Identification and Characterization of Three Novel Solemo-like Viruses in the White-Backed Planthopper, Sogatella furcifera
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Generation and Virus Discovery
2.2. Virus Detection
2.3. Insect Samples
2.4. Virus Genome Determination
2.5. Phylogenetic Analysis
2.6. RNA Extraction and Sequencing
2.7. Small RNA Analysis
2.8. The Distribution of SFSolV1 in Different Tissue Types of WBPH
2.9. Viral Detection of SFSolV1 in Different Potential Hosts
2.10. Statistical Analyses
3. Results
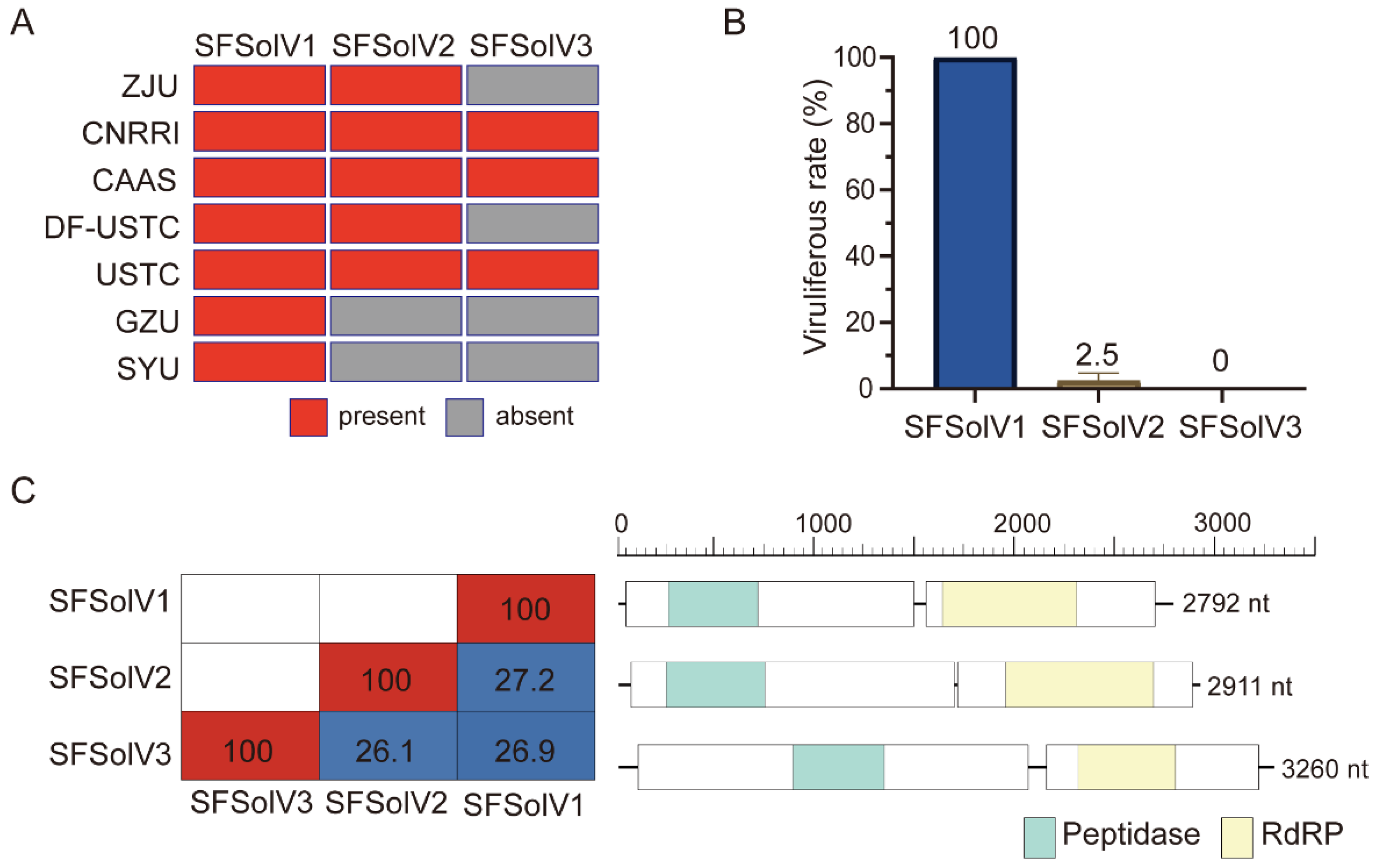
3.1. Discovery of Three Novel Solemo-like Viruses in WBPH
3.2. Genome Organization of the Novel Viruses
3.3. Phylogenetic Analysis of Novel Viruses
3.4. Profiles of Virus-Derived Small Interfering RNAs for Sogatella furcifera Solemo-like Viruses in WBPH
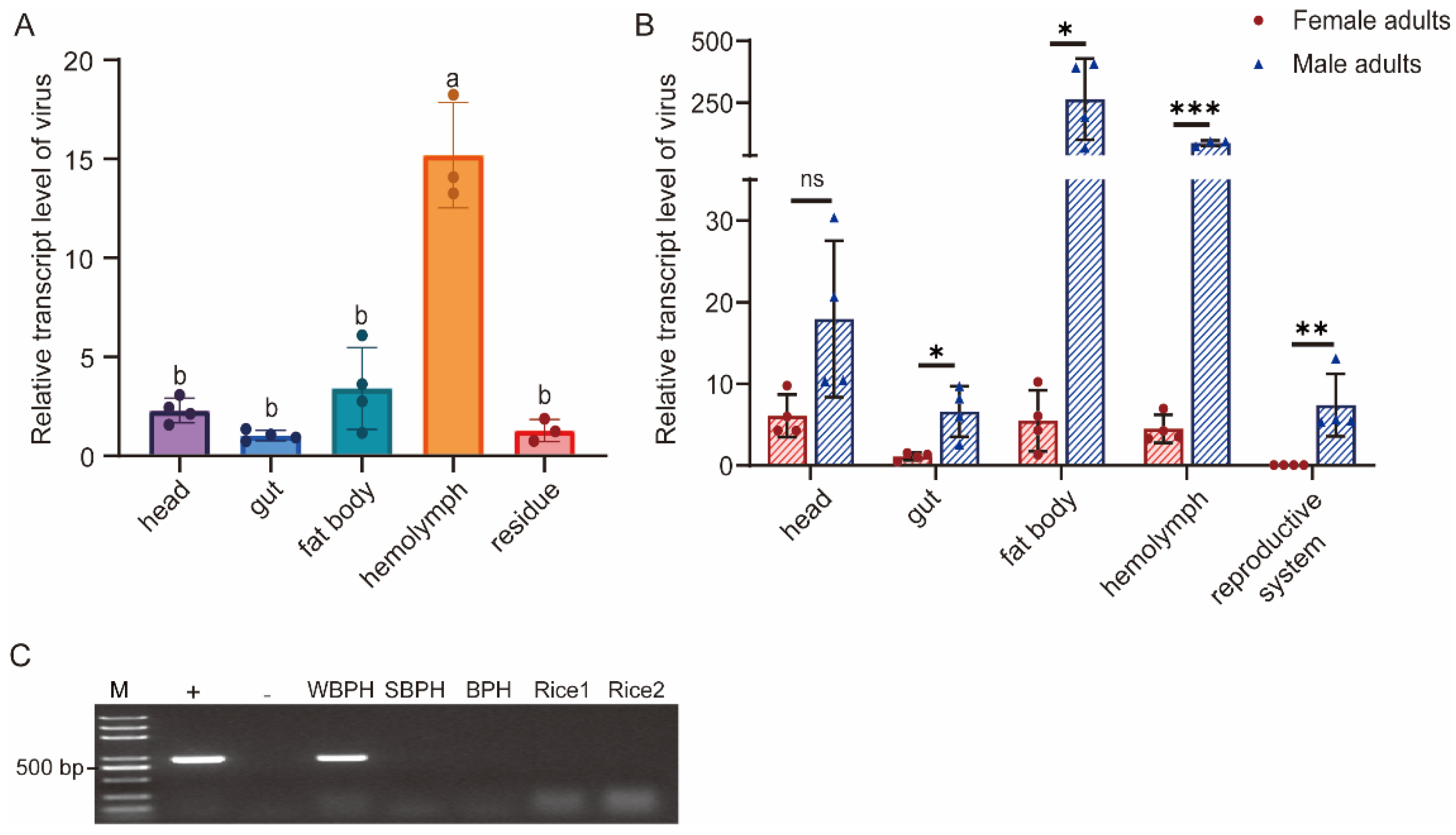
3.5. Tissue Distribution in WBPH and Host Range Detection of SFSolV1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bonning, B.C. The Insect Virome: Opportunities and Challenges. In Insect Molecular Virology: Advances and Emerging Trends; Caister Academic Press: Tours, France, 2019; ISBN 978-1-912530-08-3. [Google Scholar]
- Nouri, S.; Matsumura, E.E.; Kuo, Y.-W.; Falk, B.W. Insect-Specific Viruses: From Discovery to Potential Translational Applications. Curr. Opin. Virol. 2018, 33, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.-H.; Ye, Z.-X.; Zhang, C.-X.; Chen, J.-P.; Li, J.-M. Diversity of RNA Viruses in Agricultural Insects. Comput. Struct. Biotechnol. J. 2023, 21, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Q.; Wei, T. Complex Interactions among Insect Viruses-insect Vector-arboviruses. Insect Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, P.; Lundén, H.; Blomström, A.-L. Insect-Specific Virus Evolution and Potential Effects on Vector Competence. Virus Genes. 2019, 55, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect Pathogens as Biological Control Agents: Back to the Future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Patterson, E.I.; Villinger, J.; Muthoni, J.N.; Dobel-Ober, L.; Hughes, G.L. Exploiting Insect-Specific Viruses as a Novel Strategy to Control Vector-Borne Disease. Curr. Opin. Insect Sci. 2020, 39, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Higgs, S. The Discovery of Arthropod-Specific Viruses in Hematophagous Arthropods: An Open Door to Understanding the Mechanisms of Arbovirus and Arthropod Evolution? Annu. Rev. Entomol. 