Pine Response to Sawfly Pheromones: Effects on Sawfly’s Oviposition and Larval Growth
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Plant
2.2. Olfactory Choice Assay
2.3. Oviposition Assay
2.4. Larval Performance Study
2.5. Statistics
3. Results
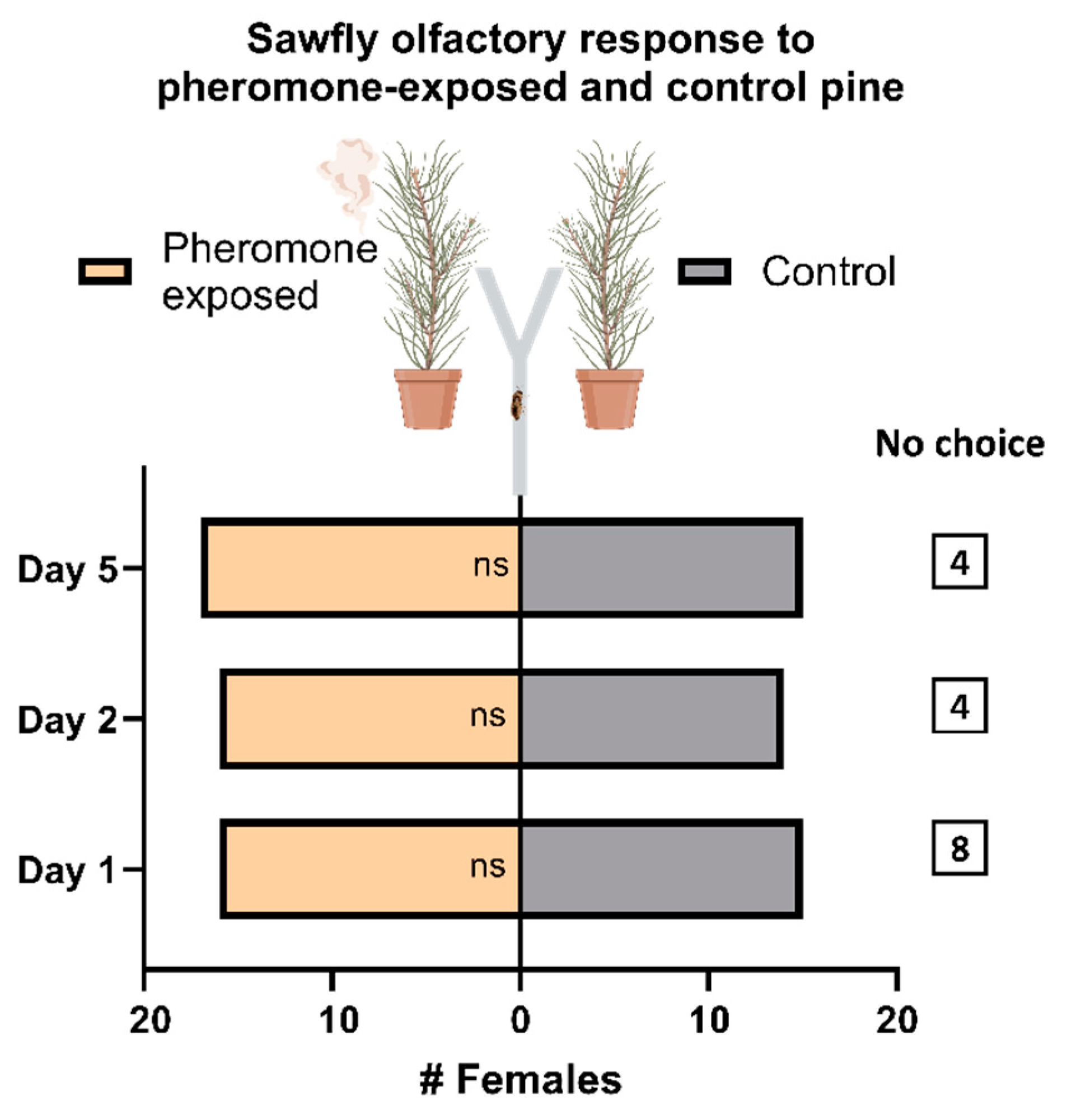
3.1. Females of D. pini Do Not Discriminate between Odor from Pheromone-Exposed and Unexposed Pine
3.2. Ovipositing D. pini Females Prefer Trees without Prior Pheromone Exposure, but If Chosen, the Number of Eggs on Pheromone-Exposed Trees Does Not Differ from That on Unexposed Trees
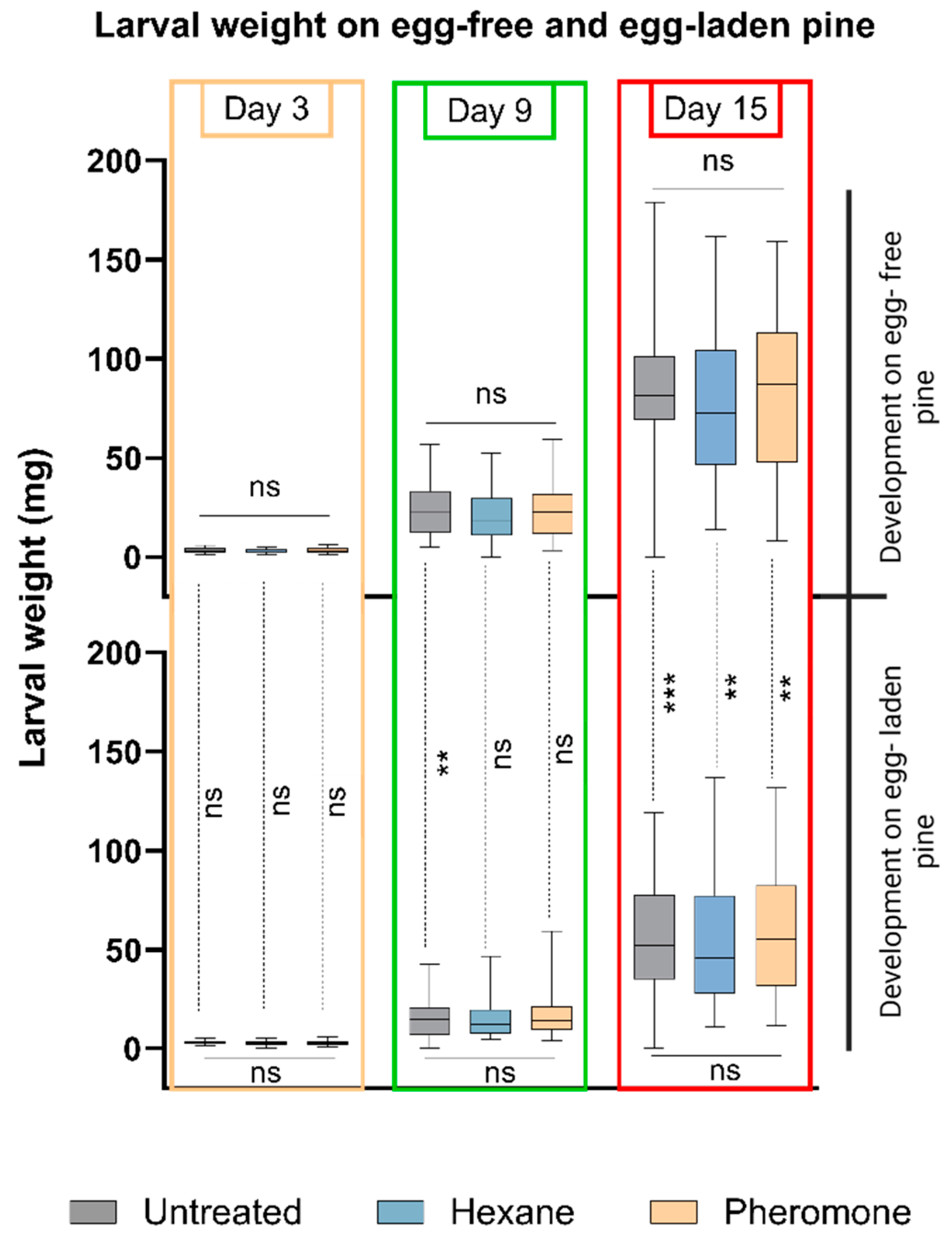
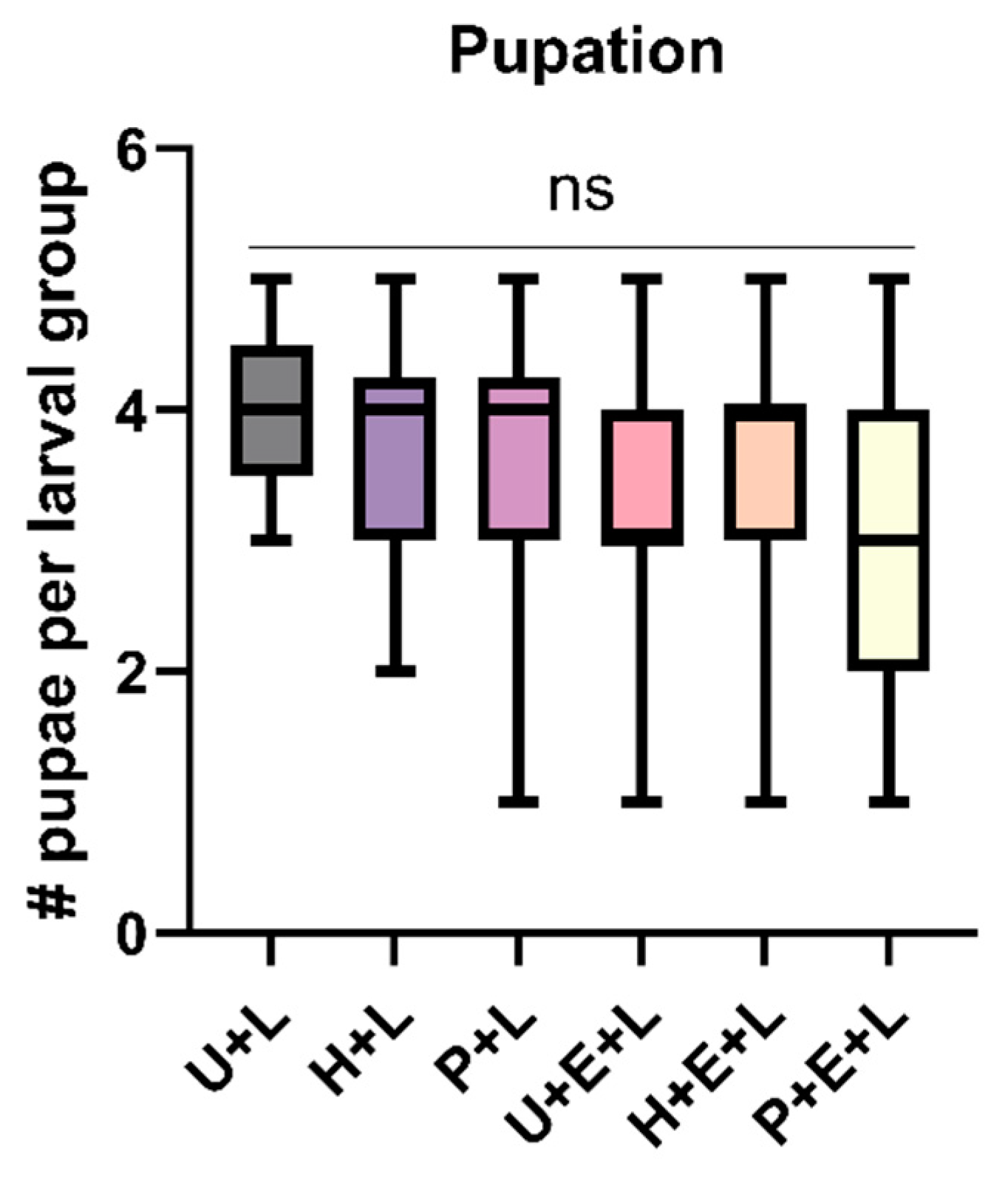
3.3. Performance of D. pini Larvae Is Not Affected by Exposure of Pine Trees to the Sawfly’s Pheromones, but by Pine Responses to Sawfly Egg Deposition
4. Discussion
4.1. No Olfactory Discrimination by D. pini between Pheromone-Exposed and Unexposed Pine
4.2. Oviposition Preference of Unexposed Pine over Pheromone-Exposed Pine upon Contact
4.3. No Detrimental Effects of Pheromone-Exposed Pine on Larval Performance
4.4. No Amplifier Effect of Pheromone Exposure on Egg-Mediated Improved Anti-Herbivore Defense
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stökl, J.; Steiger, S. Evolutionary origin of insect pheromones. Curr. Opin. Insect Sci. 2017, 24, 36–42. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Vogt, R.G. (Eds.) Insect Pheromone Biochemistry and Molecular Biology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; 750p. [Google Scholar] [CrossRef]
- Cardé, R.T. Navigation along windborne plumes of pheromone and resource-linked odors. Annu. Rev. Entomol. 2021, 66, 317–336. [Google Scholar] [CrossRef]
- Guo, X.; He, H.; Sun, J.; Kang, L. Plasticity of aggregation pheromones in insects. Curr. Opin. Insect Sci. 2023, 59, 101098. [Google Scholar] [CrossRef]
