Comprehensive Approach to Phenotype Varroa destructor Reproduction in Honey Bee Drone Brood and Its Correlation with Decreased Mite Reproduction (DMR)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Colony Sampling and Data Collection
2.2. Phenotyping Calculations
2.3. Statistics
3. Results
3.1. Data Overview and Filtering
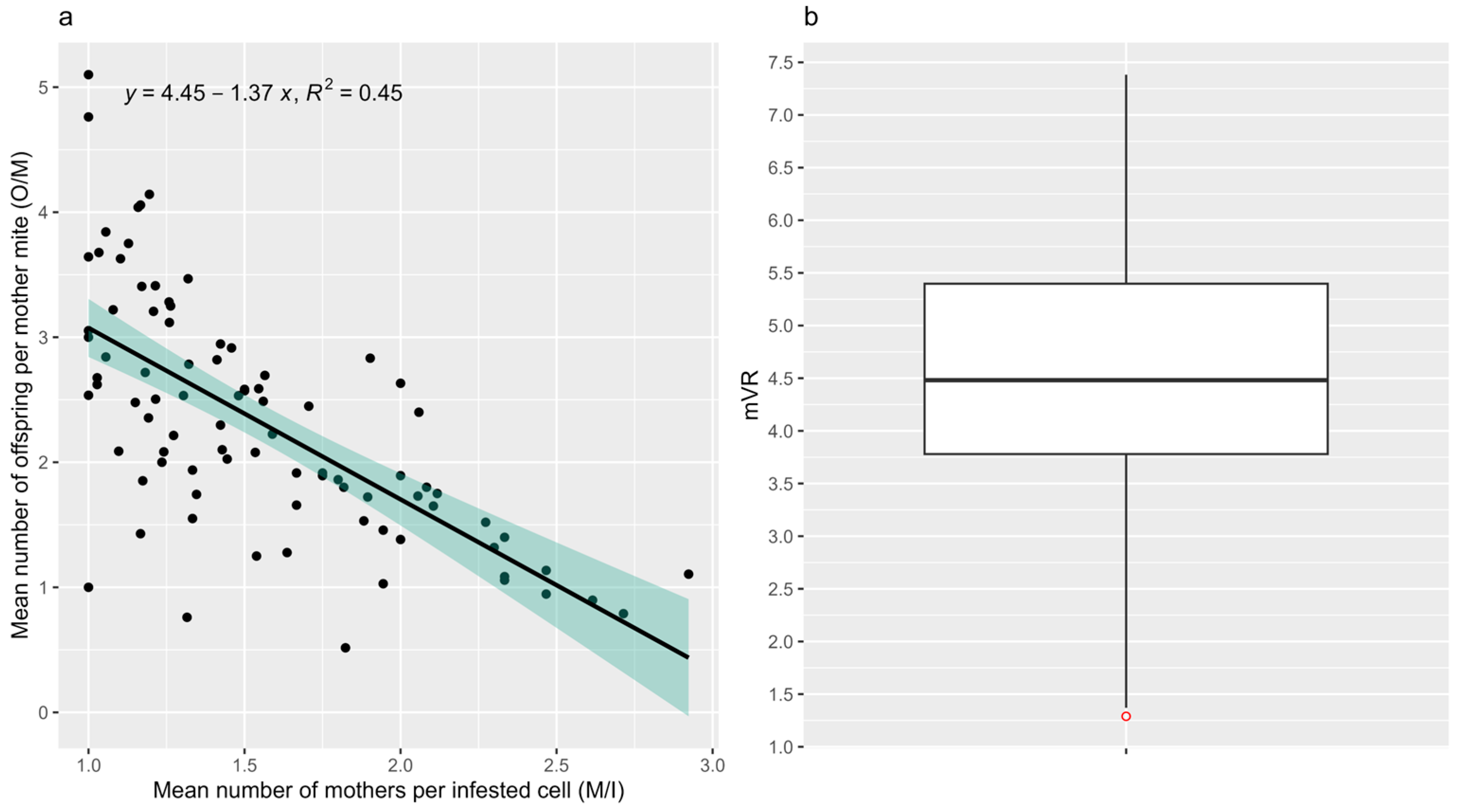
3.2. Mean V. destructor Reproduction Rate
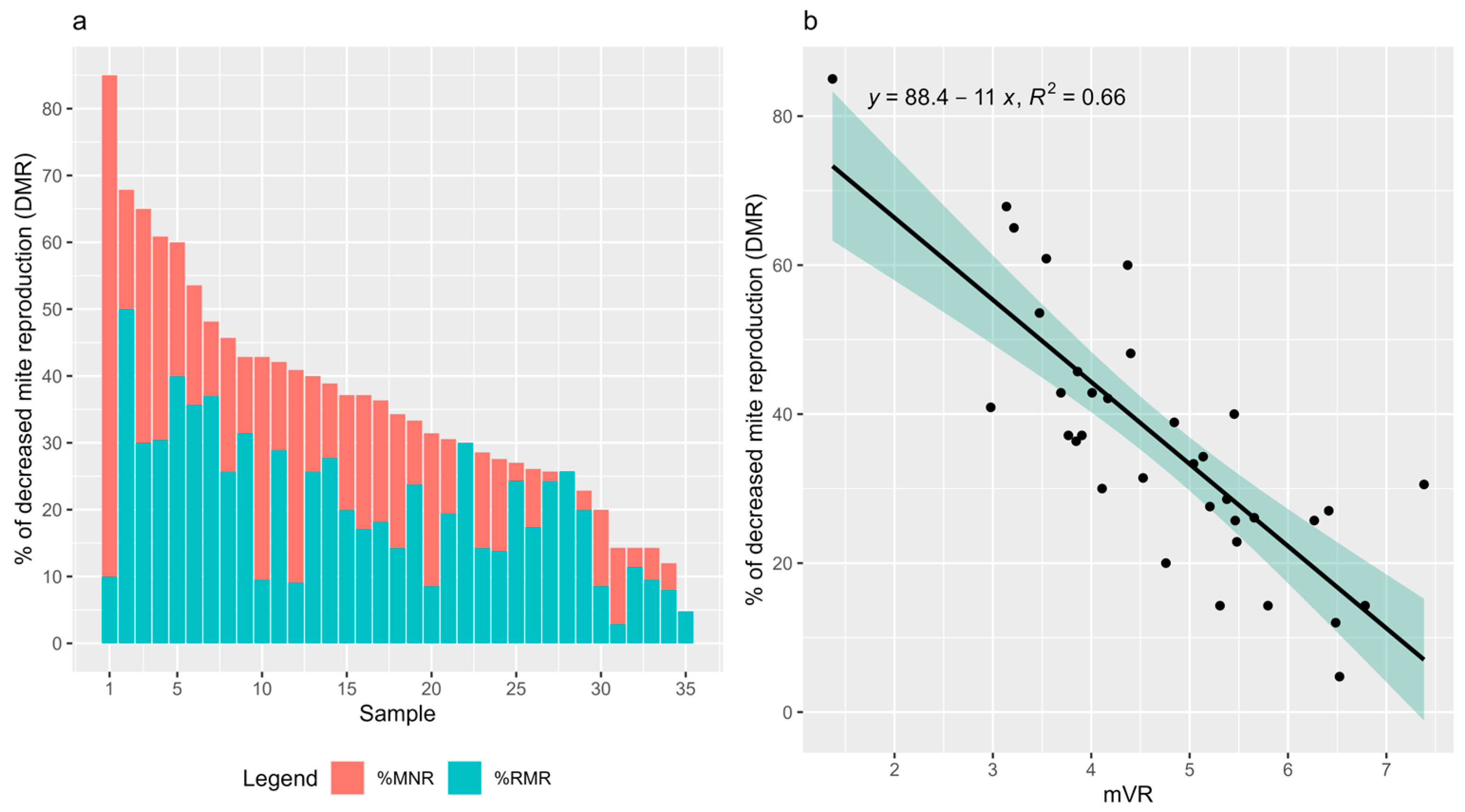
3.3. Correlation with DMR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oldroyd, B.P. Coevolution while you wait: Varroa jacobsoni, a new parasite of western honeybees. Trends Ecol. Evol. 1999, 14, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wu, J.L.; Wei, Q.H.; Liu, F.Y.; Cui, L.H.; Rueppell, O.; Xu, S.F. Life-history stage determines the diet of ectoparasitic mites on their honey bee hosts. Nat. Commun. 2024, 15, 725. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie 2010, 41, 353–363. [Google Scholar] [CrossRef]
- Genersch, E.; von der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Buchler, R.; Berg, S.; Ritter, W.; Muhlen, W.; Gisder, S.; et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef]
- van Engelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Dahle, B. The role of Varroa destructor for honey bee colony losses in Norway. J. Apic. Res. 2010, 49, 124–125. [Google Scholar] [CrossRef]
- Stahlmann-Brown, P.; Hall, R.J.; Pragert, H.; Robertson, T. Varroa Appears to Drive Persistent Increases in New Zealand Colony Losses. Insects 2022, 13, 589. [Google Scholar] [CrossRef]
- Dynes, T.L.; Berry, J.A.; Delaplane, K.S.; de Roode, J.C.; Brosi, B.J. Assessing virulence of Varroa destructor mites from different honey bee management regimes. Apidologie 2020, 51, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Hillayova, M.K.; Koreny, L.; Skvarenina, J. The local environmental factors impact the infestation of bee colonies by mite Varroa destructor. Ecol. Indic. 2022, 141, 109104. [Google Scholar] [CrossRef]
