Pattern Matters in the Aposematic Colouration of Papilio polytes Butterflies
Abstract
:Simple Summary
Abstract
1. Introduction
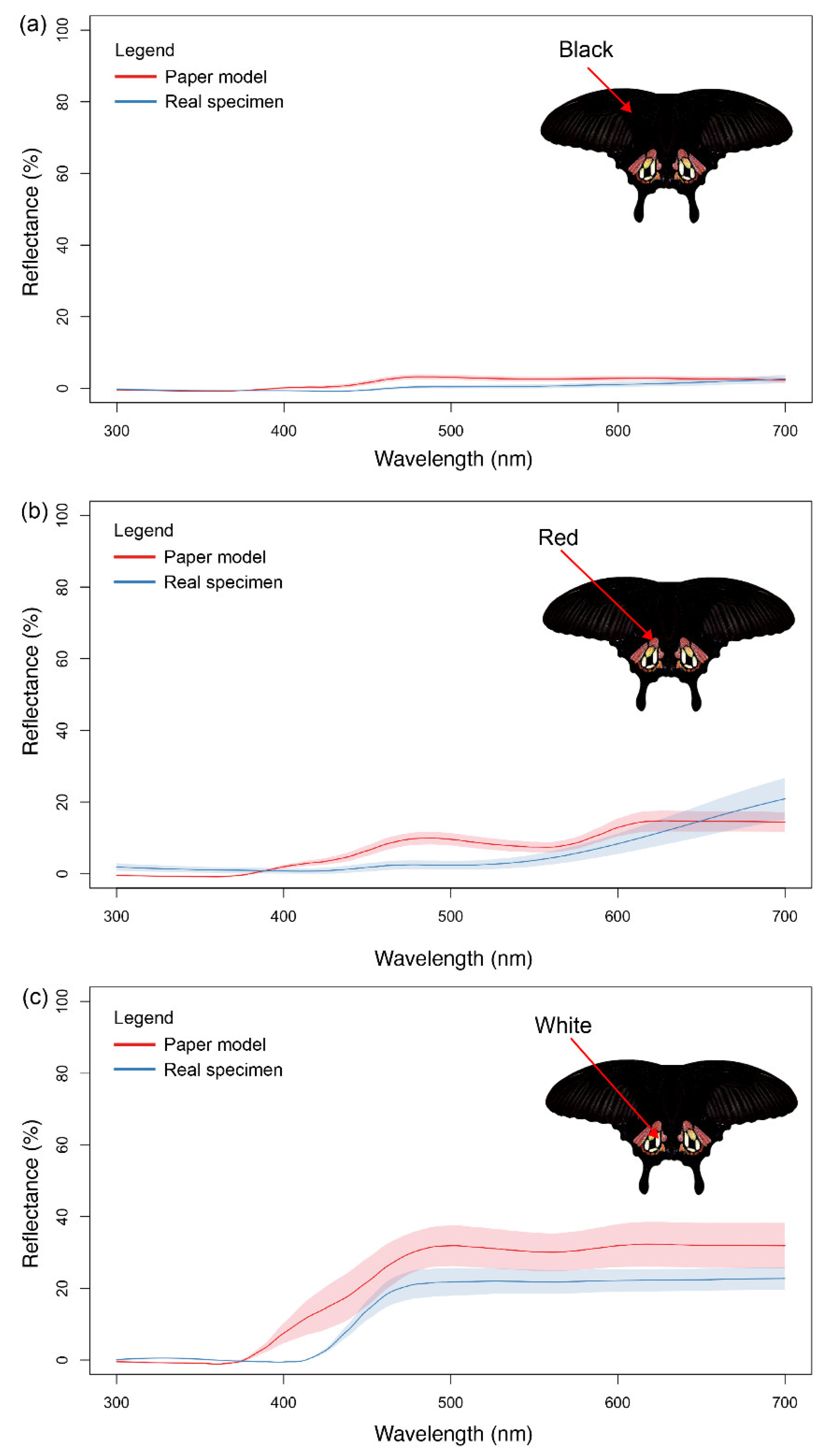
2. Materials and Methods
2.1. Preparation of Paper Butterfly Models
2.2. Deployment and Surveyance of Paper Models
2.3. Statistical Analysis
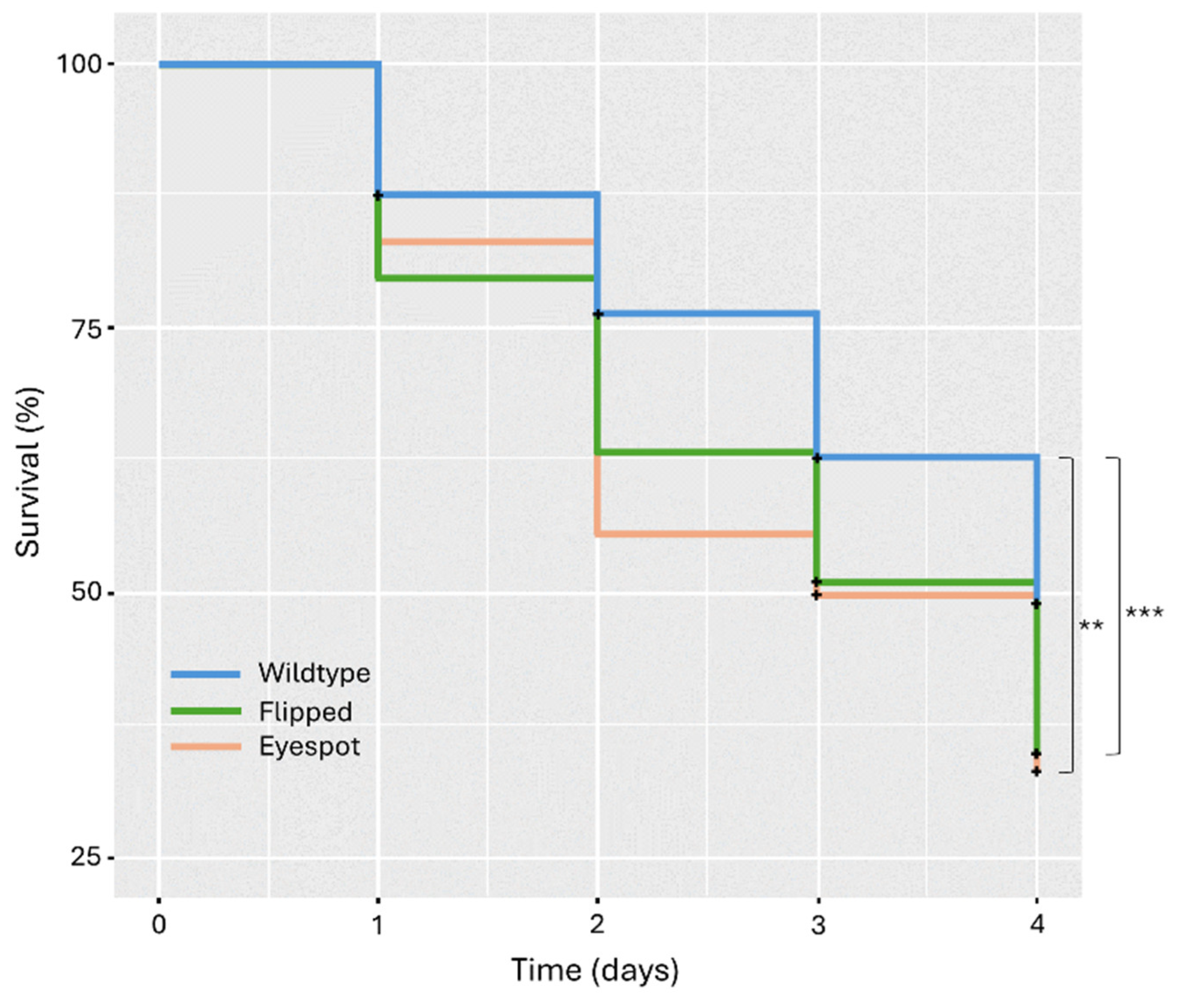
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas, B.; Valkonen, J.K.; Nokelainen, O. Aposematism. Curr. Biol. 2015, 25, R350–R351. [Google Scholar] [CrossRef] [PubMed]
- Bates, H.W. Co006Etributions to an insect fauna of the Amazon Valley (Lepidoptera: Heliconiidae). Trans. Linn. Soc. Lond. 1862, 23, 495–556. [Google Scholar] [CrossRef]
- Pasteur, G. A classificatory review of mimicry systems. Annu. Rev. Ecol. Syst. 1982, 13, 169–199. [Google Scholar] [CrossRef]
- Gamberale, G.; Tullberg, B.S. Aposematism and gregariousness: The combined effect of group size and coloration on signal repellence. Proc. R. Soc. B 1998, 265, 889–894. [Google Scholar] [CrossRef]
- Härlin, C.; Härlin, M. Towards a historization of aposematism. Evol. Ecol. 2003, 17, 197–212. [Google Scholar] [CrossRef]
- Santos, J.C.; Coloma, L.A.; Cannatella, D.C. Multiple, recurring origins of aposematism and diet specialization in poison frogs. Proc. Natl. Acad. Sci. USA 2003, 100, 12792–12797. [Google Scholar] [CrossRef] [PubMed]
