Dengue Virus Serotype 1 Effects on Mosquito Survival Differ among Geographically Distinct Aedes aegypti Populations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.2. Virus Strain
2.3. DENV-1 Challenging
2.4. Infection Rate Assay
2.5. Survival and Fecundity Assessments and Wing Length
2.6. Statistical Analysis
3. Results
3.1. Infection Rate Assay
3.2. Survival
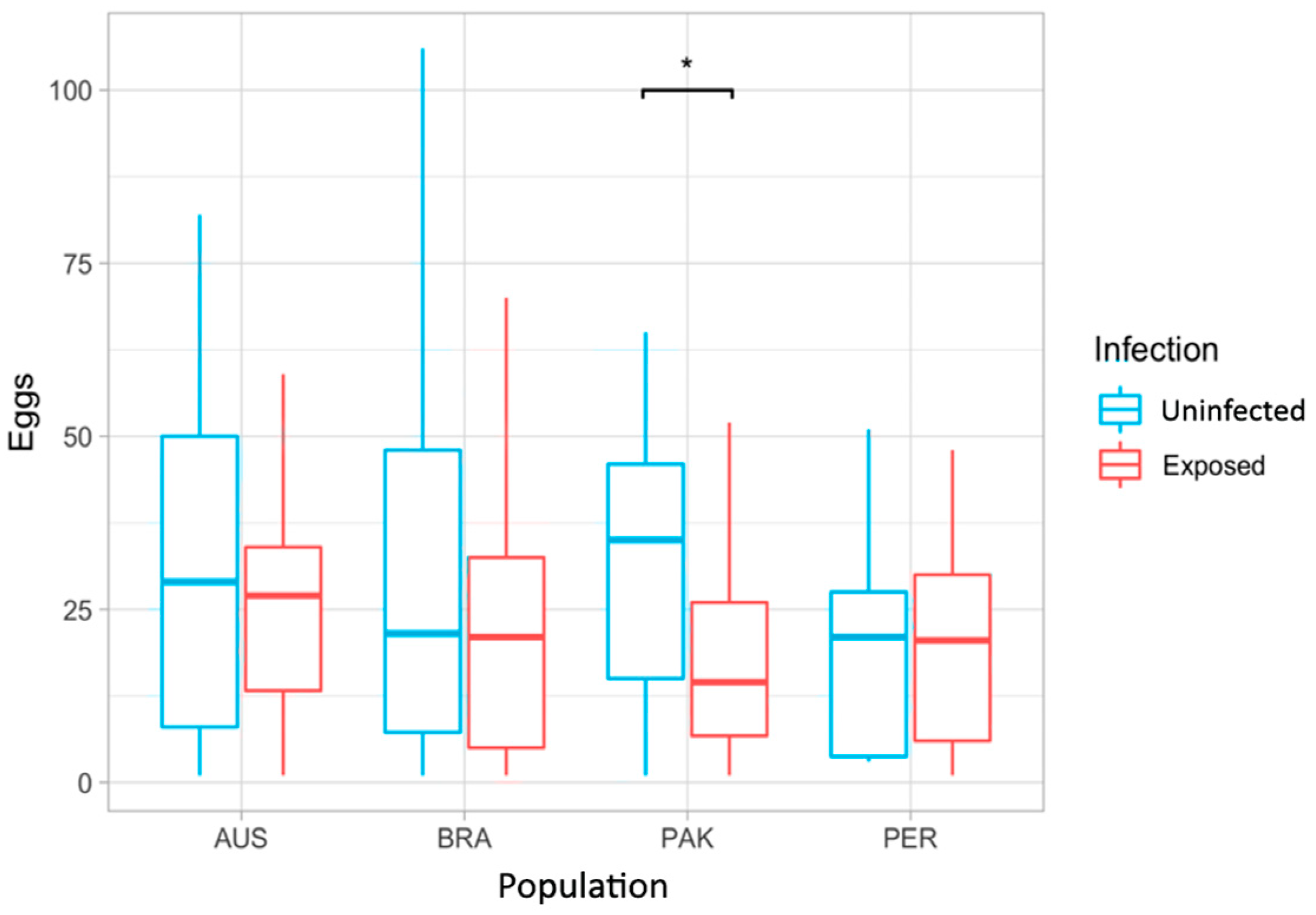
3.3. Fecundity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, S.C.; Charlier, C.; Vasilakis, N.; Lecuit, M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu. Rev. Med. 2018, 69, 395–408. [Google Scholar] [CrossRef]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes Aegypti Vector Competence Studies: A Review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Brady, O.J.; Hay, S.I. The Global Expansion of Dengue: How Aedes Aegypti Mosquitoes Enabled the First Pandemic Arbovirus. Annu. Rev. Entomol. 2020, 65, 191–208. [Google Scholar] [CrossRef]
- Golding, N.; Wilson, A.L.; Moyes, C.L.; Cano, J.; Pigott, D.M.; Velayudhan, R.; Brooker, S.J.; Smith, D.L.; Hay, S.I.; Lindsay, S.W. Integrating Vector Control across Diseases. BMC Med. 2015, 13, 249. [Google Scholar] [CrossRef]
- Shaw, W.R.; Catteruccia, F. Vector Biology Meets Disease Control: Using Basic Research to Fight Vector-Borne Diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef]
- Kramer, L.D.; Ciota, A.T. Dissecting Vectorial Capacity for Mosquito-Borne Viruses. Curr. Opin. Virol. 2015, 15, 112–118. [Google Scholar] [CrossRef]
- Garrett-Jones, C. Prognosis for Interruption of Malaria Transmission Through Assessment of the Mosquito’s Vectorial Capacity. Nature 1964, 204, 1173–1175. [Google Scholar] [CrossRef]
- Kuno, G. Review of the Factors Modulating Dengue Transmission. Epidemiol. Rev. 1995, 17, 321–335. [Google Scholar] [CrossRef] [PubMed]
- GARRETT-JONES, C. The Human Blood Index of Malaria Vectors in Relation to Epidemiological Assessment. Bull. World Health Organ. 1964, 30, 241–261. [Google Scholar] [PubMed]
- Brady, O.J.; Godfray, H.C.J.; Tatem, A.J.; Gething, P.W.; Cohen, J.M.; McKenzie, F.E.; Perkins, T.A.; Reiner, R.C.; Tusting, L.S.; Sinka, M.E.; et al. Vectorial Capacity and Vector Control: Reconsidering Sensitivity to Parameters for Malaria Elimination. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 107–117. [Google Scholar] [CrossRef]
- Maciel-de-Freitas, R. A Review on the Ecological Determinants of Aedes Aegypti (Diptera: Culicidae) Vectorial Capacity. Oecologia Aust. 2010, 14, 726–736. [Google Scholar] [CrossRef]
- Javed, N.; Bhatti, A.; Paradkar, P.N. Advances in Understanding Vector Behavioural Traits after Infection. Pathogens 2021, 10, 1376. [Google Scholar] [CrossRef]
