Binding Analysis of Sf-SR-C MAM Domain and Sf-FGFR Ectodomain to Vip3Aa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Expression and Purification
2.2. Crystallization of the Sf-MAM Domain
2.3. Data Collection and Structure Determination
2.4. Structure Docking of Sf-MAM Domain to the Vip3Aa CTD
2.5. Surface Plasmon Resonance Spectroscopy
3. Results
3.1. Protein Purification
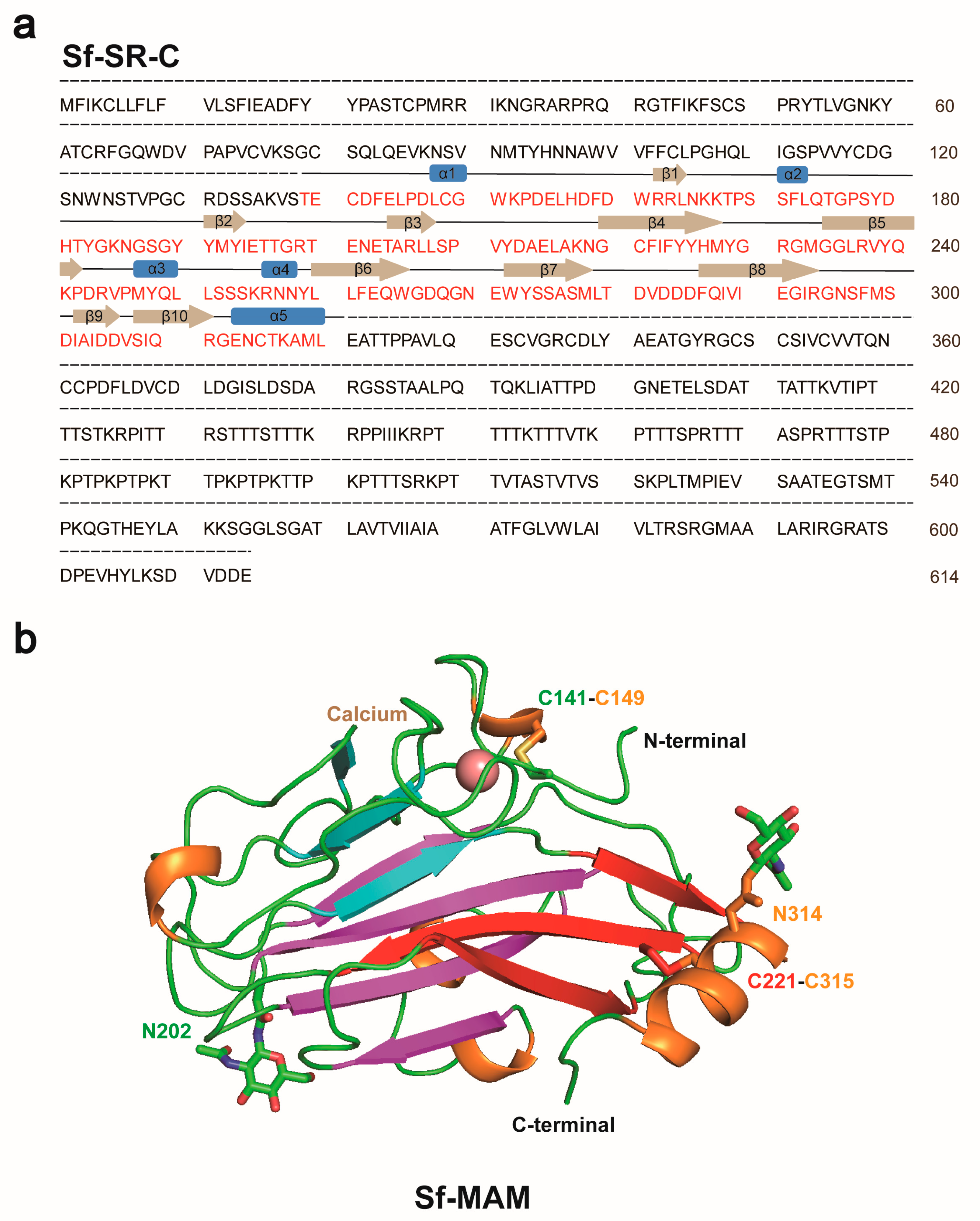
3.2. The Structure of Sf MAM Domain
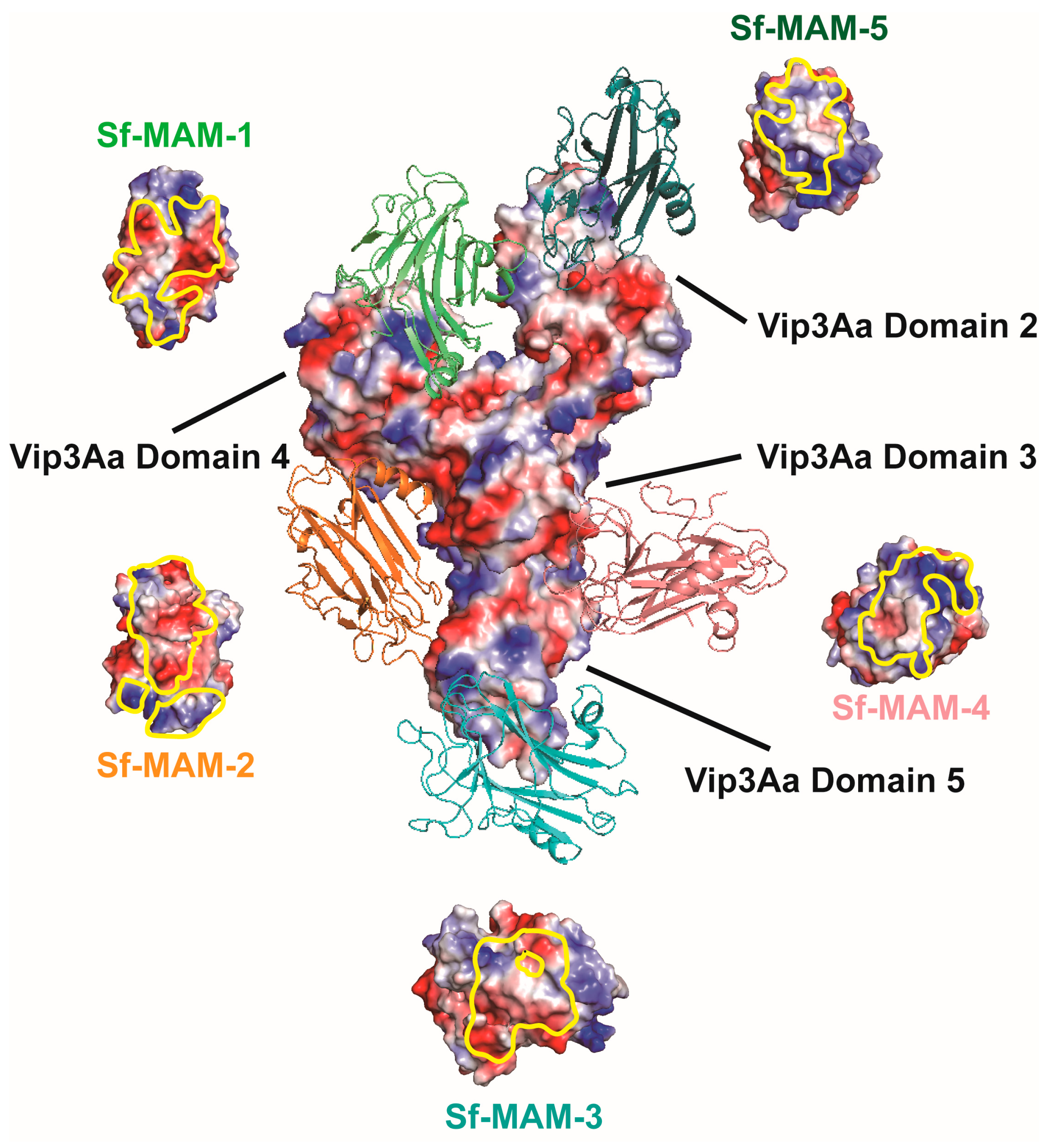
3.3. Structure Docking of the Sf-MAM Domain to the Vip3Aa CTD
3.4. In Vitro Binding of Vip3Aa to Sf-MAM and Sf-FGFR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakroun, M.; Banyuls, N.; Bel, Y.; Escriche, B.; Ferré, J. Bacterial Vegetative Insecticidal Proteins (Vip) from Entomopathogenic Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 329–350. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, H.; Kaur, S. Vegetative Insecticidal Protein (Vip): A Potential Contender from Bacillus thuringiensis for Efficient Management of Various Detrimental Agricultural Pests. Front. Microbiol. 2021, 12, 659736. [Google Scholar] [CrossRef]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A Structure-Based Nomenclature for Bacillus thuringiensis and Other Bacteria-Derived Pesticidal Proteins. J. Invertebr. Pathol. 2021, 186, 107438. [Google Scholar] [CrossRef]
- Geng, J.; Jiang, J.; Shu, C.; Wang, Z.; Song, F.; Geng, L.; Duan, J.; Zhang, J. Bacillus thuringiensis Vip1 Functions as a Receptor of Vip2 Toxin for Binary Insecticidal Activity against Holotrichia parallela. Toxins 2019, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Mei, S.Q.; Wang, T.T.; Pan, J.H.; Chen, Y.H.; Cai, J. Vip3Aa Induces Apoptosis in Cultured Spodoptera frugiperda (Sf9) Cells. Toxicon 2016, 120, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Han, L.; An, B.; Zhang, Y.; Cao, Z.; Zhan, Y.; Cai, X.; Yan, B.; Cai, J. Mitochondria and Lysosomes Participate in Vip3Aa-Induced Spodoptera frugiperda Sf9 Cell Apoptosis. Toxins 2020, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Din, S.U.; Azam, S.; Rao, A.Q.; Shad, M.; Ahmed, M.; Gul, A.; Latif, A.; Ali, M.A.; Husnain, T.; Shahid, A.A. Development of Broad-Spectrum and