- Mappes, J.; Marples, N.M.; Endler, J.A. The complex business of survival by aposematism. Trends Ecol. Evol. 2005, 20, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, D.W.; Herberstein, M.E.; Barfield, M.; Holt, R.D.; Mappes, J. Why aren’t warning signals everywhere? On the prevalence of aposematism and mimicry in communities. Biol. Rev. Cambridge Philos. Soc. 2021, 96, 2446–2460. [Google Scholar] [CrossRef] [PubMed]
- Uésugi, K. The Adaptive Significance of Batesian Mimicry in the Swallowtail Butterfly, Papilio polytes (Insecta, Papilionidae): Associative Learning in a Predator. Ethology 1996, 102, 762–775. [Google Scholar] [CrossRef]
- Caley, M.J.; Schluter, D. Predators favour mimicry in a tropical reef fish. Proc. R. Soc. B 2003, 270, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Darst, C.R.; Cummings, M.E.; Cannatella, D.C. A mechanism for diversity in warning signals: Conspicuousness versus toxicity in poison frogs. Proc. Natl. Acad. Sci. USA 2006, 103, 5852–5857. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.R.; Pfennig, D.W. Mimicry on the edge: Why do mimics vary in resemblance to their model in different parts of their geographical range? Proc. R. Soc. B 2007, 274, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, D.W.; Pfennig, D.W. Predator cognition permits imperfect coral snake mimicry. Am. Nat. 2010, 176, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.H.; Tan, Y.C.; Finkbeiner, S.D.; Briscoe, A.D.; Monteiro, A.; Kronforst, M.R. Experimental field tests of Batesian mimicry in the swallowtail butterfly Papilio polytes. Ecol. Evol. 2018, 8, 7657–7666. