Floral Trait Preferences of Three Common wild Bee Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
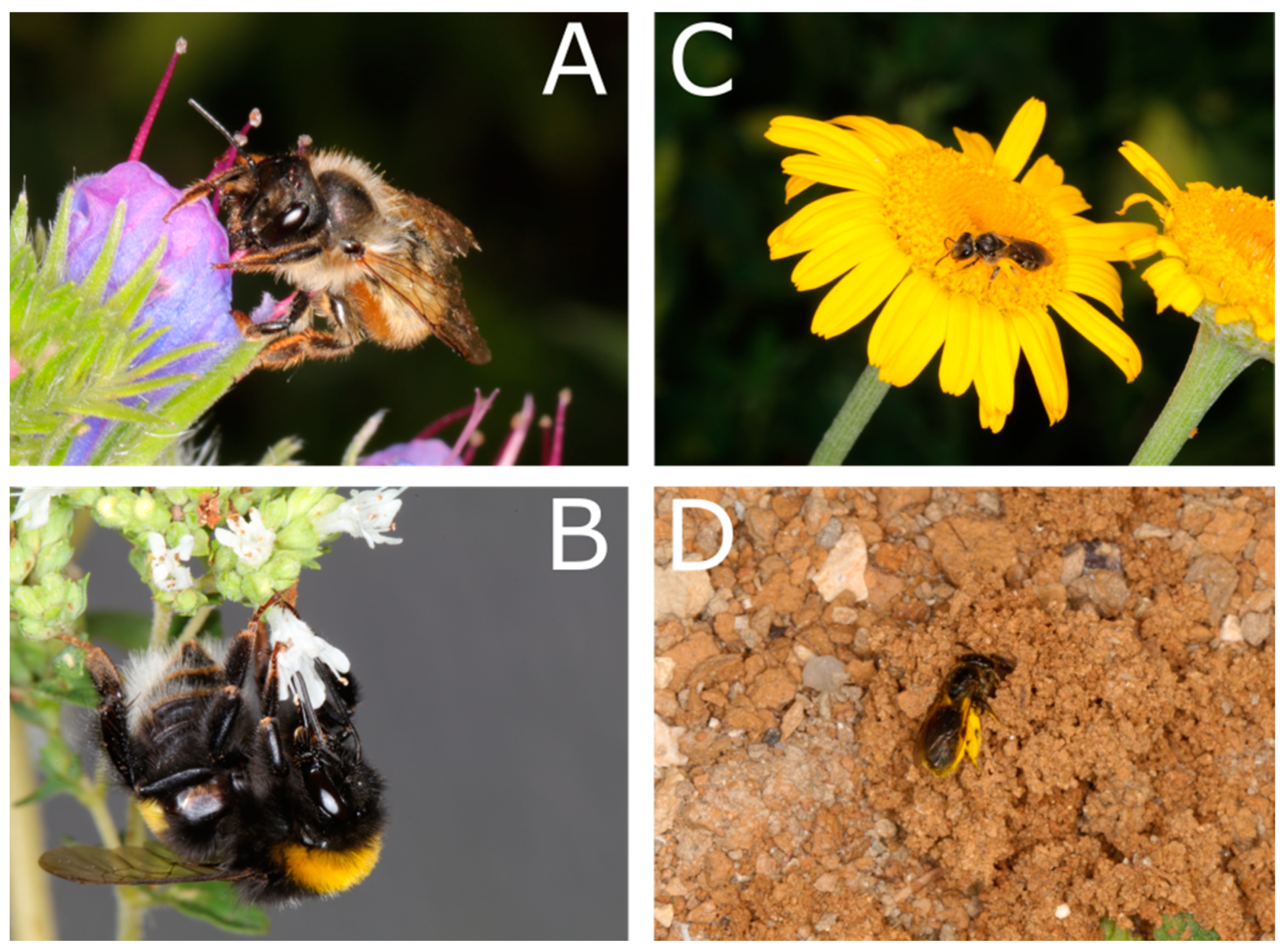
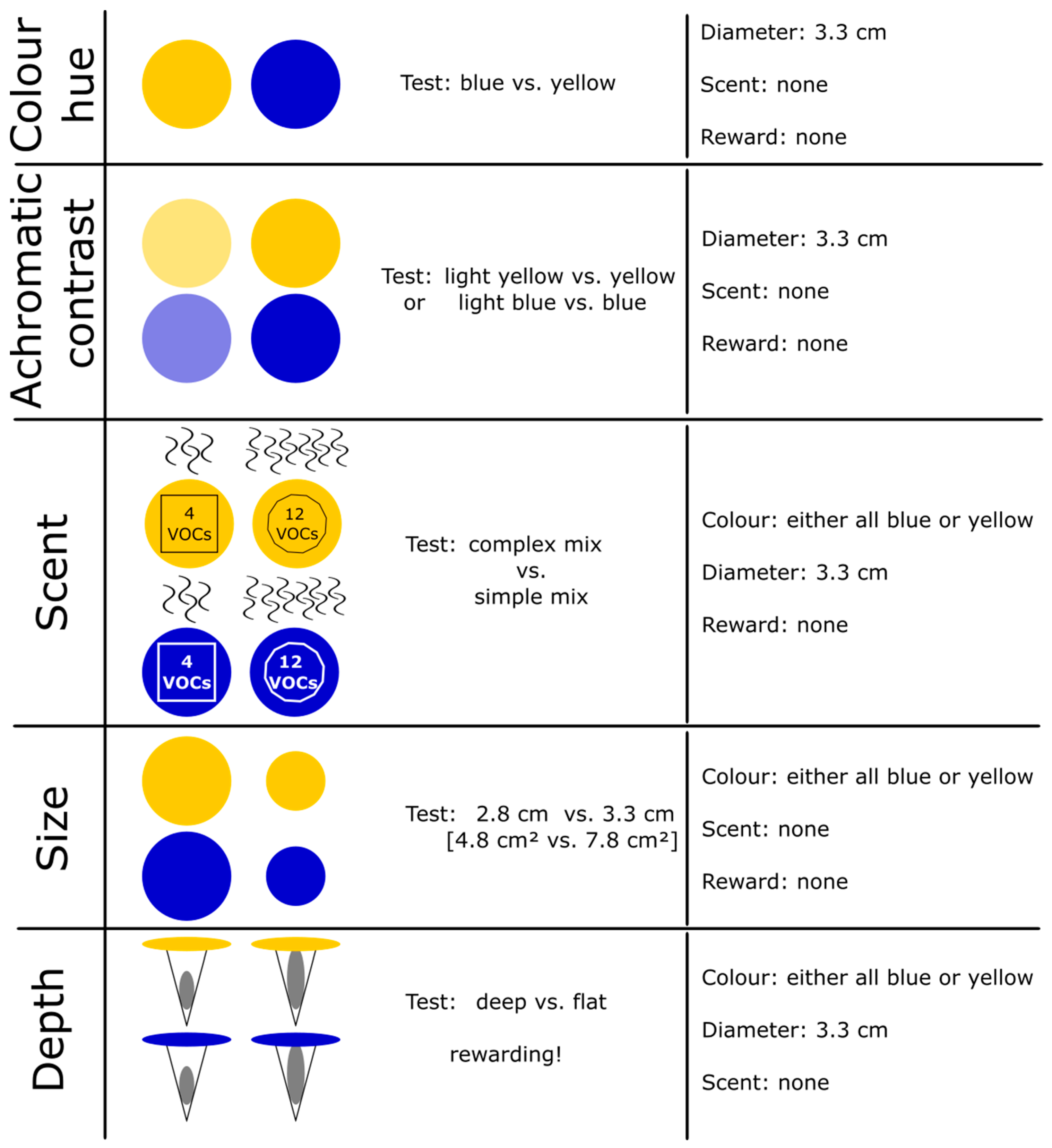
2.1. Behavioral Assays
2.2. Artificial Flowers
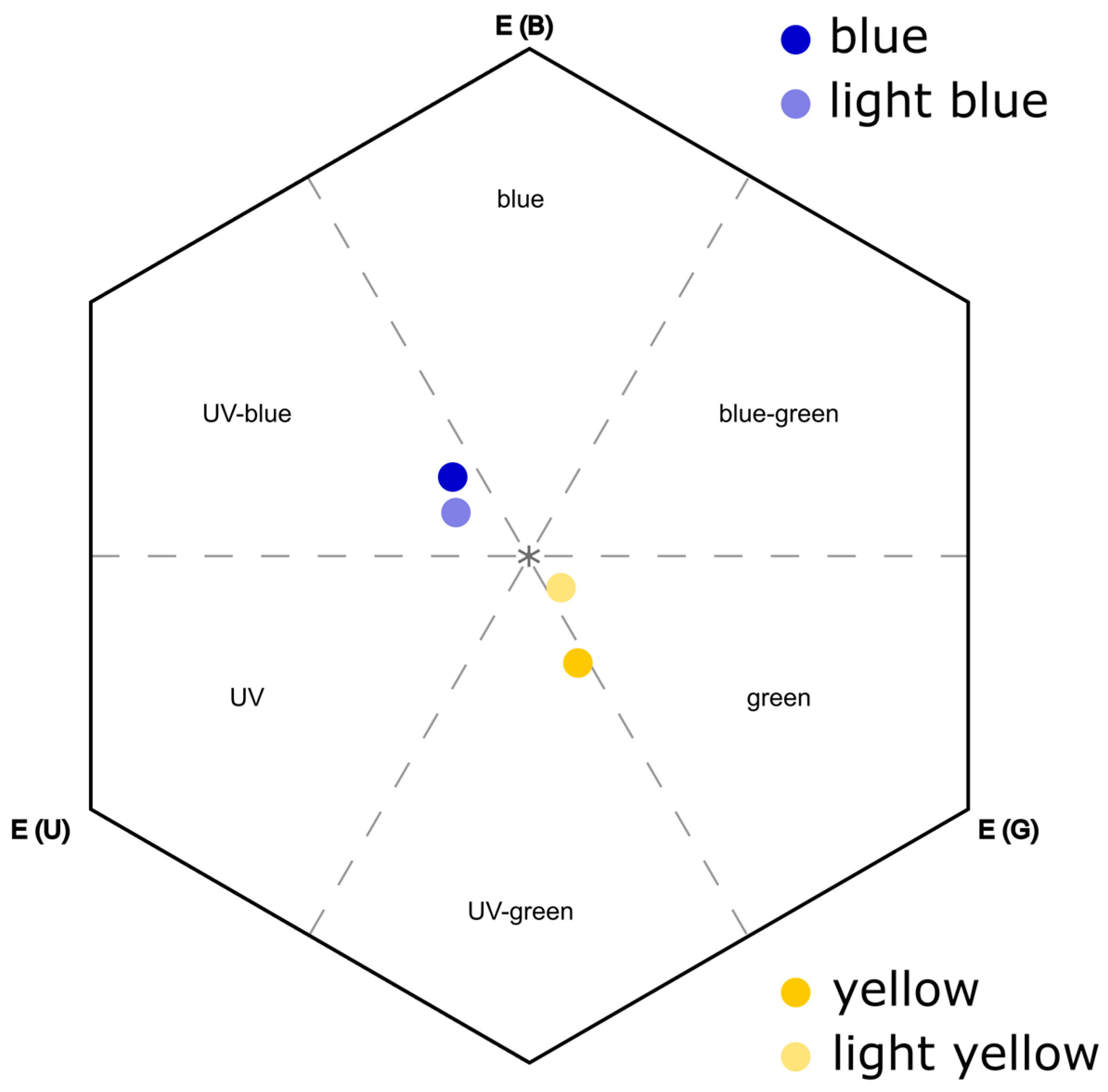
2.3. Color Measurements
2.4. Synthetic Scent Mixtures
2.5. Electrophysiological Experiments
2.6. Floral Depth Experiment with Rewarding Artificial Flowers
2.7. Statistical Analyses
3. Results
3.1. Preferences for Different Color and Size Properties
3.1.1. Cardboard Properties and Model Flower Color
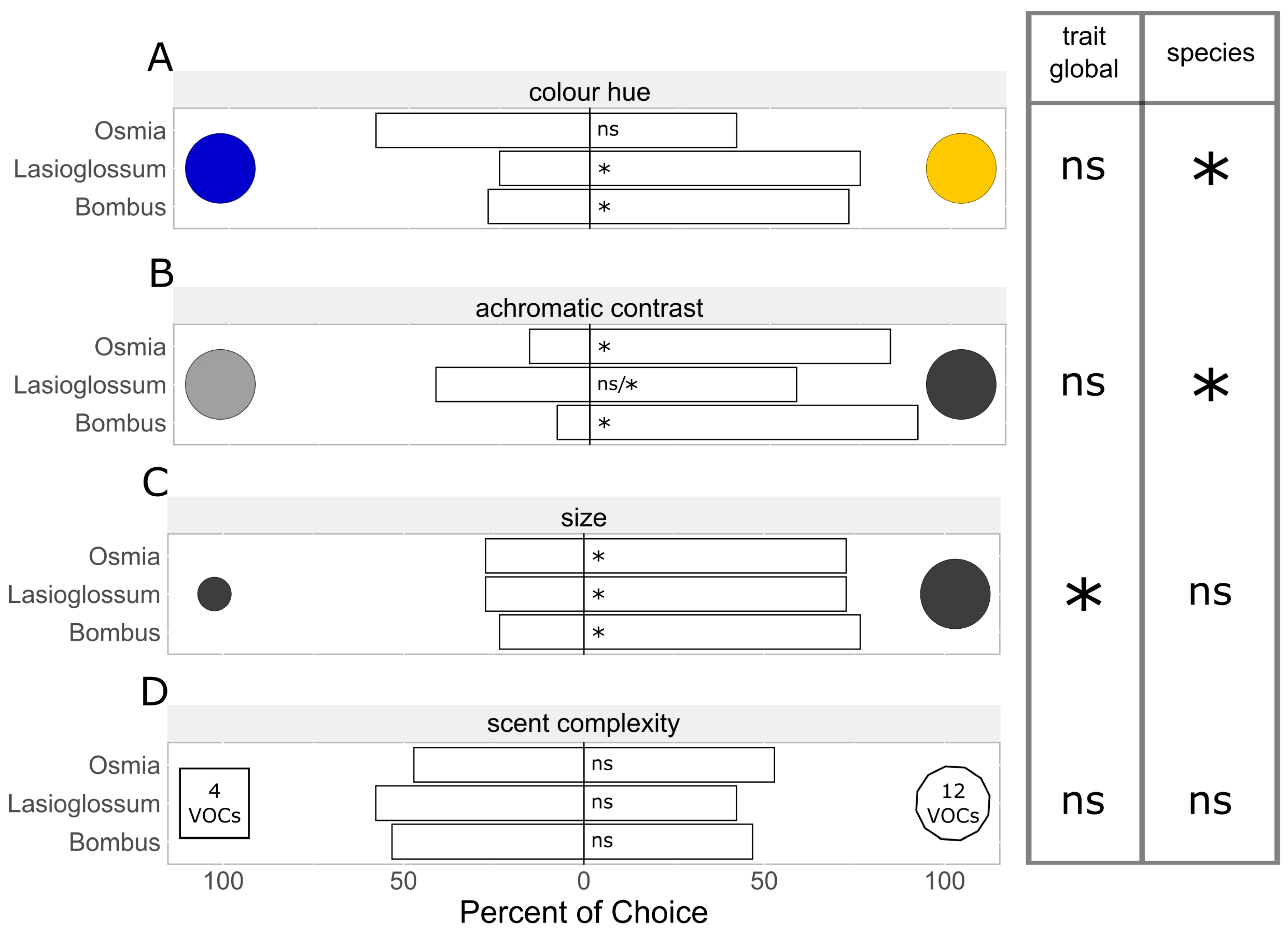
3.1.2. Color Hue Preferences
3.1.3. Achromatic Contrast Preferences
3.1.4. Size Preferences
3.2. Preferences for Scent Complexity and Antennal Responses to Scents
3.2.1. Scent Complexity Preferences
3.2.2. Antennal Responses to Scent Compounds
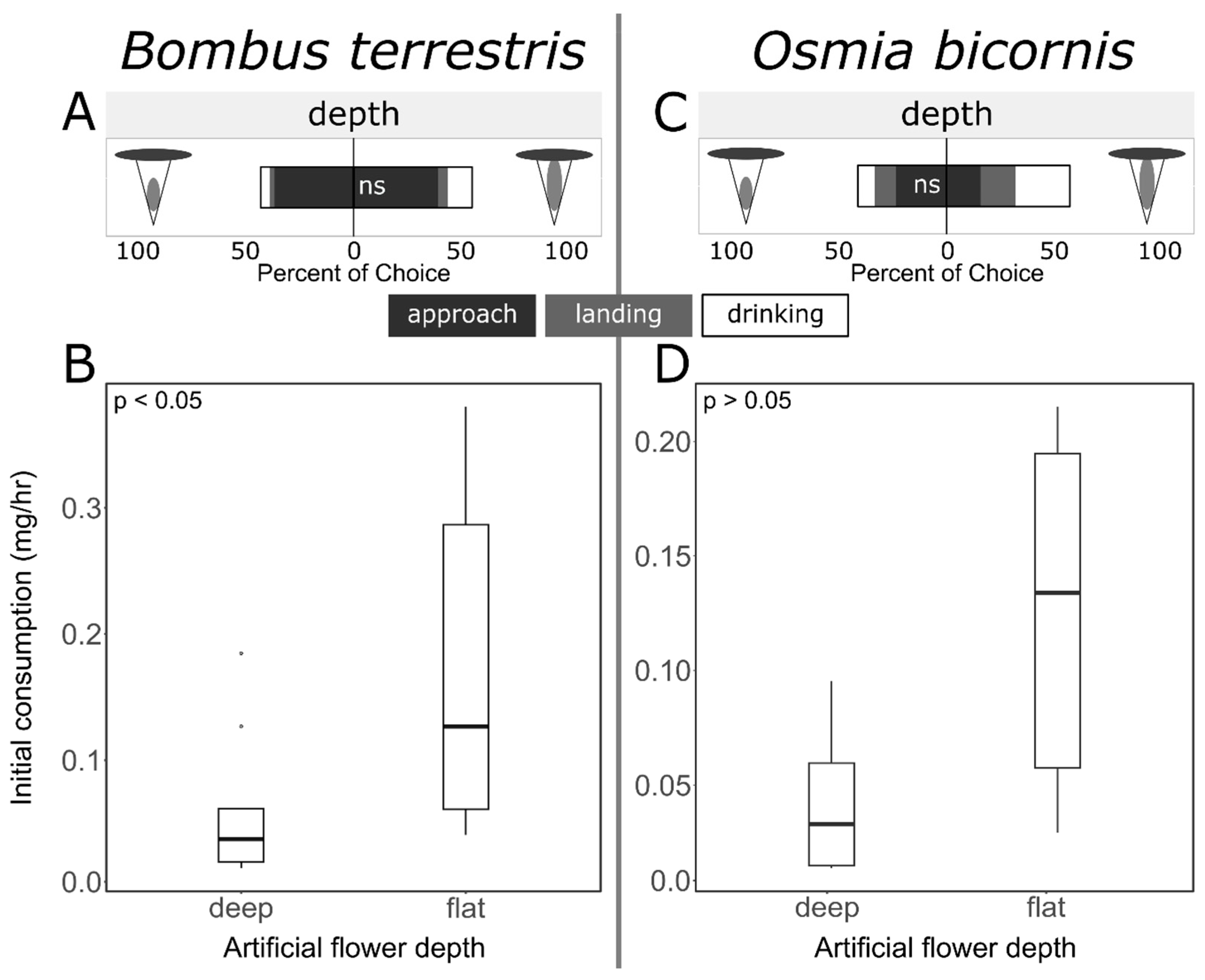
3.3. Flower Depth Preferences
4. Discussion
4.1. Preferences for Color Hue and Achromatic Contrast
4.2. Preferences for Large Corollas
4.3. Responses to Scent Complexity
4.4. Bees Prefer Low-Effort Nectar Exploitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Scheuchl, E.; Schwenninger, H.-R.; Burger, R.; Diestelhorst, O.; Kuhlmann, M.; Saure, C.; Schmid-Egger, C.; Silló, N. Die Wildbienenarten Deutschlands: Kritisches Verzeichnis und aktualisierte Checkliste der Wildbienen Deutschlands (Hymenoptera, Anthophila). Anthophila 2023, 1, 25–138. [Google Scholar]
- van der Kooi, C.J.; Dyer, A.G.; Kevan, P.G.; Lunau, K. Functional significance of the optical properties of flowers for visual signalling. Ann. Bot. 2019, 123, 263–276. [Google Scholar] [CrossRef]
- Müller, A.; Kuhlmann, M. Pollen hosts of western palaearctic bees of the genus Colletes (Hymenoptera: Colletidae): The Asteraceae paradox. Biol. J. Linn. Soc. Lond. 2008, 95, 719–733. [Google Scholar] [CrossRef]
- Gegear, R.J.; Laverty, T.M. Effect of flower complexity on relearning flower-handling skills in bumble bees. Can. J. Zool. 1995, 73, 2052–2058. [Google Scholar] [CrossRef]
- Lawson, D.A.; Chittka, L.; Whitney, H.M.; Rands, S.A. Bumblebees distinguish floral scent patterns, and can transfer these to corresponding visual patterns. Proc. Biol. Sci. 2018, 285, 20180661. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Flick, A.J.; Bauer, A.A. Phenotypic Selection on Flower Color and Floral Display Size by Three Bee Species. Front. Plant. Sci. 2020, 11, 587528. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.A.; Clayton, M.K.; Brunet, J. Floral traits influencing plant attractiveness to three bee species: Consequences for plant reproductive success. Am. J. Bot. 2017, 104, 772–781. [Google Scholar] [CrossRef] [PubMed]