2018, 63, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Ye, Z.-X.; Wang, X.; Yan, X.-T.; Zhang, Y.; He, Y.-J.; Qi, Y.-H.; Zhang, X.-D.; Zhuo, J.-C.; Lu, G.; et al. Diversity and Infectivity of the RNA Virome among Different Cryptic Species of an Agriculturally Important Insect Vector: Whitefly Bemisia Tabaci. npj Biofilms Microbiomes 2021, 7, 43. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, J.; Lin, X.; Shi, W.; Cao, C. Identification of Diverse Viruses Associated with Grasshoppers Unveils the Parallel Relationship between Host Phylogeny and Virome Composition. Virus Evol. 2022, 8, veac057. [Google Scholar] [CrossRef]
- Jia, W.; Wang, F.; Li, J.; Chang, X.; Yang, Y.; Yao, H.; Bao, Y.; Song, Q.; Ye, G. A Novel Iflavirus Was Discovered in Green Rice Leafhopper Nephotettix Cincticeps and Its Proliferation Was Inhibited by Infection of Rice Dwarf Virus. Front. Microbiol. 2021, 11, 621141. [Google Scholar] [CrossRef]
- Chiapello, M.; Bosco, L.; Ciuffo, M.; Ottati, S.; Salem, N.; Rosa, C.; Tavella, L.; Turina, M. Complexity and Local Specificity of the Virome Associated with Tospovirus-Transmitting Thrips Species. J. Virol. 2021, 95, e00597-21. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, P.; Liu, W.; Cao, M.; Wang, X. Sequence Analysis and Genomic Organization of a New Insect Iflavirus, Sogatella Furcifera Honeydew Virus 1. Arch. Virol. 2018, 163, 2001–2003. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, P.; Liu, W.; Wang, X. Sogatella Furcifera Hepe-like Virus: First Member of a Novel Hepeviridae Clade Identified in an Insect. Virus Res. 2018, 250, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, W.; Cao, M.; Massart, S.; Wang, X. Two Novel Totiviruses in the White-Backed Planthopper, Sogatella Furcifera. J. Gen. General. Virol. 2018, 99, 710–716. [Google Scholar] [CrossRef]
