The Relationships between the Population Density of Fir Bark Beetles and Niche Breadth
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
- Measurement of stem length and the diameters at the thicker and thinner ends. The average diameters at the thicker end for the trap trees (35.8 ± 6.9 SD) and the windfalls (33.7 ± 7.3 SD) were similar (t-test: t = 1.1109; df = 58; P = 0.2712). The average length of the trap trees (22.1 ± 1.96 SD) was greater than that of the windfalls (20.6 ± 2.5 SD) (t-test: t = 2.6780; df = 58; P = 0.0096);
- Division of the stems into units each covering 5% of the stem length (20 equal units). For each unit, the diameters at the thicker and thinner ends were measured;
- Division of the stems lengthwise into upper and lower sections.
- The nuptial chamber of C. piceae is approximately 0.5 cm long. Females deposit eggs in clusters. A female lays from 20 to 40 eggs. After hatching, larvae excavate 2–4 cm-long maternal galleries in all directions from the nuptial chamber [59].
- Egg gallery of P. curvidens: Males excavate nuptial chambers, from which females excavate two maternal galleries upward and downward. Later, these maternal galleries are divided into two arms to the right and to the left. Maternal galleries are about 5–10 cm long and 1 mm wide. They are mostly in the bark, but also visible on the wood [59].
- Egg gallery of P. spinidens is “star”-shaped. Maternal galleries (usually 3–8) are about 1 mm wide and 10 cm long. They start from the nuptial chamber and bend in a distance not shorter than 10 mm (egg gallery). Pupal chambers are in the sapwood or between the sapwood and bark [59].
2.2. Measures Used to Describe the Niche Breadth Parameters of Bark Beetles
2.2.1. Levin’s Measure of Niche Breadth [61]
2.2.2. Index of Niche Overlap (Morisita’s Index) [64]
2.2.3. Proportional Similarity Index (PSi) [65]
2.3. Models for Bark Beetle Niches
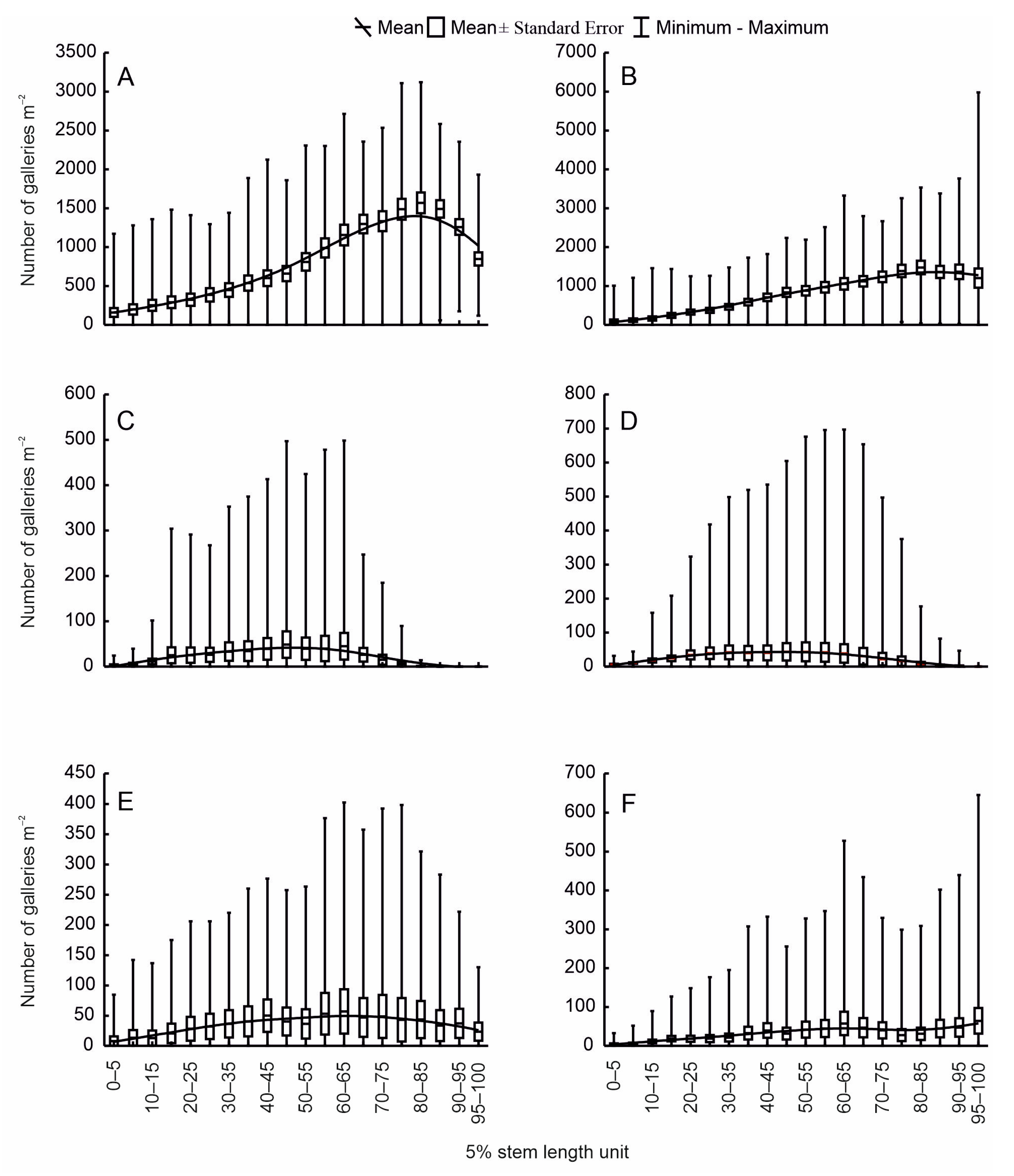
3. Results
3.1. Colonisation of Firs by Bark Beetles
3.2. Biotic Interactions
4. Discussion
4.1. Tree Infestation
4.2. Biotic Interactions
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sexton, J.P.; Montiel, J.; Shay, J.E.; Stephens, M.R.; Slatyer, R.A. Evolution of Ecological Niche Breadth. Ann. Rev. Ecol. Evol. Syst. 2017, 48, 183–206. [Google Scholar] [CrossRef]
- Carscadden, K.A.; Emery, N.C.; Arnillas, C.A.; Cadotte, M.W. Niche breadth: Causes and consequences for ecology, evolution, and conservation. Quart. Rev. Biol. 2020, 95, 179–214. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2011; pp. 1–326. [Google Scholar]