- da Silveira, I.D.; Petersen, M.T.; Sylvestre, G.; Garcia, G.A.; David, M.R.; Pavan, M.G.; Maciel-de-Freitas, R. Zika Virus Infection Produces a Reduction on Aedes Aegypti Lifespan but No Effects on Mosquito Fecundity and Oviposition Success. Front. Microbiol. 2018, 9, 3011. [Google Scholar] [CrossRef] [PubMed]
- Chahad-Ehlers, S.; Gentile, C.; Lima, J.B.P.; Peixoto, A.A.; Bruno, R.V. Analysis of Cycle Gene Expression in Aedes Aegypti Brains by In Situ Hybridization. PLoS ONE 2013, 8, e52559. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Ramos, L.F.C.; Murillo, J.R.; Torres, A.; de Carvalho, S.S.; Domont, G.B.; de Oliveira, D.M.P.; Mesquita, R.D.; Nogueira, F.C.S.; Maciel-de-Freitas, R.; et al. Comprehensive Quantitative Proteome Analysis of Aedes Aegypti Identifies Proteins and Pathways Involved in Wolbachia Pipientis and Zika Virus Interference Phenomenon. Front. Physiol. 2021, 12, 642237. [Google Scholar] [CrossRef]
- Maire, T.; Lambrechts, L.; Hol, F.J.H. Arbovirus Impact on Mosquito Behavior: The Jury Is Still Out. Trends Parasitol. 2024, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maciel-de-Freitas, R.; Sylvestre, G.; Gandini, M.; Koella, J.C. The Influence of Dengue Virus Serotype-2 Infection on Aedes Aegypti (Diptera: Culicidae) Motivation and Avidity to Blood Feed. PLoS ONE 2013, 8, e65252. [Google Scholar] [CrossRef] [PubMed]
- Maciel-de-Freitas, R.; Koella, J.C.; Lourenço-de-Oliveira, R. Lower Survival Rate, Longevity and Fecundity of Aedes Aegypti (Diptera: Culicidae) Females Orally Challenged with Dengue Virus Serotype 2. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 452–458. [Google Scholar] [CrossRef]
- Lambrechts, L.; Chevillon, C.; Albright, R.G.; Thaisomboonsuk, B.; Richardson, J.H.; Jarman, R.G.; Scott, T.W. Genetic Specificity and Potential for Local Adaptation between Dengue Viruses and Mosquito Vectors. BMC Evol. Biol. 2009, 9, 160. [Google Scholar] [CrossRef]
- Lambrechts, L. Dissecting the Genetic Architecture of Host–Pathogen Specificity. PLoS Pathog. 2010, 6, e1001019. [Google Scholar] [CrossRef]
- Gloria-Soria, A.; Ayala, D.; Bheecarry, A.; Calderon-Arguedas, O.; Chadee, D.D.; Chiappero, M.; Coetzee, M.; Elahee, K.B.; Fernandez-Salas, I.; Kamal, H.A.; et al. Global Genetic Diversity of Aedes Aegypti. Mol. Ecol. 2016, 25, 5377–5395. [Google Scholar] [CrossRef]
- Schmidt, T.L.; Chung, J.; Honnen, A.-C.; Weeks, A.R.; Hoffmann, A.A. Population Genomics of Two Invasive Mosquitoes (Aedes Aegypti and Aedes Albopictus) from the Indo-Pacific. PLoS Negl. Trop. Dis. 2020, 14, e0008463. [Google Scholar] [CrossRef]
- Fay, R.W.; Eliason, D.a. A Preferred Oviposition Site as a Surveillance Method for Aedes Aegypti. Mosq. News 1966, 26, 531–535. [Google Scholar]
- Codeço, C.T.; Lima, A.W.S.; Araújo, S.C.; Lima, J.B.P.; Maciel-de-Freitas, R.; Honório, N.A.; Galardo, A.K.R.; Braga, I.A.; Coelho, G.E.; Valle, D. Surveillance of Aedes Aegypti: Comparison of House Index with Four Alternative Traps. PLoS Negl. Trop. Dis. 2015, 9, e0003475. [Google Scholar] [CrossRef]