- Dolezal, A.G.; Carrillo-Tripp, J.; Judd, T.M.; Miller, W.A.; Bonning, B.C.; Toth, A.L. Interacting stressors matter: Diet quality and virus infection in honeybee health. Roy Soc. Open Sci. 2019, 6, 181803. [Google Scholar] [CrossRef] [PubMed]
- Annoscia, D.; Di Prisco, G.; Becchimanzi, A.; Caprio, E.; Frizzera, D.; Linguadoca, A.; Nazzi, F.; Pennacchio, F. Neonicotinoid Clothianidin reduces honey bee immune response and contributes to Varroa mite proliferation. Nat. Commun. 2020, 11, 5887. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.P.; Delaplane, K.S. Distance between honey bee Apis mellifera colonies regulates populations of Varroa destructor at a landscape scale. Apidologie 2017, 48, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Dynes, T.L.; Berry, J.A.; Delaplane, K.S.; Brosi, B.J.; de Roode, J.C. Reduced density and visually complex apiaries reduce parasite load and promote honey production and overwintering survival in honey bees. PLoS ONE 2019, 14, e0216286. [Google Scholar] [CrossRef] [PubMed]
- Smolinski, S.; Langowska, A.; Glazaczow, A. Raised seasonal temperatures reinforce autumn Varroa destructor infestation in honey bee colonies. Sci. Rep. 2021, 11, 22256. [Google Scholar] [CrossRef] [PubMed]
- Anguiano-Baez, R.; Guzman-Novoa, E.; Hamiduzzaman, M.M.; Espinosa-Montano, L.G.; Correa-Benitez, A. Varroa destructor (Mesostigmata: Varroidae) Parasitism and Climate Differentially Influence the Prevalence, Levels, and Overt Infections of DeformedWing Virus in Honey Bees (Hymenoptera: Apidae). J. Insect Sci. 2016, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. Biol. Sci. 2017, 284, 20171711. [Google Scholar] [CrossRef]
- Locke, B. Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 2016, 47, 467–482. [Google Scholar] [CrossRef]
- Mondet, F.; Beaurepaire, A.; McAfee, A.; Locke, B.; Alaux, C.; Blanchard, S.; Danka, B.; Le Conte, Y. Honey bee survival mechanisms against the parasite Varroa destructor: A systematic review of phenotypic and genomic research efforts. Int. J. Parasitol. 2020, 50, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Ruttner, F.; Marx, H.; Marx, G. Observation About a Possible Adaptation of Varroa-Jacobsoni to Apis mellifera L. in Uruguay. Apidologie 1984, 15, 43–62. [Google Scholar] [CrossRef]