- Krebs, C.J. Niche measures and resource preferences. In Ecological Methodology; Krebs, C.J., Ed.; Harper & Row: New York, NY, USA, 2014; pp. 597–653. [Google Scholar]
- Paine, T.D.; Birch, M.C.; Švihra, P. Niche breadth and resource partitioning by four sympatric species of bark beetles (Coleoptera: Scolytidae). Oecologia 1981, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, M. Ecological segregation of bark beetles (Coleoptera, Scolytidae) of spruce. Zeit. Angew. Entomol. 1986, 101, 176–187. [Google Scholar] [CrossRef]
- Amezaga, I.; Rodríguez, M.A. Resource partitioning of four sympatric bark beetles depending on swarming dates and tree species. For. Ecol. Manag. 1998, 109, 127–135. [Google Scholar] [CrossRef]
- Ayres, B.D.; Ayres, M.P.; Abrahamson, M.D.; Teale, S.A. Resource partitioning and overlap in three sympatric species of Ips bark beetles (Coleoptera: Scolytidae). Oecologia 2001, 128, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Wallin, K.F.; Kolb, T.E.; Skov, K.R.; Wagner, M. Forest management treatments, tree resistance, and bark beetle resource utilization in ponderosa pine forests of northern Arizona. For. Ecol. Manag. 2008, 255, 3263–3269. [Google Scholar] [CrossRef]
- Borkowski, A.; Skrzecz, I. Ecological segregation of bark beetles (Col., Curculionidae, Scolytinae) in Scots pine. Ecol. Res. 2016, 31, 135–144. [Google Scholar] [CrossRef]
- Borkowski, A. Resource partitioning of Scots pine by pine shoot beetles in stands under conditions of stress. Forests 2021, 12, 1336. [Google Scholar] [CrossRef]
- Tinner, W.; Colombaroli, D.; Heiri, O.; Henne, P.D.; Steinacher, M.; Untenecker, J.; Vescovi, E.; Allen, J.R.K.; Carraro, G.; Conedera, M.; et al. The past ecology of Abies alba provides new perspectives on future responses of silver fir forests to global warming. Ecol. Monogr. 2013, 83, 419–439. [Google Scholar] [CrossRef]
- Rüegg, D. Im Glarner Schutzwald alle 60 m eine Weisstanne. Schw. Zeit. Forstw. 2015, 166, 40–41. [Google Scholar] [CrossRef]
- Elling, W.; Dittmar, C.; Pfaffelmoser, K.; Rötzer, T. Dendroecological assessment of the complex causes of decline and recovery of the growth of silver fir (Abies alba Mill.) in Southern Germany. For. Ecol. Manag. 2009, 257, 1175–1187. [Google Scholar] [CrossRef]
- Nagel, T.A.; Mikac, S.; Dolinar, M.; Klopcic, M.; Keren, S.; Svoboda, M.; Diaci, J.; Boncina, A.; Paulic, V. The natural disturbance regime in forests of the Dinaric Mountains: A synthesis of evidence. For. Ecol. Manag. 2017, 388, 29–42. [Google Scholar] [CrossRef]