- Paris, V.; Bell, N.; Schmidt, T.L.; Endersby-Harshman, N.M.; Hoffmann, A.A. Evaluation of In2Care Mosquito Stations for Suppression of the Australian Backyard Mosquito, Aedes Notoscriptus (Diptera: Culicidae). J. Med. Entomol. 2023, 60, 1061–1072. [Google Scholar] [CrossRef]
- Rašić, G.; Schama, R.; Powell, R.; Maciel-de Freitas, R.; Endersby-Harshman, N.M.; Filipović, I.; Sylvestre, G.; Máspero, R.C.; Hoffmann, A.A. Contrasting Genetic Structure between Mitochondrial and Nuclear Markers in the Dengue Fever Mosquito from Rio de Janeiro: Implications for Vector Control. Evol. Appl. 2015, 8, 901–915. [Google Scholar] [CrossRef]
- Kotsakiozi, P.; Gloria-Soria, A.; Schaffner, F.; Robert, V.; Powell, J.R. Aedes Aegypti in the Black Sea: Recent Introduction or Ancient Remnant? Parasit. Vectors 2018, 11, 396. [Google Scholar] [CrossRef]
- Souto-Maior, C.; Sylvestre, G.; Braga Stehling Dias, F.; Gomes, M.G.M.; Maciel-de-Freitas, R. Model-Based Inference from Multiple Dose, Time Course Data Reveals Wolbachia Effects on Infection Profiles of Type 1 Dengue Virus in Aedes Aegypti. PLoS Negl. Trop. Dis. 2018, 12, e0006339. [Google Scholar] [CrossRef]
- Johnson, B.W.; Russell, B.J.; Lanciotti, R.S. Serotype-Specific Detection of Dengue Viruses in a Fourplex Real-Time Reverse Transcriptase PCR Assay. J. Clin. Microbiol. 2005, 43, 4977–4983. [Google Scholar] [CrossRef]
- Corrêa-Antônio, J.; David, M.R.; Couto-Lima, D.; Garcia, G.A.; Keirsebelik, M.S.G.; Maciel-de-Freitas, R.; Pavan, M.G. DENV-1 Titer Impacts Viral Blocking in WMel Aedes Aegypti with Brazilian Genetic Background. Viruses 2024, 16, 214. [Google Scholar] [CrossRef]
- Harbach, R.E.; Knight, K.L. Taxonomist’s Glossary of Mosquito Anatomy; Plexus Publications Co.: Medford, NJ, USA, 1980; pp. 1–54. ISBN 978-0937548004. [Google Scholar]
- Schwartz, A.; Koella, J.C. Trade-Offs, Conflicts of Interest and Manipulation in Plasmodium–Mosquito Interactions. Trends Parasitol. 2001, 17, 189–194. [Google Scholar] [CrossRef]
- Petersen, M.T.; Silveira, I.D.d.; Tátila-Ferreira, A.; David, M.R.; Chouin-Carneiro, T.; Van den Wouwer, L.; Maes, L.; Maciel-de-Freitas, R. The Impact of the Age of First Blood Meal and Zika Virus Infection on Aedes Aegypti Egg Production and Longevity. PLoS ONE 2018, 13, e0200766. [Google Scholar] [CrossRef]
- Petersen, M.T.; Couto-Lima, D.; Garcia, G.A.; Pavan, M.G.; David, M.R.; Maciel-de-Freitas, R. Dengue Exposure and Wolbachia WMel Strain Affects the Fertility of Quiescent Eggs of Aedes Aegypti. Viruses 2023, 15, 952. [Google Scholar] [CrossRef]
- R Development Core Team, R. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 20 April 2024).