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Ruxton, G.D. Linking the evolution and form of warning coloration in nature. Proc. R. Soc. B 2011, 279, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.; Sheppard, P.M. Sensory Discrimination and its role in the evolution of Batesian mimicry. Behaviour 1965, 24, 269–282. [Google Scholar] [CrossRef]
- Dittrich, W.H.; Gilbert, F.; Green, P.; McGregor, P.K.; Grewcock, D. Imperfect mimicry: A pigeon’s perspective. Proc. R. Soc. B 1993, 251, 195–200. [Google Scholar] [CrossRef]
- Edmunds, M. Why are there good and poor mimics? Bioll. J Linn. Soc. 2000, 70, 459–466. [Google Scholar] [CrossRef]
- Howarth, B.; Edmunds, M.; Gilbert, F. Does the abundance of hoverfly (Syrphidae) mimics depend on the numbers of their hymenopteran models? Evolution 2004, 58, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Dixit, T.; Choi, G.P.T.; Al-Mosleh, S.; Lund, J.; Troscianko, J.; Moya, C.; Mahadevan, L.; Spottiswoode, C.N. Combined measures of mimetic fidelity explain imperfect mimicry in a brood parasite–host system. Biol. Lett. 2023, 19, 20220538. [Google Scholar] [CrossRef] [PubMed]
- Wickler, W. Mimicry in Plants and Animals; Weidenfeld & Nicolson: London, UK, 1968; Available online: https://hdl.handle.net/11858/00-001M-0000-0028-D897-2 (accessed on 21 June 2024).