- Dobrowolska, D.; Bončina, A.; Klumpp, R. Ecology and silviculture of silver fir (Abies alba Mill.): A review. J. For. Res. 2017, 22, 326–335. [Google Scholar] [CrossRef]
- Larsen, J.B. Das Tannensterben: Eine neue Hypothese zur KIärung des Hintergrundes dieser rätselhaften Komplexkrankheit der Weisstanne (Abies alba Mill.). Forstwiss. Centralbl. 1986, 105, 381–396. [Google Scholar] [CrossRef]
- Konnert, M.; Bergmann, F. The geographical distribution of genetic variation of silver fir (Abies alba, Pinaceae) in relation to its migration history. Plant Syst. Evol. 1995, 196, 19–30. [Google Scholar] [CrossRef]
- Sagnard, F.; Barberot, C.; Fady, B. Structure of genetic diversity in Abies alba Mill. from southwestern Alps: Multivariate analysis of adaptive and non-adaptive traits for conservation in France. For. Ecol. Manag. 2002, 157, 175–189. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bottero, A.; Rebetez, M.; Conedera, M.; Augustin, S.; Brang, P.; Tinner, W. What is the potential of silver fir to thrive under warmer and drier climate? Eur. J. For. Res. 2019, 138, 547–560. [Google Scholar] [CrossRef]
- Henne, P.D.; Elkin, C.; Colombaroli, D.; Samartin, S.; Bugmann, H.; Heiri, O.; Tinner, W. Impacts of changing climate and land use on vegetation dynamics in a Mediterranean ecosystem: Insights from paleoecology and dynamic modeling. Landsc. Ecol. 2013, 28, 819–833. [Google Scholar] [CrossRef]
- Ruosch, M.; Spahni, R.; Joos, F.; Henne, P.D.; Knaap, W.O.; Tinner, W. Past and future evolution of Abies alba forests in Europe—Comparison of a dynamic vegetation model with palaeo data and observations. Glob. Chang. Biol. 2016, 22, 727–740. [Google Scholar] [CrossRef]
- Maiorano, L.; Cheddadi, R.; Zimmermann, N.E.; Pellissier, L.; Petitpierre, B.; Pottier, J.; Laborde, H.; Hurdu, B.I.; Pearman, P.B.; Psomas, A.; et al. Building the niche through time: Using 13,000 years of data to predict the effects of climate change on three tree species in Europe. Glob. Ecol. Biogeogr. 2013, 22, 302–317. [Google Scholar] [CrossRef]
- Zimmermann, N.E.; Schmatz, D.; Gallien, L.; Körner, C.; Huber, B.; Frehner, M.; Küchler, M.; Psomas, A. Répartition des espèces forestières et adéquation des stations. In Forêts et Changements Climatiques—Éléments pour des Stratégies D’adaptation; Pluess, A.R., Augustin, S., Brang, P., Eds.; Office Fédéral de L’environnement OFEV: Berne, Switzerland, 2016; pp. 205–228. Available online: https://www.dora.lib4ri.ch/wsl/islandora/object/wsl:10637 (accessed on 25 January 2024).