Sustainable Resistance in Cotton against Major Insects through the Combination of Bt and Plant Lectin Genes. Plant Cell Rep. 2021, 40, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, L.; Zhou, Z.; Pacheco, S.; Gómez, I.; Song, F.; Soberón, M.; Zhang, J.; Bravo, A. Specific Binding between Bacillus thuringiensis Cry9Aa and Vip3Aa Toxins Synergizes Their Toxicity against Asiatic Rice Borer (Chilo suppressalis). J. Biol. Chem. 2018, 293, 11447–11458. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Dai, H.; Jin, Z.; Shen, H.; Guan, F.; Yang, Y.; Tabashnik, B.E.; Wu, Y. Evaluating Cross-Resistance to Cry and Vip Toxins in Four Strains of Helicoverpa armigera with Different Genetic Mechanisms of Resistance to Bt Toxin Cry1Ac. Front. Microbiol. 2021, 12, 670402. [Google Scholar] [CrossRef]
- Núñez-Ramírez, R.; Huesa, J.; Bel, Y.; Ferré, J.; Casino, P.; Arias-Palomo, E. Molecular Architecture and Activation of the Insecticidal Protein Vip3Aa from Bacillus thuringiensis. Nat. Commun. 2020, 11, 3974. [Google Scholar] [CrossRef]
- Byrne, M.J.; Iadanza, M.G.; Perez, M.A.; Maskell, D.P.; George, R.M.; Hesketh, E.L.; Beales, P.A.; Zack, M.D.; Berry, C.; Thompson, R.F. Cryo-EM Structures of an Insecticidal Bt Toxin Reveal Its Mechanism of Action on the Membrane. Nat. Commun. 2021, 12, 2791. [Google Scholar] [CrossRef]
- Jiang, K.; Chen, Z.; Zang, Y.; Shi, Y.; Shang, C.; Jiao, X.; Cai, J.; Gao, X. Functional Characterization of Vip3Aa from Bacillus thuringiensis Reveals the Contributions of Specific Domains to Its Insecticidal Activity. J. Biol. Chem. 2023, 299, 103000. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Li, M.; Mao, C.; Jiang, L.; Zhang, W.; Li, M.; Geng, X.; Li, X.; Liu, S.; Yang, G.; et al. Domain III β4-β5 Loop and β14-β15 Loop of Bacillus thuringiensis Vip3Aa Are Involved in Receptor Binding and Toxicity. Toxins 2024, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Lázaro-Berenguer, M.; Paredes-Martínez, F.; Bel, Y.; Núñez-Ramírez, R.; Arias-Palomo, E.; Casino, P.; Ferré, J. Structural and Functional Role of Domain I for the Insecticidal Activity of the Vip3Aa Protein from Bacillus thuringiensis. Microb. Biotechnol. 2022, 15, 2607–2618. [Google Scholar] [CrossRef]