- Carrillo-Hernandez, M.Y.; Ruiz-Saenz, J.; Jaimes-Villamizar, L.; Robledo-Restrepo, S.M.; Martinez-Gutierrez, M. Phylogenetic and Evolutionary Analysis of Dengue Virus Serotypes Circulating at the Colombian–Venezuelan Border during 2015–2016 and 2018–2019. PLoS ONE 2021, 16, e0252379. [Google Scholar] [CrossRef]
- Gularte, J.S.; Sacchetto, L.; Demoliner, M.; Girardi, V.; da Silva, M.S.; Filippi, M.; Pereira, V.M. de A.G.; Hansen, A.W.; da Silva, L.L.; Fleck, J.D.; et al. DENV-1 Genotype V Linked to the 2022 Dengue Epidemic in Southern Brazil. J. Clin. Virol. 2023, 168, 105599. [Google Scholar] [CrossRef]
- Fritsch, H.; Moreno, K.; Lima, I.A.B.; Santos, C.S.; Costa, B.G.G.; de Almeida, B.L.; dos Santos, R.A.; Francisco, M.V.L.d.O.; Sampaio, M.P.S.; de Lima, M.M.; et al. Phylogenetic Reconstructions Reveal the Circulation of a Novel Dengue Virus-1V Clade and the Persistence of a Dengue Virus-2 III Genotype in Northeast Brazil. Viruses 2023, 15, 1073. [Google Scholar] [CrossRef]
- Vazquez, C.; Alcantara, L.C.J.; Fonseca, V.; Lima, M.; Xavier, J.; Adelino, T.; Fritsch, H.; Castro, E.; de Oliveira, C.; Schuab, G.; et al. Retrospective Spatio-Temporal Dynamics of Dengue Virus 1, 2 and 4 in Paraguay. Viruses 2023, 15, 1275. [Google Scholar] [CrossRef]
- de Souza, C.S.; Caleiro, G.S.; Claro, I.M.; de Jesus, J.G.; Coletti, T.M.; da Silva, C.A.M.; Costa, Â.A.; Inenami, M.; Ribeiro, A.C.; Felix, A.C.; et al. Phylogenetics, Epidemiology and Temporal Patterns of Dengue Virus in Araraquara, São Paulo State. Viruses 2024, 16, 274. [Google Scholar] [CrossRef]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R.; Failloux, A.-B. Differential Susceptibilities of Aedes Aegypti and Aedes Albopictus from the Americas to Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef]
- Lourenço-de-Oliveira, R.; Vazeille, M.; de Filippis, A.M.; Failloux, A. Aedes Aegypti in Brazil: Genetically Differentiated Populations with High Susceptibility to Dengue and Yellow Fever Viruses. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 43–54. [Google Scholar] [CrossRef]
- Vega-Rúa, A.; Marconcini, M.; Madec, Y.; Manni, M.; Carraretto, D.; Gomulski, L.M.; Gasperi, G.; Failloux, A.-B.; Malacrida, A.R. Vector Competence of Aedes Albopictus Populations for Chikungunya Virus Is Shaped by Their Demographic History. Commun. Biol. 2020, 3, 326. [Google Scholar] [CrossRef]
- Vega-Rúa, A.; Lourenço-de-Oliveira, R.; Mousson, L.; Vazeille, M.; Fuchs, S.; Yébakima, A.; Gustave, J.; Girod, R.; Dusfour, I.; Leparc-Goffart, I.; et al. Chikungunya Virus Transmission Potential by Local Aedes Mosquitoes in the Americas and Europe. PLoS Negl. Trop. Dis. 2015, 9, e0003780. [Google Scholar] [CrossRef]
- Fernandes, R.S.; O’Connor, O.; Bersot, M.I.L.; Girault, D.; Dokunengo, M.R.; Pocquet, N.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R. Vector Competence of Aedes Aegypti, Aedes Albopictus and Culex Quinquefasciatus from Brazil and New Caledonia for Three Zika Virus Lineages. Pathogens 2020, 9, 575. [Google Scholar] [CrossRef]