- Camazine, S. Differential Reproduction of the Mite, Varroa-Jacobsoni (Mesostigmata, Varroidae), on Africanized and European Honey-Bees (Hymenoptera, Apidae). Ann. Entomol. Soc. Am. 1986, 79, 801–803. [Google Scholar] [CrossRef]
- Locke, B.; Fries, I. Characteristics of honey bee colonies (Apis mellifera) in Sweden surviving Varroa destructor infestation. Apidologie 2011, 42, 533–542. [Google Scholar] [CrossRef]
- Le Conte, Y.; De Vaublanc, G.; Crauser, D.; Jeanne, F.; Rousselle, J.C.; Becard, J.M. Honey bee colonies that have survived Varroa destructor. Apidologie 2007, 38, 566–572. [Google Scholar] [CrossRef]
- Mondet, F.; Parejo, M.; Meixner, M.D.; Costa, C.; Kryger, P.; Andonov, S.; Servin, B.; Basso, B.; Bienkowska, M.; Bigio, G.; et al. Evaluation of Suppressed Mite Reproduction (SMR) Reveals Potential for Varroa Resistance in European Honey Bees (Apis mellifera L.). Insects 2020, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Eynard, S.E.; Sann, C.; Basso, B.; Guirao, A.L.; Le Conte, Y.; Servin, B.; Tison, L.; Vignal, A.; Mondet, F. Descriptive Analysis of the Varroa Non-Reproduction Trait in Honey Bee Colonies and Association with Other Traits Related to Varroa Resistance. Insects 2020, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- von Virag, A.; Guichard, M.; Neuditschko, M.; Dietemann, V.; Dainat, B. Decreased Mite Reproduction to Select Varroa destructor (Acari: Varroidae) Resistant Honey Bees (Hymenoptera: Apidae): Limitations and Potential Methodological Improvements. J. Econ. Entomol. 2022, 115, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Frey, E.; Odemer, R.; Blum, T.; Rosenkranz, P. Activation and interruption of the reproduction of Varroa destructor is triggered by host signals (Apis mellifera). J. Invertebr. Pathol. 2013, 113, 56–62. [Google Scholar] [CrossRef]
- Garrido, C.; Rosenkranz, P. Volatiles of the honey bee larva initiate oogenesis in the parasitic mite Varroa destructor. Chemoecology 2004, 14, 193–197. [Google Scholar]