- von Frisch, K. Der Farbensinn und Formensinn der Biene, 1st ed.; Fischer: Jena, Germany, 1914. [Google Scholar]
- Hertz, M. New Experiments on Colour Vision in Bees. J. Exp. Biol. 1939, 16, 1–8. [Google Scholar] [CrossRef]
- Vogel, S. Duftdrüsen im Dienste der Bestäubung: Über Bau und Funktion der Osmophoren; d. Akad. d. Wiss. und Literatur; Verlag: Mainz, Germany, 1963. [Google Scholar]
- Welsford, M.R.; Johnson, S.D. Solitary and social bees as pollinators of Wahlenbergia (Campanulaceae): Single-visit effectiveness, overnight sheltering and responses to flower colour. Arthropod-Plant Interact. 2012, 6, 1–14. [Google Scholar] [CrossRef]
- Knauer, A.C.; Schiestl, F.P. Bees use honest floral signals as indicators of reward when visiting flowers. Ecol. Lett. 2015, 18, 135–143. [Google Scholar] [CrossRef]
- Langridge, K.V.; Wilke, C.; Riabinina, O.; Vorobyev, M.; Hempel de Ibarra, N. Approach Direction Prior to Landing Explains Patterns of Colour Learning in Bees. Front. Physiol. 2021, 12, 697886. [Google Scholar] [CrossRef] [PubMed]
- Dötterl, S.; Schäffler, I. Flower scent of floral oil-producing Lysimachia punctata as attractant for the oil-bee Macropis fulvipes. J. Chem. Ecol. 2007, 33, 441–445. [Google Scholar] [CrossRef]
- Burger, H.; Dötterl, S.; Ayasse, M. Host-plant finding and recognition by visual and olfactory floral cues in an oligolectic bee. Funct. Ecol. 2010, 24, 1234–1240. [Google Scholar] [CrossRef]
- Milet-Pinheiro, P.; Ayasse, M.; Dötterl, S. Visual and Olfactory Floral Cues of Campanula (Campanulaceae) and Their Significance for Host Recognition by an Oligolectic Bee Pollinator. PLoS ONE 2015, 10, e0128577. [Google Scholar] [CrossRef] [PubMed]
- Steitz, I.; Brandt, K.; Biefel, F.; Minat, Ä.; Ayasse, M. Queen Recognition Signals in Two Primitively Eusocial Halictid Bees: Evolutionary Conservation and Caste-Specific Perception. Insects 2019, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Steitz, I.; Ayasse, M. Macrocyclic Lactones Act as a Queen Pheromone in a Primitively Eusocial Sweat Bee. Curr. Biol. 2020, 30, 1136–1141.e3. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.R.; Garcia, J.E.; Dyer, A.G. Comparative psychophysics of colour preferences in two species of non-eusocial Australian native halictid bees. J. Comp. Physiol. A 2021, 207, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.R.; Prendergast, K.; Symonds, M.R.E.; Shrestha, M.; Dyer, A.G. Spontaneous choices for insect-pollinated flower shapes by wild non-eusocial halictid bees. J. Exp. Biol. 2021, 224, jeb242457. [Google Scholar] [CrossRef]
- Howard, S.R.; Symonds, M.R.E. Complex preference relationships between native and non-native angiosperms and foraging insect visitors in a suburban greenspace under field and laboratory conditions. Sci. Nat. 2023, 110, 16. [Google Scholar] [CrossRef]