- Mao, Q.; Zheng, S.; Han, Q.; Chen, H.; Ma, Y.; Jia, D.; Chen, Q.; Wei, T. New Model for the Genesis and Maturation of Viroplasms Induced by Fijiviruses in Insect Vector Cells. J. Virol. 2013, 87, 6819–6828. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, D.; Xu, D.; Zhang, M. Southern Rice Black-Streaked Dwarf Virus: A White-Backed Planthopper-Transmitted Fijivirus Threatening Rice Production in Asia. Front. Microbiol. 2013, 4, 270. [Google Scholar] [CrossRef] [PubMed]
- Sõmera, M.; Fargette, D.; Hébrard, E.; Sarmiento, C.; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Solemoviridae 2021. J. Gen. Virol. 2021, 102, 001707. [Google Scholar] [CrossRef]
- LaTourrette, K.; Holste, N.M.; Garcia-Ruiz, H. Polerovirus Genomic Variation. Virus Evol. 2021, 7, veab102. [Google Scholar] [CrossRef]
- Sõmera, M.; Sarmiento, C.; Truve, E. Overview on Sobemoviruses and a Proposal for the Creation of the Family Sobemoviridae. Viruses 2015, 7, 3076–3115. [Google Scholar] [CrossRef]
- Longue, R.D.S.; Traore, V.S.E.; Zinga, I.; Asante, M.D.; Bouda, Z.; Neya, J.B.; Barro, N.; Traore, O. Pathogenicity of Rice Yellow Mottle Virus and Screening of Rice Accessions from the Central African Republic. Virol. J. 2018, 15, 6. [Google Scholar] [CrossRef]
- Miller, W.A.; Lozier, Z. Yellow Dwarf Viruses of Cereals: Taxonomy and Molecular Mechanisms. Annu. Rev. Phytopathol. 2022, 60, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Antipov, D.; Raiko, M.; Lapidus, A.; Pevzner, P.A. METAVIRALSPADES: Assembly of Viruses from Metagenomic Data. Bioinformatics 2020, 36, 4126–4129. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.-Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving Coverage, Classification and Access to Protein Sequence Annotations. Nucleic Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of Phylogenies after Removing Divergent and Ambiguously Aligned Blocks from Protein Sequence Alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A Fast, Scalable and User-Friendly Tool for Maximum Likelihood Phylogenetic Inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- Li, J.; Andika, I.B.; Shen, J.; Lv, Y.; Ji, Y.; Sun, L.; Chen, J. Characterization of Rice Black-Streaked Dwarf Virus- and Rice Stripe Virus-Derived siRNAs in Singly and Doubly Infected Insect Vector Laodelphax Striatellus. PLoS ONE 2013, 8, e66007. [Google Scholar] [CrossRef]