- Aubry, F.; Dabo, S.; Manet, C.; Filipović, I.; Rose, N.H.; Miot, E.F.; Martynow, D.; Baidaliuk, A.; Merkling, S.H.; Dickson, L.B.; et al. Enhanced Zika Virus Susceptibility of Globally Invasive Aedes Aegypti Populations. Science 2020, 370, 991–996. [Google Scholar] [CrossRef]
- Fansiri, T.; Pongsiri, A.; Klungthong, C.; Ponlawat, A.; Thaisomboonsuk, B.; Jarman, R.G.; Scott, T.W.; Lambrechts, L. No Evidence for Local Adaptation of Dengue Viruses to Mosquito Vector Populations in Thailand. Evol. Appl. 2016, 9, 608–618. [Google Scholar] [CrossRef]
- Fansiri, T.; Fontaine, A.; Diancourt, L.; Caro, V.; Thaisomboonsuk, B.; Richardson, J.H.; Jarman, R.G.; Ponlawat, A.; Lambrechts, L. Genetic Mapping of Specific Interactions between Aedes Aegypti Mosquitoes and Dengue Viruses. PLoS Genet. 2013, 9, e1003621. [Google Scholar] [CrossRef]
- Lambrechts, L.; Quillery, E.; Noël, V.; Richardson, J.H.; Jarman, R.G.; Scott, T.W.; Chevillon, C. Specificity of Resistance to Dengue Virus Isolates Is Associated with Genotypes of the Mosquito Antiviral Gene Dicer-2. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122437. [Google Scholar] [CrossRef]
- Lambrechts, L. Quantitative Genetics of Aedes Aegypti Vector Competence for Dengue Viruses: Towards a New Paradigm? Trends Parasitol. 2011, 27, 111–114. [Google Scholar] [CrossRef]
- Dabo, S.; Henrion-Lacritick, A.; Lecuyer, A.; Jiolle, D.; Paupy, C.; Ayala, D.; da Veiga Leal, S.; Badolo, A.; Vega-Rúa, A.; Sylla, M.; et al. Extensive Variation and Strain-Specificity in Dengue Virus Susceptibility among African Aedes Aegypti Populations. PLoS Negl. Trop. Dis. 2024, 18, e0011862. [Google Scholar] [CrossRef]
- Kain, M.P.; Skinner, E.B.; Athni, T.S.; Ramirez, A.L.; Mordecai, E.A.; van den Hurk, A.F. Not All Mosquitoes Are Created Equal: A Synthesis of Vector Competence Experiments Reinforces Virus Associations of Australian Mosquitoes. PLoS Negl. Trop. Dis. 2022, 16, e0010768. [Google Scholar] [CrossRef]
- Lequime, S.; Fontaine, A.; Ar Gouilh, M.; Moltini-Conclois, I.; Lambrechts, L. Genetic Drift, Purifying Selection and Vector Genotype Shape Dengue Virus Intra-Host Genetic Diversity in Mosquitoes. PLOS Genet. 2016, 12, e1006111. [Google Scholar] [CrossRef]
- Ladner, J.T.; Grubaugh, N.D.; Pybus, O.G.; Andersen, K.G. Precision Epidemiology for Infectious Disease Control. Nat. Med. 2019, 25, 206–211. [Google Scholar] [CrossRef]
- Van den Eynde, C.; Sohier, C.; Matthijs, S.; De Regge, N. Japanese Encephalitis Virus Interaction with Mosquitoes: A Review of Vector Competence, Vector Capacity and Mosquito Immunity. Pathogens 2022, 11, 317. [Google Scholar] [CrossRef]