- Ryder, J.T.; Cherrill, A.; Prew, R.; Shaw, J.; Thorbek, P.; Walters, K.F.A. Impact of enhanced Osmia bicornis (Hymenoptera: Megachilidae) populations on pollination and fruit quality in commercial sweet cherry (Prunus avium L.) orchards. J. Apic. Res. 2020, 59, 77–87. [Google Scholar] [CrossRef]
- Kęsy, M.; Banaszak–Cibicka, W.; Dylewski, Ł.; Fliszkiewicz, M. Effect of Osmia bicornis supplemental pollination on seed yield of forest seed orchards. Apidologie 2023, 54, 32. [Google Scholar] [CrossRef]
- Cordeiro, G.D.; Dötterl, S. Global warming impairs the olfactory floral signaling in strawberry. BMC Plant Biol. 2023, 23, 549. [Google Scholar] [CrossRef] [PubMed]
- Klatt, B.K.; Burmeister, C.; Westphal, C.; Tscharntke, T.; von Fragstein, M. Flower volatiles, crop varieties and bee responses. PLoS ONE 2013, 8, e72724. [Google Scholar] [CrossRef]
- Parachnowitsch, A.L.; Raguso, R.A.; Kessler, A. Phenotypic selection to increase floral scent emission, but not flower size or colour in bee-pollinated Penstemon digitalis. New Phytol. 2012, 195, 667–675. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. Pollination mode determines floral scent. Biochem. Syst. Ecol. 2015, 61, 44–53. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and Distribution of Floral Scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Dötterl, S.; Gershenzon, J. Chemistry, biosynthesis and biology of floral volatiles: Roles in pollination and other functions. Nat. Prod. Rep. 2023, 40, 1901–1937. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.; Dötterl, S.; Francke, W.; Ayasse, M.; Milet-Pinheiro, P. Flower Visitors of Campanula: Are Oligoleges More Sensitive to Host-Specific Floral Scents Than Polyleges? J. Chem. Ecol. 2017, 43, 4–12. [Google Scholar] [CrossRef]
- Burger, H.; Marquardt, M.; Babucke, K.; Heuel, K.C.; Ayasse, M.; Dötterl, S.; Galizia, C.G. Neural and behavioural responses of the pollen-specialist bee Andrena vaga to Salix odours. J. Exp. Biol. 2021, 224, jeb242166. [Google Scholar] [CrossRef]
- Cardé, R.T.; Willis, M.A. Navigational strategies used by insects to find distant, wind-borne sources of odor. J. Chem. Ecol. 2008, 34, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.R.; Parachnowitsch, A.L. Working Towards a Holistic View on Flower Traits—How Floral Scents Mediate Plant–Animal Interactions in Concert with Other Floral Characters. J. Indian Inst. Sci. 2015, 95, 43–68. [Google Scholar]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F.P. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A. Start making scents: The challenge of integrating chemistry into pollination ecology. Entomol. Exp. Appl. 2008, 128, 196–207. [Google Scholar] [CrossRef]