- Jiang, K.; Hou, X.Y.; Tan, T.T.; Cao, Z.L.; Mei, S.Q.; Yan, B.; Chang, J.; Han, L.; Zhao, D.; Cai, J. Scavenger Receptor-C Acts as a Receptor for Bacillus thuringiensis Vegetative Insecticidal Protein Vip3Aa and Mediates the Internalization of Vip3Aa via Endocytosis. PLoS Pathog. 2018, 14, e1007347. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Hou, X.; Han, L.; Tan, T.; Cao, Z.; Cai, J. Fibroblast Growth Factor Receptor, a Novel Receptor for Vegetative Insecticidal Protein Vip3Aa. Toxins 2018, 10, 546. [Google Scholar] [CrossRef]
- Shan, Y.; Jin, M.; Chakrabarty, S.; Yang, B.; Li, Q.; Cheng, Y.; Zhang, L.; Xiao, Y. Sf-FGFR and Sf-SR-C Are Not the Receptors for Vip3Aa to Exert Insecticidal Toxicity in Spodoptera frugiperda. Insects 2022, 13, 547. [Google Scholar] [CrossRef]
- Minor, W.; Cymborowski, M.; Otwinowski, Z.; Chruszcz, M. HKL-3000: The Integration of Data Reduction and Structure Solution—From Diffraction Images to an Initial model in Minutes. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 62 Pt 8, 859–866. [Google Scholar] [CrossRef]
- Adams, P.D.; Grosse-Kunstleve, R.W.; Hung, L.W.; Ioerger, T.R.; McCoy, A.J.; Moriarty, N.W.; Read, R.J.; Sacchettini, J.C.; Sauter, N.K.; Terwilliger, T.C. PHENIX: Building New Software for Automated Crystallographic Structure Determination. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002, 58 Pt 11, 1948–1954. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-Building Tools for Molecular Graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60 Pt 12, 2126–2132. [Google Scholar] [CrossRef]
- Janson, G.; Zhang, C.; Prado, M.G.; Paiardini, A. PyMod 2.0: Improvements in Protein Sequence-Structure Analysis and Homology Modeling within PyMOL. Bioinformatics 2017, 33, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.H.; Vreven, T.; Weng, Z. ZDOCK Server: Interactive Docking Prediction of Protein-Protein Complexes and Symmetric Multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zhang, Y.; Chen, Z.; Wu, D.; Cai, J.; Gao, X. Structural and Functional Insights into the C-terminal Fragment of Insecticidal Vip3A Toxin of Bacillus thuringiensis. Toxins 2020, 12, 438. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, Z.; Kerns, D.L. Resistance of Spodoptera frugiperda to Cry1, Cry2, and Vip3Aa Proteins in Bt Corn and Cotton in the Americas: Implications for the Rest of the World. J. Econ. Entomol. 2022, 115, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Dively, G.P.; Kuhar, T.P.; Taylor, S.V.; Doughty, H.; Holmstrom, K.; Gilrein, D.O.; Nault, B.A.; Ingerson-Mahar, J.; Huseth, A.; Reisig, D.; et al. Extended Sentinel Monitoring of Helicoverpa Zea Resistance to Cry and Vip3Aa Toxins in Bt Sweet Corn: Assessing Changes in Phenotypic and Allele Frequencies of Resistance. Insects 2023, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Guo, C.; Fisher, P.B.; Subjeck, J.R.; Wang, X.Y. Scavenger Receptors: Emerging Roles in Cancer Biology and Immunology. Adv. Cancer Res. 2015, 128, 309–364. [Google Scholar] [CrossRef] [PubMed]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger Receptors in Homeostasis and Immunity. Nat. Rev. Immunol. 2013, 13, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Fierro, N.A.; Rivera-Toledo, E.; Ávila-Horta, F.; Anaya-Covarrubias, J.Y.; Mendlovic, F. Scavenger Receptors in the Pathogenesis of Viral Infections. Viral Immunol. 2022, 35, 175–191. [Google Scholar] [CrossRef]
- Zani, I.A.; Stephen, S.L.; Mughal, N.A.; Russell, D.; Homer-Vanniasinkam, S.; Wheatcroft, S.B.; Ponnambalam, S. Scavenger Receptor Structure and Function in Health and Disease. Cells 2015, 4, 178–201. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, M.; Chen, X.; Fan, S.; Lan, J. Binding Analysis of Sf-SR-C MAM Domain and Sf-FGFR Ectodomain to Vip3Aa. Insects 2024, 15, 428. https://doi.org/10.3390/insects15060428
Wang C, Li M, Chen X, Fan S, Lan J. Binding Analysis of Sf-SR-C MAM Domain and Sf-FGFR Ectodomain to Vip3Aa. Insects. 2024; 15(6):428. https://doi.org/10.3390/insects15060428
Chicago/Turabian StyleWang, Chenghai, Min Li, Xiling Chen, Shilong Fan, and Jun Lan. 2024. "Binding Analysis of Sf-SR-C MAM Domain and Sf-FGFR Ectodomain to Vip3Aa" Insects 15, no. 6: 428. https://doi.org/10.3390/insects15060428
APA StyleWang, C., Li, M., Chen, X., Fan, S., & Lan, J. (2024). Binding Analysis of Sf-SR-C MAM Domain and Sf-FGFR Ectodomain to Vip3Aa. Insects, 15(6), 428. https://doi.org/10.3390/insects15060428