- Macias, M.; Andreu, L.; Bosch, O.; Camarero, J.J.; Gutiérrez, E. Increasing aridity is enhancing silver fir Abies alba (Mill.) water stress in its South-Western distribution limit. Clim. Chang. 2006, 79, 289–313. [Google Scholar] [CrossRef]
- Rigling, A.; Stähli, M. Erkenntnisse aus der Trockenheit 2018 für die zukünftige Waldentwicklung. Schweiz. Z. Forstwes. 2020, 171, 242–248. Available online: https://www.dora.lib4ri.ch/wsl/islandora/object/wsl%3A24569 (accessed on 22 February 2024). [CrossRef]
- Urban, J. Diagnostics of bark beetles of the genus Pityokteines Fuchs important in forestry. J. For. Sci. 2002, 48, 329–341. [Google Scholar] [CrossRef]
- Serin, M.; Erdem, M.; Yuksel, B.; Akbulut, S. Determination of life cycles of bark beetles damaging efficiently on Fir (Abies bornmülleriana Mattf.) forests and preventive measures against their. Tech. Bul. 2005, 12, 1–84. [Google Scholar]
- Yildiz, O.; Eşen, D.; Akbulut, S. Effects of different ecological and silvicultural factors on beetle catches in the Turkish fir (Abies bornmülleriana Mattf.) ecosystems. J. Pest. Sci. 2007, 80, 145–150. [Google Scholar] [CrossRef]
- Pernek, M.; Lackovic, N. The role of bark beetles in silver fir decline and possible use of pheromone traps for the monitoring. Sum. List 2011, 135, 114–121. Available online: https://hrcak.srce.hr/72319 (accessed on 22 January 2024).
- Durand-Gillmann, M.; Cailleret, M.; Boivin, T.; Nageleisen, L.M.; Davi, H. Individual vulnerability factors of Silver fir (Abies alba Mill.) to parasitism by two contrasting biotic agents: Mistletoe (Viscum album L. ssp abietis) and bark beetles (Coleoptera: Curculionidae: Scolytinae) during a decline process. Ann. For. Sci. 2014, 71, 659–673. [Google Scholar] [CrossRef]
- Justesen, M.J.; Hansen, A.K.; Thomsen, I.M.; Byriel, D.B.; Ro-Poulsen, H.; Ravn, H.P. Contributions to the knowledge on biology and phenology of Cryphalus piceae (Coleoptera: Curculionidae: Scolytinae). Scand. J. For. Res. 2020, 35, 468–475. [Google Scholar] [CrossRef]
- Ukleja-Dobrowolska, D. Zamieranie jodły, wciąż nie wyjaśnione zjawisko. Sylwan 1989, 133, 59–67. [Google Scholar]
- Podlaski, R. Accuracy assessment of a small-area method for estimating the spatial distribution of the degree of tree damage. Environ. Monit. Assess. 2007, 135, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Mauri, A.; de Rigo, D.; Caudullo, G. Abies alba in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Durrant, T.H., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 7–9. Available online: https://www.researchgate.net/publication/299403032 (accessed on 22 January 2024).