- Garrido, C.; Rosenkranz, P. The reproductive program of female Varroa destructor mites is triggered by its host, Apis mellifera. Exp. Appl. Acarol. 2003, 31, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, B.J.G.; De Smet, L.; Blacquiere, T.; Maebe, K.; Khalenkow, M.; Van Poucke, M.; Dahle, B.; Neumann, P.; Nguyen, K.B.; Smagghe, G.; et al. Honey bee predisposition of resistance to ubiquitous mite infestations. Sci. Rep. 2019, 9, 7794. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. Suppressed mite reproduction explained by the behaviour of adult bees. J. Apic. Res. 2005, 44, 21–23. [Google Scholar] [CrossRef]
- Harris, J.W.; Danka, R.G.; Villa, J.D. Changes in Infestation, Cell Cap Condition, and Reproductive Status of Varroa destructor (Mesostigmata: Varroidae) in Brood Exposed to Honey Bees with Varroa Sensitive Hygiene. Ann. Entomol. Soc. Am. 2012, 105, 512–518. [Google Scholar] [CrossRef]
- Kim, S.H.; Mondet, F.; Herve, M.; Mercer, A. Honey bees performing varroa sensitive hygiene remove the most mite-compromised bees from highly infested patches of brood. Apidologie 2018, 49, 335–345. [Google Scholar] [CrossRef]
- Sprau, L.; Hasselmann, M.; Rosenkranz, P. Reproduction of Varroa destructor does not elicit varroa sensitive hygiene (VSH) or recapping behaviour in honey bee colonies (Apis mellifera). Apidologie 2021, 52, 1048–1059. [Google Scholar] [CrossRef]
- Martin, S.J.; Hawkins, G.P.; Brettell, L.E.; Reece, N.; Correia-Oliveira, M.E.; Allsopp, M.H. Varroa destructorreproduction and cell re-capping in mite-resistant Apis mellifera populations. Apidologie 2020, 51, 369–381. [Google Scholar] [CrossRef]
- Guichard, M.; Dietemann, V.; Neuditschko, M.; Dainat, B. Advances and perspectives in selecting resistance traits against the parasitic mite Varroa destructor in honey bees. Genet Sel Evol 2020, 52, 71. [Google Scholar] [CrossRef] [PubMed]
- Büchler, R.C.C.; Mondet, F.; Kezic, N.; Kovacic, M. Screening for Low Varroa Mite Reproduction (SMR) and Recapping in European Honey Bees. 2017. Available online: https://www.beebreeding.net/wp-content/uploads/2017/11/RNSBB_SMR-recapping_protocol_2017_09_11.pdf (accessed on 1 May 2024).
