scholar] [CrossRef]
- Storm, J.; Kilpinen, O. Modelling the subgenual organ of the honeybee, Apis mellifera. Biol. Cybern. 1998, 78, 175–182. [Google Scholar] [CrossRef]
- Field, L.H. Sensory physiology. In The Biology of Wetas, King Crickets and Their Allies; Field, L.H., Ed.; CABI Publishing: Wallingford, UK, 2001; pp. 429–458. [Google Scholar]
- Sansom, T.M.; Oberst, S.; Richter, A.; Lai, J.C.S.; Saadatfar, M.; Nowotny, M.; Evans, T.A. Low radiodensity µCT scans to reveal detailed morphology of the termite leg and its subgenual organ. Arthropod Struct. Dev. 2022, 70, 101191. [Google Scholar] [CrossRef]
- Jarman, A.P. Development of the auditory organ (Johnston’s organ) in Drosophila. In Development of Auditory and Vestibular Systems, 4th ed.; Romand, R., Varela-Nieto, I., Eds.; Academic Press: Oxford, UK, 2014; pp. 31–61. [Google Scholar]
- Dambach, M. Vibrational responses. In Cricket Behaviour and Neurobiology; Huber, F., Moore, T.E., Loher, W., Eds.; Cornell University Press: Ithaca, NY, USA, 1989; pp. 179–197. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strauß, J. Functional Morphology of Leg Mechanosensory Organs in Early Postembryonic Development in the Stick Insect (Sipyloidea chlorotica). Insects 2024, 15, 392. https://doi.org/10.3390/insects15060392
Strauß J. Functional Morphology of Leg Mechanosensory Organs in Early Postembryonic Development in the Stick Insect (Sipyloidea chlorotica). Insects. 2024; 15(6):392. https://doi.org/10.3390/insects15060392
Chicago/Turabian StyleStrauß, Johannes. 2024. "Functional Morphology of Leg Mechanosensory Organs in Early Postembryonic Development in the Stick Insect (Sipyloidea chlorotica)" Insects 15, no. 6: 392. https://doi.org/10.3390/insects15060392
APA StyleStrauß, J. (2024). Functional Morphology of Leg Mechanosensory Organs in Early Postembryonic Development in the Stick Insect (Sipyloidea chlorotica). Insects, 15(6), 392. https://doi.org/10.3390/insects15060392