- González, M.A.; Pavan, M.G.; Fernandes, R.S.; Busquets, N.; David, M.R.; Lourenço-Oliveira, R.; García-Pérez, A.L.; Maciel-de-Freitas, R. Limited Risk of Zika Virus Transmission by Five Aedes Albopictus Populations from Spain. Parasit. Vectors 2019, 12, 150. [Google Scholar] [CrossRef]
- Luz, P.M.; Codeço, C.T.; Massad, E.; Struchiner, C.J. Uncertainties Regarding Dengue Modeling in Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 2003, 98, 871–878. [Google Scholar] [CrossRef]
- Schmid-Hempel, P.; Ebert, D. On the Evolutionary Ecology of Specific Immune Defence. Trends Ecol. Evol. 2003, 18, 27–32. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Evolutionary Ecology of Insect Immune Defenses. Annu. Rev. Entomol. 2005, 50, 529–551. [Google Scholar] [CrossRef]
- Sanchez-Vargas, I.; Travanty, E.A.; Keene, K.M.; Franz, A.W.E.; Beaty, B.J.; Blair, C.D.; Olson, K.E. RNA Interference, Arthropod-Borne Viruses, and Mosquitoes. Virus Res. 2004, 102, 65–74. [Google Scholar] [CrossRef]
- Sánchez-Vargas, I.; Scott, J.C.; Poole-Smith, B.K.; Franz, A.W.E.; Barbosa-Solomieu, V.; Wilusz, J.; Olson, K.E.; Blair, C.D. Dengue Virus Type 2 Infections of Aedes Aegypti Are Modulated by the Mosquito’s RNA Interference Pathway. PLoS Pathog. 2009, 5, e1000299. [Google Scholar] [CrossRef]
- Black, W.C.; Bennett, K.E.; Gorrochótegui-Escalante, N.; Barillas-Mury, C.V.; Fernández-Salas, I.; de Lourdes Muñoz, M.; Farfán-Alé, J.A.; Olson, K.E.; Beaty, B.J. Flavivirus Susceptibility in Aedes Aegypti. Arch. Med. Res. 2002, 33, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Nasci, R.S. The Size of Emerging and Host-Seeking Aedes Aegypti and the Relation of Size to Blood-Feeding Success in the Field. J. Am. Mosq. Control Assoc. 1986, 2, 61–62. [Google Scholar] [PubMed]
- Nasci, R.S. Relationship Between Adult Mosquito (Diptera: Culicidae) Body Size and Parity in Field Populations. Environ. Entomol. 1986, 15, 874–876. [Google Scholar] [CrossRef]
- Kay, B.H.; Muir, L.E. Aedes Aegypti Survival and Dispersal Estimated by Mark-Release-Recapture in Northern Australia. Am. J. Trop. Med. Hyg. 1998, 58, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Maciel-de-Freitas, R.; Codeço, C.T.; Lourenço-de-Oliveira, R. Body Size-Associated Survival and Dispersal Rates of Aedes Aegypti in Rio de Janeiro. Med. Vet. Entomol. 2007, 21, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Tun-Lin, W.; Burkot, T.R.; Kay, B.H. Effects of Temperature and Larval Diet on Development Rates and Survival of the Dengue Vector Aedes Aegypti in North Queensland, Australia. Med. Vet. Entomol. 2000, 14, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.W.; Chow, E.; Strickman, D.; Kittayapong, P.; Wirtz, R.A.; Lorenz, L.H.; Edman, J.D. Blood-Feeding Patterns of Aedes Aegypti (Diptera: Culicidae) Collected in a Rural Thai Village. J. Med. Entomol. 1993, 30, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.W.; Amerasinghe, P.H.; Morrison, A.C.; Lorenz, L.H.; Clark, G.G.; Strickman, D.; Kittayapong, P.; Edman, J.D. Longitudinal Studies of Aedes Aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Blood Feeding Frequency. J. Med. Entomol. 2000, 37, 89–101. [Google Scholar] [CrossRef]