- Kessler, D.; Diezel, C.; Clark, D.G.; Colquhoun, T.A.; Baldwin, I.T. Petunia flowers solve the defence/apparency dilemma of pollinator attraction by deploying complex floral blends. Ecol. Lett. 2013, 16, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.S.; Dornhaus, A.; Papaj, D.R. Flowers help bees cope with uncertainty: Signal detection and the function of floral complexity. J. Exp. Biol. 2011, 214, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Lunau, K.; Maier, E.J. Innate colour preferences of flower visitors. J. Comp. Physiol. A 1995, 177, 1–19. [Google Scholar] [CrossRef]
- Lunau, K. Colour saturation triggers innate reactions to flower signals: Flower dummy experiments with bumblebees. J. Comp. Physiol. A 1990, 166, 827–834. [Google Scholar] [CrossRef]
- Leonard, A.S.; Papaj, D.R. ‘X’ marks the spot: The possible benefits of nectar guides to bees and plants. Funct. Ecol. 2011, 25, 1293–1301. [Google Scholar] [CrossRef]
- Muth, F.; Papaj, D.R.; Leonard, A.S. Colour learning when foraging for nectar and pollen: Bees learn two colours at once. Biol. Lett. 2015, 11, 20150628. [Google Scholar]
- Ne’eman, G.; Ne’eman, R. Factors determining visual detection distance to real flowers by bumble bees. J. Poll. Ecol. 2017, 20, 1–12. [Google Scholar] [CrossRef]
- Stang, M.; Klinkhamer, P.G.L.; Waser, N.M.; Stang, I.; van der Meijden, E. Size-specific interaction patterns and size matching in a plant-pollinator interaction web. Ann. Bot. 2009, 103, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Inouye, D. Species Structure of Bumblebee Communities in North America and Europe. In The Role of Arthropods in Forest Ecosystems; Mattson, W.J., Ed.; Springer: Berlin, Heidelberg, 1977; pp. 35–40. ISBN 978-3-642-88448-1. [Google Scholar]
- Inouye, D.W. The effect of proboscis and corolla tube lengths on patterns and rates of flower visitation by bumblebees. Oecologia 1980, 45, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Harder, L.D. Flower handling efficiency of bumble bees: Morphological aspects of probing time. Oecologia 1983, 57, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Fründ, J.; Linsenmair, K.E.; Blüthgen, N. Pollinator diversity and specialization in relation to flower diversity. Oikos 2010, 119, 1581–1590. [Google Scholar] [CrossRef]
- Kuppler, J.; Neumüller, U.; Mayr, A.V.; Hopfenmüller, S.; Weiß, K.; Prosi, R.; Schanowski, A.; Schwenninger, H.-R.; Ayasse, M.; Burger, H. Favourite plants of wild bees. Agric. Ecosyst. Environ. 2023, 342, 108266. [Google Scholar] [CrossRef]