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard methods for varroa research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Fuchs, S.; Langenbach, K. Multiple Infestation of Apis-Mellifera L Brood Cells and Reproduction in Varroa-Jacobsoni Oud. Apidologie 1989, 20, 257–266. [Google Scholar] [CrossRef]
- Floris, I.; Pusceddu, M.; Satta, A. How the Infestation Level of Varroa destructor Affects the Distribution Pattern of Multi-Infested Cells in Worker Brood of Apis mellifera (vol 7, 136, 2020). Vet. Sci. 2020, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Ghamdi, A.A.; Hoopingarner, R. Reproductive Biology of Varroa jacobsoni Oud. in Worker and Drone Brood of the Honey Bee Apis mellifera L. under Michigan Conditions. Pak. J. Biol. Sci. 2003, 6, 756–761. [Google Scholar] [CrossRef]
- Eguaras, M.; Marcangeli, J.; Fernandez, N.A. Influence of Parasitic Intensity on Varroa-Jacobsoni Oud Reproduction. J. Apic. Res. 1994, 33, 155–159. [Google Scholar] [CrossRef]
- Tofilski, A. Honey Bee. Available online: http://www.honeybee.drawwing.org (accessed on 1 May 2024).
- Martin, S.J. Life and Death of Varroa; International BeeResearch Association: Cardiff, Wales, 1997. [Google Scholar]
- Lee, K.V.; Moon, R.D.; Burkness, E.C.; Hutchison, W.D.; Spivak, M. Practical Sampling Plans for Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) Colonies and Apiaries. J. Econ. Entomol. 2010, 103, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Claeys Bouuaert, D.; Danneels, E.; Villers, V.; Brunain, M.; Lefebre, R.; De Smet, L.; de Graaf, D.C. Can a simple selection program predict winter mortality? Preliminary evaluation of the Flemish honey bee breeding program steers towards selection by exclusion. Bee World 2023, 100, 15–19. [Google Scholar] [CrossRef]
- Martin, G. Heritability of Apis mellifera recapping behavior and suppressed mite reproduction as resistance traits towards Varroa destructor. Front. Insect Sci. 2023, 3, 1135187. [Google Scholar]
- Le Conte, Y.; Meixner, M.D.; Brandt, A.; Carreck, N.L.; Costa, C.; Mondet, F.; Buchler, R. Geographical Distribution and Selection of European Honey Bees Resistant to Varroa destructor. Insects 2020, 11, 873. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefebre, R.; Claeys Bouuaert, D.; Bossuyt, E.; De Smet, L.; Brunain, M.; Danneels, E.; de Graaf, D.C. Comprehensive Approach to Phenotype Varroa destructor Reproduction in Honey Bee Drone Brood and Its Correlation with Decreased Mite Reproduction (DMR). Insects 2024, 15, 397. https://doi.org/10.3390/insects15060397
Lefebre R, Claeys Bouuaert D, Bossuyt E, De Smet L, Brunain M, Danneels E, de Graaf DC. Comprehensive Approach to Phenotype Varroa destructor Reproduction in Honey Bee Drone Brood and Its Correlation with Decreased Mite Reproduction (DMR). Insects. 2024; 15(6):397. https://doi.org/10.3390/insects15060397
Chicago/Turabian StyleLefebre, Regis, David Claeys Bouuaert, Emma Bossuyt, Lina De Smet, Marleen Brunain, Ellen Danneels, and Dirk C. de Graaf. 2024. "Comprehensive Approach to Phenotype Varroa destructor Reproduction in Honey Bee Drone Brood and Its Correlation with Decreased Mite Reproduction (DMR)" Insects 15, no. 6: 397. https://doi.org/10.3390/insects15060397