- Sherratt, T.N. The evolution of imperfect mimicry. Behav. Ecol. 2002, 13, 821–826. [Google Scholar] [CrossRef]
- Ruxton, G.D.; Allen, W.H.; Sherratt, T.N.; Speed, M.P. Avoiding Attack: The Evolutionary Ecology of Crypsis Aposematism and Mimicry; Oxford University Press: Oxford, UK, 2018; Available online: https://cronfa.swan.ac.uk/Record/cronfa43559 (accessed on 20 June 2024).
- Westerman, E.L.; Antonson, N.D.; Kreutzmann, S.; Peterson, A.; Pineda, S.; Kronforst, M.R.; Olson-Manning, C.F. Behaviour before beauty: Signal weighting during mate selection in the butterfly Papilio polytes. Ethology 2019, 125, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.H.; Reader, T.; Gilbert, F. Why many Batesian mimics are inaccurate: Evidence from hoverfly colour patterns. Proc. R. Soc. B 2016, 283, 20161585. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Tatsuta, H.; Tsuji, K. Mimicry genes reduce pre-adult survival rate in Papilio polytes: A possible new mechanism for maintaining female-limited polymorphism in Batesian mimicry. J Evol. Biol. 2020, 33, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Ritland, D.B. Revising a classic butterfly mimicry scenario: Demonstration of Müllerian mimicry between Florida viceroys (Limenitis archippus floridensis) and queens (Danaus gilippus berenice). Evolution 1991, 45, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Côté, I.M.; Cheney, K.L. A protective function for aggressive mimicry? Proc. R. Soc. B 2007, 274, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, H.; Brien, M.N.; Morochz, C.; Salazar, P.A.; Rastas, P.; Nadeau, N.J. Limited genetic parallels underlie convergent evolution of quantitative pattern variation in mimetic butterflies. J Evol. Biol. 2020, 33, 1516–1529. [Google Scholar] [CrossRef] [PubMed]
- Joron, M.; Papa, R.; Beltrán, M.; Chamberlain, N.; Mavárez, J.; Baxter, S.W.; Abanto, M.; Bermingham, E.; Humphray, S.; Rogers, J.; et al. A conserved supergene locus controls colour pattern diversity in Heliconius butterflies. PLoS Biol. 2006, 4, e303. [Google Scholar] [CrossRef] [PubMed]
- Khew, S.K. A Field Guide to the Butterflies of Singapore, 2nd ed.; Ink on Communications Pte. Ltd.: Singapore, 2015. [Google Scholar]
- Euw, J.V.; Reichstein, T.; Rothschild, M. Aristolochic acid-I in the swallowtail butterfly Pachlioptera aristolochiae (Fabr.)(Papilionidae). Isr. J Chem. 1968, 6, 659–670. [Google Scholar] [CrossRef]
- Wu, T.-S.; Leu, Y.-L.; Chan, Y.-Y. Aristolochic Acids as a Defensive Substance for the Aristolochiaceous Plant-Feeding Swallowtail Butterfly, Pachliopta aristolochiae interpositus. J. Chin. Chem. Soc. 2000, 47, 221–226. [Google Scholar] [CrossRef]
- Sato, Y.; Tsurui-Sato, K.; Katoh, M.; Kimura, R.; Tatsuta, H.; Tsuji, K. Population genetic structure and evolution of Batesian mimicry in Papilio polytes from the Ryukyu Islands, Japan, analyzed by genotyping-by-sequencing. Ecol. Evol. 2020, 11, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Shimajiri, T.; Otaki, J.M. Phenotypic Plasticity of the Mimetic Swallowtail Butterfly Papilio polytes: Color Pattern Modifications and Their Implications in Mimicry Evolution. Insects 2022, 13, 649. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Iga, M.; Yamaguchi, J.; Saito, K.; Kataoka, H.; Suzuki, Y.; Sugano, S.; Fujiwara, H. Molecular basis of wing coloration in a Batesian mimic butterfly, Papilio polytes. Sci. Rep. 2013, 3, 3184. [Google Scholar] [CrossRef]