- Rottler, A.-M.; Schulz, S.; Ayasse, M. Wax Lipids Signal Nest Identity in Bumblebee Colonies. J. Chem. Ecol. 2013, 39, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Wilms, J.; Eltz, T. Foraging scent marks of bumblebees: Footprint cues rather than pheromone signals. Sci. Nat. 2008, 95, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Peitsch, D.; Fietz, A.; Hertel, H.; de Souza, J.; Ventura, D.F.; Menzel, R. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A 1992, 170, 23–40. [Google Scholar] [CrossRef]
- Chittka, L. The colour hexagon: A chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. J. Comp. Physiol. A 1992, 170, 533–543. [Google Scholar] [CrossRef]
- Menzel, R.; Steinmann, E.; de Souza, J.; Backhaus, W. Spectral Sensitivity of Photoreceptors and Colour Vision in the Solitary Bee, Osmia rufa. J. Exp. Biol. 1988, 136, 35–52. [Google Scholar] [CrossRef]
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data, and Formulae, Wiley classics library ed.; John Wiley & Sons: New York, NY, USA, 2000; ISBN 9780471399186. [Google Scholar]
- The R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Bryer, J.; Speerschneider, K. Package ‘Likert’: Analysis and Visualization Likert Items. 2016. Available online: http://github.com/jbryer/likert (accessed on 4 February 2023).
- Splitt, A.; Schulz, M.; Skórka, P. Current state of knowledge on the biology and breeding of the solitary bee—Osmia bicornis. J. Apic. Res. 2022, 61, 163–179. [Google Scholar] [CrossRef]
- Nicholls, E.; de Ibarra, N.H. Bees associate colour cues with differences in pollen rewards. J. Exp. Biol. 2014, 217, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Balfour, N.J.; Ratnieks, F.L.W. Why Petals? Naïve, but Not Experienced Bees, Preferentially Visit Flowers with Larger Visual Signals. Insects 2023, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.P.; Sullivan, M.S.; Poppy, G.M. The influence of floral character on the foraging behaviour of the hoverfly, Episyrphus balteatus. Entomol. Exp. Appl. 1999, 93, 157–164. [Google Scholar] [CrossRef]
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Koethe, S.; Bossems, J.; Dyer, A.G.; Lunau, K. Colour is more than hue: Preferences for compiled colour traits in the stingless bees Melipona mondury and M. quadrifasciata. J. Comp. Physiol. A 2016, 202, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Hempel de Ibarra, N.; Vorobyev, M. Flower patterns are adapted for detection by bees. J. Comp. Physiol. A 2009, 195, 319–323. [Google Scholar] [CrossRef]
- Kantsa, A.; Raguso, R.A.; Dyer, A.G.; Olesen, J.M.; Tscheulin, T.; Petanidou, T. Disentangling the role of floral sensory stimuli in pollination networks. Nat. Commun. 2018, 9, 1041. [Google Scholar] [CrossRef]
- Westrich, P. Die Wildbienen Deutschlands; Verlag Eugen Ulmer: Stuttgart, Germany, 2019. [Google Scholar]