- Schulz, S. (Ed.) The Chemistry of Pheromones and Other Semiochemicals I; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; Volume 239, 241p. [Google Scholar] [CrossRef]
- Schulz, S. (Ed.) The Chemistry of Pheromones and Other Semiochemicals II; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; Volume 240, 333p. [Google Scholar] [CrossRef]
- Symonds, M.R.E.; Elgar, M.A. The evolution of pheromone diversity. Trends Ecol. Evol. 2008, 23, 220–228. [Google Scholar] [CrossRef]
- Engl, T.; Kaltenpoth, M. Influence of microbial symbionts on insect pheromones. Nat. Prod. Rep. 2018, 35, 386–397. [Google Scholar] [CrossRef]
- Fleischer, J.; Krieger, J. Insect pheromone receptors—Key elements in sensing intraspecific chemical signals. Front. Cell. Neurosci. 2018, 12, 425. [Google Scholar] [CrossRef]
- Helms, A.M.; De Moraes, C.M.; Tooker, J.F.; Mescher, M.C. Exposure of Solidago altissima plants to volatile emissions of an insect antagonist (Eurosta solidaginis) deters subsequent herbivory. Proc. Natl. Acad. Sci. USA 2013, 110, 199–204. [Google Scholar] [CrossRef]
- Helms, A.M.; De Moraes, C.M.; Tröger, A.; Alborn, H.T.; Francke, W.; Tooker, J.F.; Mescher, M.C. Identification of an insect-produced olfactory cue that primes plant defenses. Nat. Commun. 2017, 8, 337. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2016, 91, 1118–1133. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; Van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; De Moraes, C.M. Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef]
- Appel, H.M.; Cocroft, R.B. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 2014, 175, 1257–1266. [Google Scholar] [CrossRef]
- Hilker, M.; Fatouros, N.E. Resisting the onset of herbivore attack: Plants perceive and respond to insect eggs. Curr. Opin. Plant Biol. 2016, 32, 9–16. [Google Scholar] [CrossRef]