- Nishikawa, H.; Iijima, T.; Kajitani, R.; Yamaguchi, J.; Ando, T.; Suzuki, Y.; Sugano, S.; Fujiyama, A.; Kosugi, S.; Hirakawa, H.; et al. A genetic mechanism for female-limited Batesian mimicry in Papilio butterfly. Nat. Genet. 2015, 47, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M. The role of eyespots as anti-predator mechanisms, principally demonstrated in the Lepidoptera. Biol. Rev. Camb. Phil. Soc. 2005, 80, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Hopkins, E.; Hinde, W.; Adcock, A.; Connolly, Y.; Trościanko, T.; Cuthill, I.C. Field experiments on the effectiveness of ‘eyespots’ as predator deterrents. Anim. Behav. 2007, 74, 1215–1227. [Google Scholar] [CrossRef]
- Stevens, M.; Hardman, C.J.; Stubbins, C.L. Conspicuousness, not eye mimicry, makes “eyespots” effective antipredator signals. Behav. Ecol. 2008, 19, 525–531. [Google Scholar] [CrossRef]
- Kodandaramaiah, U. Eyespot evolution: Phylogenetic insights from Junonia and related butterfly genera (Nymphalidae: Junoniini). Evol. Dev. 2009, 11, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Kodandaramaiah, U.; Vallin, A.; Wiklund, C. Fixed eyespot display in a butterfly thwarts attacking birds. Anim. Behav. 2009, 77, 1415–1419. [Google Scholar] [CrossRef]
- Blut, C.; Wilbrandt, J.; Fels, D.; Girgel, E.; Lunau, K. The ‘sparkle’ in fake eyes–the protective effect of mimic eyespots in lepidoptera. Entomologia Experimentalis Applicata 2012, 143, 231–244. [Google Scholar] [CrossRef]
- Kodandaramaiah, U.; Lindenfors, P.; Tullberg, B.S. Deflective and intimidating eyespots: A comparative study of eyespot size and position in Junonia butterflies. Ecol. Evol. 2013, 3, 4518–4524. [Google Scholar] [CrossRef] [PubMed]
- Kjernsmo, K.; Merilaita, S. Eyespots divert attacks by fish. Proc. R. Soc. B 2013, 280, 20131458. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Ruxton, G.D. The key role of behaviour in animal camouflage. Biol. Rev. 2018, 94, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Schachat, S.R.; Piel, W.H.; Monteiro, A. Attack risk for butterflies changes with eyespot number and size. R. Soc. Open Sci. 2016, 3, 150614. [Google Scholar] [CrossRef] [PubMed]
- Kjernsmo, K.; Grönholm, M.; Merilaita, S. Size and contrast increase the divertive effect of eyespots. Behav. Ecol. 2018, 30, 159–165. [Google Scholar] [CrossRef]
- Chan, I.Z.W.; Ngan, Z.C.; Lin, N.; Lee, Y.; Gowri, V.; Monteiro, A. Predation favours Bicyclus anynana butterflies with fewer forewing eyespots. Proc. R. Soc. B 2021, 288, 20202840. [Google Scholar] [CrossRef] [PubMed]
- De Bona, S.; Valkonen, J.K.; Lopez-Sepulcre, A.; Mappes, J. Predator mimicry, not conspicuousness, explains the efficacy of butterfly eyespots. Proc. R. Soc. B 2015, 282, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Merilaita, S.; Vallin, A.; Kodandaramaiah, U.; Dimitrova, M.; Ruuskanen, S.; Laaksonen, T. Number of eyespots and their intimidating effect on naïve predators in the peacock butterfly. Behav. Ecol. 2011, 22, 1326–1331. [Google Scholar] [CrossRef]
- Finkbeiner, S.D.; Briscoe, A.D.; Reed, R.D. The benefit of being a social butterfly: Communal roosting deters predation. Proc. R. Soc. B 2012, 279, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.L.Q.; Monteiro, A. Yellow and the Novel Aposematic Signal, Red, Protect Delias Butterflies from Predators. PLoS ONE 2017, 12, e0168243. [Google Scholar] [CrossRef] [PubMed]
- Butterflies of Singapore: A Tribute to Nature’s Flying Jewels. Available online: https://butterflycircle.blogspot.com/2011/10/life-history-of-common-mormon.html (accessed on 21 June 2024).