- Della Bennet, G.; Kelly, D.; Clemens, J. Food plants and foraging distances for the native bee Lasioglossum sordidum in Christchurch Botanic Gardens. N. Z. J. Ecol. 2018, 42, 40–47. [Google Scholar]
- Dötterl, S.; Milchreit, K.; Schäffler, I. Behavioural plasticity and sex differences in host finding of a specialized bee species. J. Comp. Physiol. A 2011, 197, 1119–1126. [Google Scholar] [CrossRef]
- Müller, H. Alpenblumen, ihre Befruchtung durch Insekten und ihre Anpassungen an dieselben; W. Engelmann: Leipzig, Germany, 1881. [Google Scholar]
- Goulson, D.; Cruise, J.L.; Sparrow, K.R.; Harris, A.J.; Park, K.J.; Tinsley, M.C.; Gilburn, A.S. Choosing rewarding flowers; perceptual limitations and innate preferences influence decision making in bumblebees and honeybees. Behav. Ecol. Sociobiol. 2007, 61, 1523–1529. [Google Scholar] [CrossRef]
- Rohde, K.; Papiorek, S.; Lunau, K. Bumblebees (Bombus terrestris) and honeybees (Apis mellifera) prefer similar colours of higher spectral purity over trained colours. J. Comp. Physiol. A 2013, 199, 197–210. [Google Scholar] [CrossRef]
- Giurfa, M.; Vorobyev, M.; Kevan, P.; Menzel, R. Detection of coloured stimuli by honeybees: Minimum visual angles and receptor specific contrasts. J. Comp. Physiol. A 1996, 178, 699–709. [Google Scholar] [CrossRef]
- Ashman, T.-L.; Stanton, M. Seasonal Variation in Pollination Dynamics of Sexually Dimorphic Sidalcea Oregana SSP. Spicata (Malvaceae). Ecology 1991, 72, 993–1003. [Google Scholar] [CrossRef]
- Rowe, L.; Gibson, D.; Bahlai, C.A.; Gibbs, J.; Landis, D.A.; Isaacs, R. Flower traits associated with the visitation patterns of bees. Oecologia 2020, 193, 511–522. [Google Scholar] [CrossRef]
- Blarer, A.; Keasar, T.; Shmida, A. Possible Mechanisms for the Formation of Flower Size Preferences by Foraging Bumblebees. Ethology 2002, 108, 341–351. [Google Scholar] [CrossRef]
- Kessler, D.; Gase, K.; Baldwin, I.T. Field experiments with transformed plants reveal the sense of floral scents. Science 2008, 321, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Bromenshenk, J.; Henderson, C.; Seccomb, R.; Rice, S.; Etter, R.; Bender, S.; Rodacy, P.; Shaw, J.; Seldomridge, N.; Spangler, L.; et al. Can Honey Bees Assist in Area Reduction and Landmine Detection? J. Conv. Weapons Destr. 2003, 7, 5. [Google Scholar]
- Dobson, H.E.M.; Bergström, G. The ecology and evolution of pollen odors. Plant Syst. Evol. 2000, 222, 63–87. [Google Scholar] [CrossRef]
- Dobson, H.E.M.; Groth, I.; Bergström, G. Pollen advertisement: Chemical contrasts between whole-flower and pollen odors. Am. J. Bot. 1996, 83, 877–885. [Google Scholar] [CrossRef]