- Pashalidou, F.G.; Eyman, L.; Sims, J.; Buckley, J.; Fatouros, N.E.; De Moraes, C.M.; Mescher, M.C. Plant volatiles induced by herbivore eggs prime defences and mediate shifts in the reproductive strategy of receiving plants. Ecol. Lett. 2020, 23, 1097–1106. [Google Scholar] [CrossRef]
- Bittner, N.; Hundacker, J.; Achotegui-Castells, A.; Anderbrant, O.; Hilker, M. Defense of Scots pine against sawfly eggs (Diprion pini) is primed by exposure to sawfly sex pheromones. Proc. Natl. Acad. Sci. USA 2019, 116, 24668–24675. [Google Scholar] [CrossRef]
- Anderbrant, O.; Hansson, B.S.; Hallberg, E.; Geri, C.; Varama, M.; Hedenström, E.; Högberg, H.-E.; Fägerhag, J.; Edlund, H.; Wassgren, A.-B.; et al. Electrophysiological and morphological characteristics of pheromone receptors in male pine sawflies, Diprion pini (Hymenoptera: Diprionidae), and behavioural response to some compounds. J. Insect Physiol. 1995, 41, 395–401. [Google Scholar] [CrossRef]
- Anderbrant, O.; Östrand, F.; Bergström, G.; Wassgren, A.-B.; Auger-Rozenberg, M.-A.; Geri, C.; Hedenström, E.; Högberg, H.-E.; Herz, A.; Heitland, W. Release of sex pheromone and its precursors in the pine sawfly Diprion pini (Hym., Diprionidae). Chemoecology 2005, 15, 147–151. [Google Scholar] [CrossRef]
- Holdcraft, R.; Rodriguez-Saona, C.; Stelinski, L. Pheromone autodetection: Evidence and implications. Insects 2016, 7, 17. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; dos Reis, M.; Donoghue, P.C.J. Dating tips for divergence-time estimation. Trends Genet. 2015, 31, 637–650. [Google Scholar] [CrossRef]
- Hundacker, J.; Linda, T.; Hilker, M.; Lortzing, V.; Bittner, N. The impact of insect egg deposition on Pinus sylvestris transcriptomic and phytohormonal responses to larval herbivory. Tree Physiol. 2024, 44, tpae008. [Google Scholar] [CrossRef]