- Low, X.H.; Monteiro, A. Dorsal Forewing White Spots of Male Papilio polytes (Lepidoptera: Papilionidae) not Maintained by Female Mate Choice. J. Insect Behav. 2018, 31, 29–41. [Google Scholar] [CrossRef]
- R Core Team. R A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 21 June 2024).
- Therneau, T. A Package for Survival Analysis in R (Version 3.5–5). 2023. Available online: https://CRAN.R-project.org/package=survival (accessed on 21 June 2024).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Ghim, M.M.; Hodos, W. Spatial contrast sensitivity of birds. J. Comp. Physiol. A 2006, 192, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Kiltie, R.A. Scaling of visual acuity with body size in mammals and birds. Funct. Ecol. 2000, 14, 226–234. [Google Scholar] [CrossRef]
- Blackwell, B.F.; Fernández-Juricic, E.; Seamans, T.W.; Dolan, T. Avian visual system configuration and behavioural response to object approach. Anim. Behav. 2009, 77, 673–684. [Google Scholar] [CrossRef]
- Thery, M.; Gomez, D. Insect colours and visual appearance in the eyes of their predators. Adv. Insect Physiol. 2010, 38, 267–353. [Google Scholar] [CrossRef]
- Prudic, K.L.; Stoehr, A.M.; Wasik, B.R.; Monteiro, A. Eyespots deflect predator attack increasing fitness and promoting the evolution of phenotypic plasticity. Proc. R. Soc. B 2015, 282, 20141531. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, M.; Gamberale-Stille, G. Colour and pattern similarity in mimicry: Evidence for a hierarchical discriminative learning of different components. Anim. Behav. 2012, 84, 881–887. [Google Scholar] [CrossRef]
- Barnett, S.A. Experiments on ‘neophobia’ in wild and laboratory rats. Br. J. Psychol. 1958, 49, 195–201. [Google Scholar] [CrossRef]
- Sweatt, J.D. Chapter 4–Rodent Behavioural Learning and Memory Models. In Mechanisms of Memory, 2nd ed.; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Roper, T.J. Effects of Novelty on Taste-Avoidance learning in chicks. Behaviour 1993, 125, 265–281. [Google Scholar] [CrossRef]
- Adamová-Ježová, D.; Hospodková, E.; Fuchsová, L.; Štys, P.; Exnerová, A. Through experience to boldness? Deactivation of neophobia towards novel and aposematic prey in three European species of tits (Paridae). Behav. Process. 2016, 131, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Marples, N.M.; Kelly, D. Neophobia and Dietary Conservatism: Two distinct processes? Evol. Ecol. 1999, 13, 641–653. [Google Scholar] [CrossRef]
- Doherty, S.; Cowie, R.J. The Effects of Early Feeding Experience on Long-term Seed Choice by Canaries (Serinus canaria). Ethology 1994, 97, 177–189. [Google Scholar] [CrossRef]
- Marples, N.M.; Roper, T.J.; Harper, D.G. Responses of wild birds to novel prey: Evidence of Dietary Conservatism. Oikos 1998, 83, 161. [Google Scholar] [CrossRef]
- Evens, R.; Beenaerts, N.; Neyens, T.; Witters, N.; Smeets, K.; Artois, T. Proximity of breeding and foraging areas affects foraging effort of a crepuscular, insectivorous bird. Sci. Rep. 2018, 8, 3008. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.; Chan, I.Z.W.; Monteiro, A. Pattern Matters in the Aposematic Colouration of Papilio polytes Butterflies. Insects 2024, 15, 465. https://doi.org/10.3390/insects15070465
Lim H, Chan IZW, Monteiro A. Pattern Matters in the Aposematic Colouration of Papilio polytes Butterflies. Insects. 2024; 15(7):465. https://doi.org/10.3390/insects15070465
Chicago/Turabian StyleLim, Huile, Ian Z. W. Chan, and Antónia Monteiro. 2024. "Pattern Matters in the Aposematic Colouration of Papilio polytes Butterflies" Insects 15, no. 7: 465. https://doi.org/10.3390/insects15070465
APA StyleLim, H., Chan, I. Z. W., & Monteiro, A. (2024). Pattern Matters in the Aposematic Colouration of Papilio polytes Butterflies. Insects, 15(7), 465. https://doi.org/10.3390/insects15070465