- Dobson, H.E.M.; Ayasse, M.; O’Neal, K.A.; Jacka, J.A. Is flower selection influenced by chemical imprinting to larval food provisions in the generalist bee Osmia bicornis (Megachilidae)? Apidologie 2012, 43, 698–714. [Google Scholar] [CrossRef]
- Wright, G.A.; Schiestl, F.P. The evolution of floral scent: The influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct. Ecol. 2009, 23, 841–851. [Google Scholar] [CrossRef]
- Knauer, A.C.; Kokko, H.; Schiestl, F.P. Pollinator behaviour and resource limitation maintain honest floral signalling. Funct. Ecol. 2021, 35, 2536–2549. [Google Scholar] [CrossRef]
- Pyke, G.H.; Starr, C.K. Optimal Foraging Theory. In Encyclopedia of Social Insects; Springer International Publishing: Cham, Germany, 2021; pp. 677–685. [Google Scholar]
- Howell, A.D.; Alarcón, R. Osmia bees (Hymenoptera: Megachilidae) can detect nectar-rewarding flowers using olfactory cues. Anim. Behav. 2007, 74, 199–205. [Google Scholar] [CrossRef]
- Burger, H.; Dötterl, S.; Häberlein, C.M.; Schulz, S.; Ayasse, M. An arthropod deterrent attracts specialised bees to their host plants. Oecologia 2012, 168, 727–736. [Google Scholar] [CrossRef]
- Cariveau, D.P.; Nayak, G.K.; Bartomeus, I.; Zientek, J.; Ascher, J.S.; Gibbs, J.; Winfree, R. The Allometry of Bee Proboscis Length and Its Uses in Ecology. PLoS ONE 2016, 11, e0151482. [Google Scholar] [CrossRef] [PubMed]
- Westerkamp, C.; Classen-Bockhoff, R. Bilabiate flowers: The ultimate response to bees? Ann. Bot. 2007, 100, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Stöbbe, J.; Schramme, J.; Claßen-Bockhoff, R. Training experiments with Bombus terrestris and Apis mellifera on artificial ‘Salvia’ flowers. Flora 2016, 221, 92–99. [Google Scholar] [CrossRef]
- Herrel, A. Ecology and Biomechanics: A Mechanical Approach to the Ecology of Animals and Plants; CRC Press: Hoboken, NJ, USA, 2006; ISBN 9781420001594. [Google Scholar]
- Inouye, D.W. Resource Partitioning in Bumblebees: Experimental Studies of Foraging Behavior. Ecology 1978, 59, 672–678. [Google Scholar] [CrossRef]
- Venjakob, C.; Ruedenauer, F.A.; Klein, A.-M.; Leonhardt, S.D. Variation in nectar quality across 34 grassland plant species. Plant Biol. 2022, 24, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.S. The ecological significance of toxic nectar. Oikos 2000, 91, 409–420. [Google Scholar] [CrossRef]
- Vanderplanck, M.; Gilles, H.; Nonclercq, D.; Duez, P.; Gerbaux, P. Asteraceae Paradox: Chemical and Mechanical Protection of Taraxacum Pollen. Insects 2020, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.; Buttala, S.; Koch, H.; Ayasse, M.; Johnson, S.D.; Stevenson, P.C. Nectar cardenolides and floral volatiles mediate a specialized wasp pollination system. J. Exp. Biol. 2024, 227, jeb246156. [Google Scholar] [CrossRef] [PubMed]