- Hilker, M.; Weitzel, C. Oviposition deterrence by chemical signals of conspecific larvae in Diprion pini (Hymenoptera: Diprionidae) and Phyllodecta vulgatissima (Coleoptera: Chrysomelidae). Entomol. Gen. 1991, 15, 293–302. [Google Scholar] [CrossRef]
- Beyaert, I.; Köpke, D.; Stiller, J.; Hammerbacher, A.; Yoneya, K.; Schmidt, A.; Gershenzon, J.; Hilker, M. Can insect egg deposition ‘warn’ a plant of future feeding damage by herbivorous larvae? Proc. R. Soc. B 2012, 279, 101–108. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef]
- Kegge, W.; Weldegergis, B.T.; Soler, R.; Eijk, M.V.; Dicke, M.; Voesenek, L.A.C.J.; Pierik, R. Canopy light cues affect emission of constitutive and methyl jasmonate-induced volatile organic compounds in Arabidopsis thaliana. New Phytol. 2013, 200, 861–874. [Google Scholar] [CrossRef]
- Meents, A.K.; Mithöfer, A. Plant–plant communication: Is there a role for volatile damage-associated molecular patterns? Front. Plant Sci. 2020, 11, 583275. [Google Scholar] [CrossRef]
- Schuman, M.C. Where, when, and why do plant volatiles mediate ecological signaling? The answer is blowing in the wind. Annu. Rev. Plant Biol. 2023, 74, 609–633. [Google Scholar] [CrossRef]
- Auer, T.O.; Khallaf, M.A.; Silbering, A.F.; Zappia, G.; Ellis, K.; Álvarez-Ocaña, R.; Arguello, J.R.; Hansson, B.S.; Jefferis, G.S.X.E.; Caron, S.J.C.; et al. Olfactory receptor and circuit evolution promote host specialization. Nature 2020, 579, 402–408. [Google Scholar] [CrossRef]
- Hilker, M.; Fatouros, N.E. Plant responses to insect egg deposition. Annu. Rev. Entomol. 2015, 60, 493–515. [Google Scholar] [CrossRef]
- Mumm, R.; Schrank, K.; Wegener, R.; Schulz, S.; Hilker, M. Chemical analysis of volatiles emitted by Pinus sylvestris after induction by insect oviposition. J. Chem. Ecol. 2003, 29, 1235–1252. [Google Scholar] [CrossRef]