- Podlaski, R.; Wojdan, D.; Żelezik, M. A quantitative approach for assessing bark beetle infestations: A study of Pityokteines spinidens Reitt. egg gallery densities in windthrown Abies alba Mill. Ecol. Ind. 2020, 109, 105789. [Google Scholar] [CrossRef]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Bentz, B.J.; Jönsson, A.M. Modeling bark beetle responses to climate. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 533–553. [Google Scholar]
- Biedermann, P.H.W.; Müller, J.; Grégoire, J.C.; Gruppe, A.; Hagge, J.; Hammerbacher, A.; Hofstetter, R.W.; Kandasamy, D.; Kolarik, M.; Kostovcik, M.; et al. Bark Beetle Population Dynamics in the Anthropocene: Challenges and Solutions. Trends Ecol. Evol. 2019, 34, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Olsson, P.O.; Eklundh, L.; Jamali, S.; Ardö, J. Features predisposing forest to bark beetle outbreaks and their dynamics during drought. For. Ecol. Manag. 2022, 523, 120480. [Google Scholar] [CrossRef]
- Thompson, J.N. The Coevolutionary Process, 1st ed.; University of Chicago Press: Chicago, IL, USA, 1994; pp. 1–383. [Google Scholar]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. Bioscience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Kausrud, K.L.; Økland, B.; Skarpaas, O.; Grégoire, J.C. Population dynamics in changing environments: The case of an eruptive forest pest species. Biol. Rev. 2012, 87, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Weed, A.S.; Bentz, B.; Ayres, M.P. Population dynamics of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 157–176. [Google Scholar] [CrossRef]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 40, 1426–1435. [Google Scholar] [CrossRef]
- Berryman, A.A. What causes population cycles of forest Lepidoptera? Trends Ecol. Evol. 1996, 11, 28–32. [Google Scholar] [CrossRef]
- Candau, J.N.; Fleming, R.A. Landscape-scale spatial distribution of spruce budworm defoliation in relation to bioclimatic conditions. Can. J. For. Res. 2005, 35, 2218–2232. [Google Scholar] [CrossRef]
- Friedenberg, N.A.; Sarkar, S.; Kouchoukos, N.; Billings, R.F.; Ayres, M.P. Temperature extremes, density dependence, and southern pine beetle (Coleoptera: Curculionidae) population dynamics in east Texas. Environ. Entomol. 2008, 37, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, B.S.; Raffa, K.F. Evolution of tree killing in bark beetles (Coleoptera: Curculionidae): Trade-offs between the maddening crowds and a sticky situation. Can. Entomol. 2013, 145, 471–495. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Bookwaiter, J.; Davis, T.; Klepzig, K.D. Symbiotic associations of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 209–245. [Google Scholar] [CrossRef]
- Wegensteiner, R.; Wermelinger, B.; Herrmann, M. Natural enemies of bark beetles: Predators, parasitoids, pathogens, and nematodes. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 247–304. Available online: https://www.dora.lib4ri.ch/wsl/islandora/object/wsl:12159 (accessed on 22 February 2024).
- Hlásny, T.; Krokene, P.; Liebhold, A.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.; Schelhaas, M.J.; Seidl, R.; Svoboda, M.; et al. Living with Bark Beetles: Impacts, Outlook and Management Options; From Science to Policy 8; European Forest Institute: Joensuu, Finland, 2019; Volume 33, pp. 1–52. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Ingram, T.; Stutz, W.E.; Snowberg, L.K.; Lau, O.L.; Paull, J.S. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc. R. Soc. B 2010, 277, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; Post, D.M. Does intraspecific competition promote variation? A test via synthesis. Ecol. Evol. 2016, 6, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pereira, R.; Rudolf, V.H.W.; Souza, F.L.; Araújo, M.S. Drivers of individual niche variation in coexisting species. J. Anim. Ecol. 2018, 87, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, J.L.; Szałach, G.; Żarnowiecki, G. Klimat. In Monografia Świętokrzyskiego Parku Narodowego; Cieśliński, S., Kowalkowski, A., Eds.; ŚPN: Bodzentyn, Kraków, Poland, 2000; pp. 129–145. [Google Scholar]
- Forest Data Bank in Poland. Available online: https://www.bdl.lasy.gov.pl (accessed on 29 January 2024).