| Test | Species | Data | Preferred | Estimated | Std. Error | z-Value | p-Value |
|---|---|---|---|---|---|---|---|
| hue | L. villosulum | all | yellow | 1.099 | 0.320 | 3.430 | <0.001 |
| B. terrestris | all | yellow | 0.937 | 0.155 | 6.047 | <0.001 | |
| O. bicornis | all | no preference | −0.375 | 0.392 | −0.957 | 0.339 | |
| size | L. villosulum | all | large | −0.981 | 0.229 | −2.898 | 0.004 |
| B. terrestris | all | large | −1.187 | 0.269 | −4.409 | <0.001 | |
| O. bicornis | all | large | −0.833 | 0.379 | −2.199 | 0.028 | |
| achromatic contrast | O. bicornis | all | high contrast | −1.609 | 0.447 | −3.599 | <0.001 |
| B. terrestris | all | high contrast | −2.311 | 0.303 | −7.635 | <0.001 | |
| L. villosulum | all | non | −0.296 | 0.245 | −1.208 | 0.227 | |
| yellow vs. light yellow | high contrast | −0.981 | 0.391 | −2.509 | 0.0121 | ||
| scent | O. bicornis | all | non | −0.114 | 0.144 | −0.791 | 0.429 |
| B. terrestris | all | non | 0.128 | 0.099 | 1.289 | 0.197 | |
| L. villosulum | all | non | 0.310 | 0.281 | 1.105 | 0.269 |
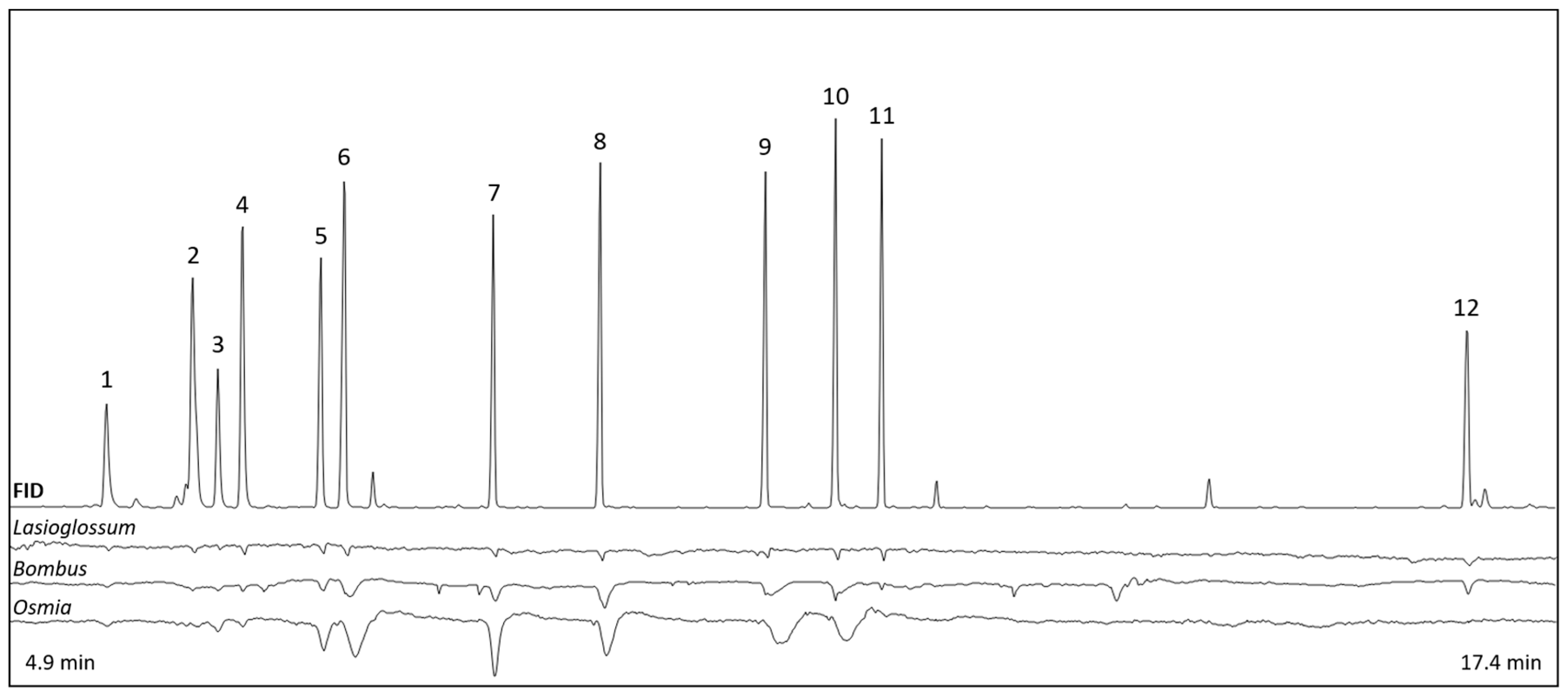
| No. # | Substance | RI | Substance Class | Mix | Electrophysiological Responses | ||
|---|---|---|---|---|---|---|---|
| O. bicornis (n = 10) | B. terrestris (n = 10) | L. villosulum (n = 6) | |||||
| 1 | β-myrcene | 986 | monoterpene | 3 | 80% | 80% | 66% |
| 2 | eucalyptol | 1032 | monoterpene | 2 | 70% | 40% | 100% |
| 3 | (E)-β-ocimene | 1045 | monoterpene | 1 | 100% | 100% | 100% |
| 4 | γ-terpinene | 1058 | monoterpene | 2 | 100% | 90% | 100% |
| 5 | β-linalool | 1099 | monoterpene | 1 | 100% | 100% | 100% |
| 6 | 2-phenylethanol | 1112 | benzenoid | 2 | 100% | 100% | 100% |
| 7 | methyl salicylate | 1194 | benzenoid | 3 | 100% | 100% | 100% |
| 8 | 2-phenethyl acetate | 1255 | benzenoid | 1 | 100% | 100% | 100% |
| 9 | eugenol | 1353 | benzenoid | 3 | 100% | 100% | 100% |
| 10 | benzyl isovalerate | 1396 | benzenoid | 1 | 100% | 90% | 100% |
| 11 | β-caryophyllene | 1426 | sesquiterpene | 3 | 60% | 80% | 100% |
| 12 | hexahydro farnesyl acetone | 1840 | sesquiterpene | 2 | 50% | 90% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heuel, K.C.; Haßlberger, T.A.; Ayasse, M.; Burger, H. Floral Trait Preferences of Three Common wild Bee Species. Insects 2024, 15, 427. https://doi.org/10.3390/insects15060427
Heuel KC, Haßlberger TA, Ayasse M, Burger H. Floral Trait Preferences of Three Common wild Bee Species. Insects. 2024; 15(6):427. https://doi.org/10.3390/insects15060427
Chicago/Turabian StyleHeuel, Kim C., Tim A. Haßlberger, Manfred Ayasse, and Hannah Burger. 2024. "Floral Trait Preferences of Three Common wild Bee Species" Insects 15, no. 6: 427. https://doi.org/10.3390/insects15060427