- Ribeiro, J.; Malta, M.; Galaghar, A.; Silva, F.; Afonso, L.P.; Medeiros, R.; Sousa, H. P53 Deregulation in Epstein-Barr Virus-Associated Gastric Cancer. Cancer Lett. 2017, 404, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, N.; Noda, H. Nonpathogenic Nilaparvata Lugens Reovirus Is Transmitted to the Brown Planthopper through Rice Plant. Virology 1995, 207, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhang, X.-D.; Xu, Z.-T.; Ye, Z.-X.; Zhang, Y.; Chen, J.-P.; Zhang, C.-X.; Li, J.-M. Complete Sequence and Genetic Characterization of a Novel Insect-Specific Reovirus Discovered from Laodelphax Striatellus. Virology 2022, 570, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.A.; Wainaina, J.M.; Dominguez-Huerta, G.; Pelletier, E.; Guo, J.; Mohssen, M.; Tian, F.; Pratama, A.A.; Bolduc, B.; Zablocki, O.; et al. Cryptic and Abundant Marine Viruses at the Evolutionary Origins of Earth’s RNA Virome. Science 2022, 376, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Dolja, V.V.; Krupovic, M.; Koonin, E.V. Deep Roots and Splendid Boughs of the Global Plant Virome. Annu. Rev. Phytopathol. 2020, 58, 23–53. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhu, Y.; Liu, W.; Zou, C.; Jia, B.; Chen, Z.-Q.; Han, Y.; Wu, J.; Yang, D.-L.; Zhang, Z.; et al. Discovery of Aphid-Transmitted Rice Tiller Inhibition Virus from Native Plants through Metagenomic Sequencing. PLoS Pathog. 2023, 19, e1011238. [Google Scholar] [CrossRef] [PubMed]
- Gammon, D.B.; Mello, C.C. RNA Interference-Mediated Antiviral Defense in Insects. Curr. Opin. Insect Sci. 2015, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.-D.; Tian, J.-H.; Chen, L.-J.; Chen, X.; Li, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the Invertebrate RNA Virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Remnant, E.; Baty, J.; Bulgarella, M.; Dobelmann, J.; Quinn, O.; Gruber, M.; Lester, P. A Diverse Viral Community from Predatory Wasps in Their Native and Invaded Range, with a New Virus Infectious to Honey Bees. Viruses 2021, 13, 1431. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, L.; Atoni, E.; Zeng, W.; Hu, X.; Matthijnssens, J.; Yuan, Z.; Xia, H. Stability of the Virome in Lab- and Field-Collected Aedes Albopictus Mosquitoes across Different Developmental Stages and Possible Core Viruses in the Publicly Available Virome Data of Aedes Mosquitoes. mSystems 2020, 5, e00640-20. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, D.; Yuan, F.; Yan, Y.; Wang, Z.; Liu, P.; Yu, Q.; Zhang, X.; Wang, X.; Zheng, A. Replication Is the Key Barrier during the Dual-Host Adaptation of Mosquito-Borne Flaviviruses. Proc. Natl. Acad. Sci. USA 2022, 119, e2110491119. [Google Scholar] [CrossRef]
- de Almeida, J.P.; Aguiar, E.R.; Armache, J.N.; Olmo, R.P.; Marques, J.T. The Virome of Vector Mosquitoes. Curr. Opin. Virol. 2021, 49, 7–12. [Google Scholar] [CrossRef]
- Olmo, R.P.; Martins, N.E.; Aguiar, E.R.G.R.; Marques, J.T.; Imler, J.-L. The Insect Reservoir of Biodiversity for Viruses and for Antiviral Mechanisms. An. Acad. Bras. CiÊNc. 2019, 91, e20190122. [Google Scholar] [CrossRef]
- Olmo, R.P.; Todjro, Y.M.H.; Aguiar, E.R.G.R.; de Almeida, J.P.P.; Ferreira, F.V.; Armache, J.N.; de Faria, I.J.S.; Ferreira, A.G.A.; Amadou, S.C.G.; Silva, A.T.S.; et al. Mosquito Vector Competence for Dengue Is Modulated by Insect-Specific Viruses. Nat. Microbiol. 2023, 8, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, N.; Koizumi, M.; Watanabe, H.; Noda, H. Complete Nucleotide Sequence of the Nilaparvata Lugens Reovirus: A Putative Member of the Genus Fijivirus. J. Gen. General. Virol. 1996, 77, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Bonning, B.C.; Saleh, M.-C. The Interplay Between Viruses and RNAi Pathways in Insects. Annu. Rev. Entomol. 2021, 66, 61–79. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, Y.; Wang, P.; Xiao, X. Mosquito Defense Strategies against Viral Infection. Trends Parasitol. 2016, 32, 177–186. [Google Scholar] [CrossRef]
- Liao, Z.; Mao, Q.; Li, J.; Lu, C.; Wu, W.; Chen, H.; Chen, Q.; Jia, D.; Wei, T. Virus-Induced Tubules: A Vehicle for Spread of Virions into Ovary Oocyte Cells of an Insect Vector. Front. Microbiol. 2017, 8, 475. [Google Scholar] [CrossRef]
- Huo, Y.; Yu, Y.; Liu, Q.; Liu, D.; Zhang, M.; Liang, J.; Chen, X.; Zhang, L.; Fang, R. Rice Stripe Virus Hitchhikes the Vector Insect Vitellogenin Ligand-Receptor Pathway for Ovary Entry. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180312. [Google Scholar] [CrossRef] [PubMed]