- Grégoire, J.C.; Evans, H.F. Damage and control of BAWBILT organisms an overview. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.C., Evans, H.F., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 19–39. [Google Scholar]
- Kolk, A.; Starzyk, J.R. The Atlas of Forest Insect Pests, 1st ed.; The Polish Forest Research Institute: Warszawa, Poland, 1996; pp. 1–705. [Google Scholar]
- Anderson, V.L.; McLean, R.A. Design of Experiments: A Realistic Approach, 1st ed.; Taylor & Francis: London, UK, 2019; pp. 1–440. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments, 1st ed.; Princeton University Press: Princeton, NJ, USA, 1968; pp. 1–132. [Google Scholar]
- Hurlbert, S.H. The measurement of niche overlap and some relatives. Ecology 1978, 59, 67–77. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 2013; pp. 1–915. [Google Scholar]
- Morisita, M. Measuring of interspecific association and similarity between communities. Mem. Faculty Sci. Kyushu Univ. Ser. E 1959, 3, 65–80. [Google Scholar]
- Bolnick, D.I.; Yang, L.H.; Fordyce, J.A.; Davis, J.M.; Svanbäck, R. Measuring individual-level resource specialization. Ecology 2002, 83, 2936–2941. Available online: https://climate.copernicus.eu/ (accessed on 15 January 2024). [CrossRef]
- Hutchinson, G.E. Homage to Santa Rosalia, or why are there so many kinds of Animals? Am. Nat. 1959, 93, 145–159. Available online: http://www.jstor.org/stable/2458768 (accessed on 7 January 2024). [CrossRef]
- Chong, E.K.P.; Żak, S.H. An Introduction to Optimization, 2nd ed.; Wiley: New York, NY, USA, 2001; pp. 1–476. [Google Scholar]
- Huang, S.; Yang, Y.; Wang, Y. A critical look at Procedures for Validating Growth and Yield Models. In Modelling Forest Systems; Amaro, A., Reed, D., Soares, P., Eds.; CABI: Oxford, UK, 2003; pp. 271–293. [Google Scholar]
- Temesgen, H.; Gadow, K. Generalized height-diameter models-an application for major tree species in complex stands of interior British Columbia. Eur. J. For. Res. 2004, 123, 45–51. [Google Scholar] [CrossRef]
- White, H.A. Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica 1980, 48, 817–838. [Google Scholar] [CrossRef]
- Borden, J.H. Disruption of semiochemical-mediated aggregation in bark beetles. In Insect Pheromone Research: New Directions; Cardé, R.T., Minks, A.K., Eds.; Springer: Boston, MA, USA, 1997; pp. 421–438. [Google Scholar]
- Sauvard, D. General biology of bark beetles. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.C., Evans, H.F., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 63–89. [Google Scholar] [CrossRef]
- Pffefer, A.; Zumr, V. Communities of Coleoptera on the silver fir (Abies alba). Acta Entomol. Bohemoslov. 1983, 80, 1–9. [Google Scholar]
- Göthlin, E.; Schroeder, L.M.; Lindelöw, Ǻ. Attacks by Ips typographus and Pityogenes chalcographus on windthrown spruces (Picea abies) during the two years following a storm felling. Scand. J. For. Res. 2000, 15, 542–549. [Google Scholar] [CrossRef]
- Komonen, A.; Laatikainen, A.; Similä, M.; Martikainen, P. Ytimennävertäjien kasvainsyönti trombin kaataman suojelumännikön ympäristössä Höytiäisen saaressa Pohjois-Karjalassa. Mets. Aik. 2009, 2, 127–134. [Google Scholar] [CrossRef][Green Version]