- Sylvestre, G.; Gandini, M.; Maciel-de-Freitas, R. Age-Dependent Effects of Oral Infection with Dengue Virus on Aedes Aegypti (Diptera: Culicidae) Feeding Behavior, Survival, Oviposition Success and Fecundity. PLoS ONE 2013, 8, e59933. [Google Scholar] [CrossRef]
- Lima-Camara, T.N.; Bruno, R.V.; Luz, P.M.; Castro, M.G.; Lourenço-de-Oliveira, R.; Sorgine, M.H.F.; Peixoto, A.A. Dengue Infection Increases the Locomotor Activity of Aedes Aegypti Females. PLoS ONE 2011, 6, e17690. [Google Scholar] [CrossRef]
- Garcia, G.d.A.; Sylvestre, G.; Aguiar, R.; da Costa, G.B.; Martins, A.J.; Lima, J.B.P.; Petersen, M.T.; Lourenço-de-Oliveira, R.; Shadbolt, M.F.; Rašić, G.; et al. Matching the Genetics of Released and Local Aedes Aegypti Populations Is Critical to Assure Wolbachia Invasion. PLoS Negl. Trop. Dis. 2019, 13, e0007023. [Google Scholar] [CrossRef]
- Dutra, H.L.C.; dos Santos, L.M.B.; Caragata, E.P.; Silva, J.B.L.; Villela, D.A.M.; Maciel-de-Freitas, R.; Moreira, L.A. From Lab to Field: The Influence of Urban Landscapes on the Invasive Potential of Wolbachia in Brazilian Aedes Aegypti Mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003689. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keirsebelik, M.S.G.; David, M.R.; Pavan, M.G.; Couto-Lima, D.; Palomino, M.; Rahman, R.U.; Hoffmann, A.A.; Bahia, A.C.; Caljon, G.; Maciel-de-Freitas, R. Dengue Virus Serotype 1 Effects on Mosquito Survival Differ among Geographically Distinct Aedes aegypti Populations. Insects 2024, 15, 393. https://doi.org/10.3390/insects15060393
Keirsebelik MSG, David MR, Pavan MG, Couto-Lima D, Palomino M, Rahman RU, Hoffmann AA, Bahia AC, Caljon G, Maciel-de-Freitas R. Dengue Virus Serotype 1 Effects on Mosquito Survival Differ among Geographically Distinct Aedes aegypti Populations. Insects. 2024; 15(6):393. https://doi.org/10.3390/insects15060393
Chicago/Turabian StyleKeirsebelik, Milan S. G., Mariana R. David, Márcio Galvão Pavan, Dinair Couto-Lima, Miriam Palomino, Rafi Ur Rahman, Ary A. Hoffmann, Ana C. Bahia, Guy Caljon, and Rafael Maciel-de-Freitas. 2024. "Dengue Virus Serotype 1 Effects on Mosquito Survival Differ among Geographically Distinct Aedes aegypti Populations" Insects 15, no. 6: 393. https://doi.org/10.3390/insects15060393
APA StyleKeirsebelik, M. S. G., David, M. R., Pavan, M. G., Couto-Lima, D., Palomino, M., Rahman, R. U., Hoffmann, A. A., Bahia, A. C., Caljon, G., & Maciel-de-Freitas, R. (2024). Dengue Virus Serotype 1 Effects on Mosquito Survival Differ among Geographically Distinct Aedes aegypti Populations. Insects, 15(6), 393. https://doi.org/10.3390/insects15060393