- Davis, J.S.; Glover, A.N.; Everson, K.M.; Coyle, D.R.; Linnen, C.R. Identification, biology, and management of conifer sawflies (Hymenoptera: Diprioninae) in eastern North America. J. Integr. Pest Manag. 2023, 14, 13. [Google Scholar] [CrossRef]
- Wagner, M.R.; Raffa, K.F. Sawfly Life History Adaptations to Woody Plants; Academic Press: San Diego, CA, USA, 1993; 581p. [Google Scholar]
- Neustupa, J. Asymmetry and integration of cellular morphology in Micrasterias compereana. BMC Evol. Biol. 2017, 17, 1. [Google Scholar] [CrossRef]
- Heisswolf, A.; Gabler, D.; Obermaier, E.; Müller, C. Olfactory versus contact cues in host plant recognition of a monophagous chrysomelid beetle. J. Insect Behav. 2007, 20, 247–266. [Google Scholar] [CrossRef]
- Anton, S.; Cortesero, A.-M. Plasticity in chemical host plant recognition in herbivorous insects and its implication for pest control. Biology 2022, 11, 1842. [Google Scholar] [CrossRef]
- Saitta, V.; Rebora, M.; Piersanti, S.; Salerno, G. Visual and chemical cues in the host plant selection of the melon ladybird Chnootriba elaterii (Coleoptera: Coccinellidae). Arthropod-Plant Interact. 2023. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference–performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef]
- Jones, L.C.; Rafter, M.A.; Walter, G.H. Insects allocate eggs adaptively across their native host plants. Arthropod-Plant Interact. 2019, 13, 181–191. [Google Scholar] [CrossRef]
- Jaenike, J. On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 1978, 14, 350–356. [Google Scholar] [CrossRef]
- Valladares, G.; Lawton, J.H. Host-plant selection in the holly leaf-miner: Does mother know best? J. Anim. Ecol. 1991, 60, 227. [Google Scholar] [CrossRef]
- Thompson, J.N. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl. 1988, 47, 3–14. [Google Scholar] [CrossRef]