- Öhrn, P.; Björklund, N.; Långström, B. Occurrence, performance and shoot damage of Tomicus piniperda in pine stands in southern Sweden after storm-felling. J. Appl. Entomol. 2018, 142, 854–861. [Google Scholar] [CrossRef]
- Zabecki, W. Reakcje kambio—I ksylofagicznych owadów na imisje przemysłowe w drzewostanach jodłowych Ojcowskiego Parku Narodowego. Prądnik 1990, 1, 167–174. [Google Scholar]
- Weslien, J.; Lindelöw, Å. Trapping a local population of spruce bark beetles Ips typographus (L.): Population size and origin of trapped beetles. Hol. Ecol. 1989, 12, 511–514. [Google Scholar] [CrossRef]
- Duelli, P.; Zahradnik, P.; Knižek, M.; Kalinova, B. Migration in spruce bark beetles (Ipstypographus L.) and the efficiency of pheromone traps. J. Appl. Entomol. 1997, 121, 297–303. [Google Scholar] [CrossRef]
- Botterweg, P.F. Dispersal and flight behaviour of the spruce bark beetle Ips typographus in relation to sex, size and fat content. Zeit. Angew. Entomol. 1982, 94, 466–489. [Google Scholar] [CrossRef]
- Nuorteva, M.; Nuorteva, P. The infestation of timber by bark beetles (Col., Scolytidae) and their natural enemies in the Finnish south-western archipelago. Ann. Entomol. Fenn. 1968, 34, 56–65. [Google Scholar]
- Nilssen, A.C. Spatial attack pattern of the bark beetle Tomicus piniperda L. (Col., Scolytidae). Nor. J. Entomol. 1978, 25, 171–175. [Google Scholar]
- Lieutier, F.; Långström, B.; Faccoli, M. The Genus Tomicus. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.C., Evans, H.F., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 371–426. [Google Scholar]
- Podlaski, R.; Borkowski, A. Method for estimating density of Cryphalus piceae (Ratz.) brood galleries using a regression model. J. Appl. Entomol. 2009, 133, 402–409. [Google Scholar] [CrossRef]
- Witrylak, M. Biologia, ekologia i znaczenie gospodarcze wgryzonia jodłowca Cryphalus piceae (Ratz.) (Coleoptera, Scolytidae) w górskich drzewostanach Leśnego Zakładu Doświadczalnego w Krynicy. Sylwan 1995, 139, 51–66. [Google Scholar]
- Price, P.W. Insect Ecology, 3rd ed.; Wiley: New York, NY, USA, 1997; pp. 1–872. [Google Scholar]
- Inbar, M.; Wool, D. Phloem-feeding specialists sharing a host tree: Resource partitioning minimises interference competition among galling aphid species. Oikos 1995, 73, 109–119. [Google Scholar] [CrossRef]
- Szujecki, A. Ecology of Forest Insects, 2nd ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–602. [Google Scholar] [CrossRef]
- Jamin, J.K. Contribution à l’étude du dépérissement provoqué par les coléoptères scolytides sur le pin sylvestre en région centre . Ecol. Nat. Ing. Tech. Eaux For. 1977, 1–84. Available online: https://infodoc.agroparistech.fr/index.php?lvl=notice_display&id=97146 (accessed on 15 January 2024).
- Saarenmaa, H. Modeling the spatial pattern and intraspecific competition in Tomicus piniperda (Coleoptera: Scolytidae). Commun. Inst. For. Fenn. 1983, 118, 1–40. Available online: http://urn.fi/URN:ISBN:951-40-0634-8 (accessed on 30 January 2024).