- Rittschof, C.C.; Pattanaik, S.; Johnson, L.; Matos, L.F.; Brusini, J.; Wayne, M.L. Sigma Virus and Male Reproductive Success in Drosophila Melanogaster. Behav. Ecol. Sociobiol. 2013, 67, 529–540. [Google Scholar] [CrossRef]
- Chen, Q.; Godfrey, K.; Liu, J.; Mao, Q.; Kuo, Y.-W.; Falk, B.W. A Nonstructural Protein Responsible for Viral Spread of a Novel Insect Reovirus Provides a Safe Channel for Biparental Virus Transmission to Progeny. J. Virol. 2019, 93, e00702-19. [Google Scholar] [CrossRef] [PubMed]
- Wan, J. Arboviruses and Symbiotic Viruses Cooperatively Hijack Insect Sperm-Specific Proteins for Paternal Transmission. Nat. Commun. 2023, 14, 1289. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yang, L.; Yang, X.; Li, T.; Graham, R.I.; Wu, K.; Wilson, K. Novel Partiti-like Viruses Are Conditional Mutualistic Symbionts in Their Normal Lepidopteran Host, African Armyworm, but Parasitic in a Novel Host, Fall Armyworm. PLoS Pathog. 2020, 16, e1008467. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fang, Q.; Wang, B.; Yan, Z.; Hong, J.; Bao, Y.; Kuhn, J.H.; Werren, J.H.; Song, Q.; Ye, G. A Novel Negative-Stranded RNA Virus Mediates Sex Ratio in Its Parasitoid Host. PLoS Pathog. 2017, 13, e1006201. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.-N.; Ye, Z.-X.; Chen, M.-N.; Ren, P.-P.; Ning, C.; Sun, Z.-T.; Chen, J.-P.; Zhang, C.-X.; Li, J.-M.; Mao, Q. Identification and Characterization of Three Novel Solemo-like Viruses in the White-Backed Planthopper, Sogatella furcifera. Insects 2024, 15, 394. https://doi.org/10.3390/insects15060394
Yuan J-N, Ye Z-X, Chen M-N, Ren P-P, Ning C, Sun Z-T, Chen J-P, Zhang C-X, Li J-M, Mao Q. Identification and Characterization of Three Novel Solemo-like Viruses in the White-Backed Planthopper, Sogatella furcifera. Insects. 2024; 15(6):394. https://doi.org/10.3390/insects15060394
Chicago/Turabian StyleYuan, Jing-Na, Zhuang-Xin Ye, Meng-Nan Chen, Peng-Peng Ren, Chao Ning, Zong-Tao Sun, Jian-Ping Chen, Chuan-Xi Zhang, Jun-Min Li, and Qianzhuo Mao. 2024. "Identification and Characterization of Three Novel Solemo-like Viruses in the White-Backed Planthopper, Sogatella furcifera" Insects 15, no. 6: 394. https://doi.org/10.3390/insects15060394