- Thompson, J.N.; Pellmyr, O. Evolution of oviposition behavior and host preference in Lepidoptera. Annu. Rev. Entomol. 1991, 36, 65–89. [Google Scholar] [CrossRef]
- Mayhew, P.J. Herbivore host choice and optimal bad motherhood. Trends Ecol. Evol. 2001, 16, 165–167. [Google Scholar] [CrossRef]
- Clark, K.E.; Hartley, S.E.; Johnson, S.N. Does mother know best? The preference-performance hypothesis and parent-offspring conflict in aboveground-belowground herbivore life cycles. Ecol. Entomol. 2011, 36, 117–124. [Google Scholar] [CrossRef]
- Janz, N. Evolutionary ecology of oviposition strategies. In Chemoecology of Insect Eggs and Egg Deposition; Hilker, M., Meiners, T., Eds.; Wiley: Hoboken, NJ, USA, 2003; pp. 349–376. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Plant responses to insect herbivory: Interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ. 2012, 35, 441–453. [Google Scholar] [CrossRef]
- Zebelo, S.A.; Maffei, M.E. Role of early signalling events in plant-insect interactions. J. Exp. Bot. 2015, 66, 435–448. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman-Soad, A.; Bittner, N.; Hilker, M. Pine Response to Sawfly Pheromones: Effects on Sawfly’s Oviposition and Larval Growth. Insects 2024, 15, 458. https://doi.org/10.3390/insects15060458
Rahman-Soad A, Bittner N, Hilker M. Pine Response to Sawfly Pheromones: Effects on Sawfly’s Oviposition and Larval Growth. Insects. 2024; 15(6):458. https://doi.org/10.3390/insects15060458
Chicago/Turabian StyleRahman-Soad, Asifur, Norbert Bittner, and Monika Hilker. 2024. "Pine Response to Sawfly Pheromones: Effects on Sawfly’s Oviposition and Larval Growth" Insects 15, no. 6: 458. https://doi.org/10.3390/insects15060458
APA StyleRahman-Soad, A., Bittner, N., & Hilker, M. (2024). Pine Response to Sawfly Pheromones: Effects on Sawfly’s Oviposition and Larval Growth. Insects, 15(6), 458. https://doi.org/10.3390/insects15060458