- Huss, M.; Byström, P.; Persson, L. Resource heterogeneity, diet shifts and intra-cohort competition: Effects on size divergence in YOY Fish. Oecologia 2008, 158, 249–257. Available online: https://www.jstor.org/stable/40309743 (accessed on 23 April 2024). [CrossRef] [PubMed]
- Araújo, M.S.; Bolnick, D.I.; Layman, C.A. The ecological causes of individual specialisation. Ecol. Lett. 2011, 14, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, Y.; Zhu, S.; Li, J.; Li, S.; Li, X. Individual dietary specialization reduces intraspecific competition, rather than feeding activity, in black amur bream Megalobrama terminalis. Sci. Rep. 2020, 10, 17961. [Google Scholar] [CrossRef] [PubMed]
- Raffa, K.F.; Grégoire, J.C.; Lindgren, B.S. Natural history and ecology of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2015; pp. 1–40. [Google Scholar] [CrossRef]
- Podlaski, R.; Borkowski, A. Estimating stem infestation density of Pityokteines curvidens (Germ.) on windfalls: A statistical approach. J. Pest. Sci. 2009, 82, 357–365. [Google Scholar] [CrossRef]
- Öhrn, P. Seasonal Flight Patterns of the Spruce Bark Beetle (Ips typographus) in Sweden. Phenology, Voltinism and Development. Licentiate Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2012. [Google Scholar]
- Lekander, B.; Bejer-Petersen, B.; Kangas, E.; Bakke, A. The distribution of bark beetles in the Nordic countries. Acta Entomol. Fenn. 1977, 32, 252. [Google Scholar]



| No. Equation | Parameters of Regression | Value of Parameter | SE | Value t-Statistics | P | |
|---|---|---|---|---|---|---|
| 4 | a0 | 0.3082 | 0.0286 | 10.7790 | <0.001 | 0.6689 |
| a1 | 0.0005 | <0.001 | 10.9641 | <0.001 | ||
| 5 | a0 | –0.5786 | 0.0830 | –6.9684 | <0.001 | 0.7669 |
| a1 | 0.1939 | 0.0139 | 13.9682 | <0.001 | ||
| 6 | a0 | 0.1028 | 0.0161 | 6.3960 | <0.001 | 0.8065 |
| a1 | 0.0106 | 0.0016 | 6.4416 | <0.001 | ||
| b0 | 0.4274 | 0.0495 | 8.6354 | <0.001 | ||
| b1 | 0.0006 | 0.0002 | 2.6094 | 0.0127 | ||
| 7 | a0 | 0.1126 | 0.0216 | 5.2157 | <0.001 | 0.8432 |
| a1 | 0.0113 | 0.0039 | 2.9022 | 0.0068 | ||
| b0 | 0.2629 | 0.0461 | 5.7043 | <0.001 | ||
| b1 | 0.0025 | 0.0004 | 6.7106 | <0.001 |
| No. Equation | Normal Distribution | Homogeneity of Variances | ||
|---|---|---|---|---|
| Shapiro–Wilk Test | White’s Test | |||
| W | P | W | P | |
| 5 | 0.9795 | 0.4095 | 4.5198 | 0.1044 |
| 6 | 0.9791 | 0.5964 | 1.8190 | 0.4027 |
| 7 | 0.9679 | 0.3890 | 2.9418 | 0.2297 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borkowski, A. The Relationships between the Population Density of Fir Bark Beetles and Niche Breadth. Insects 2024, 15, 422. https://doi.org/10.3390/insects15060422
Borkowski A. The Relationships between the Population Density of Fir Bark Beetles and Niche Breadth. Insects. 2024; 15(6):422. https://doi.org/10.3390/insects15060422
Chicago/Turabian StyleBorkowski, Andrzej. 2024. "The Relationships between the Population Density of Fir Bark Beetles and Niche Breadth" Insects 15, no. 6: 422. https://doi.org/10.3390/insects15060422
APA StyleBorkowski, A. (2024). The Relationships between the Population Density of Fir Bark Beetles and Niche Breadth. Insects, 15(6), 422. https://doi.org/10.3390/insects15060422






