Compatibility of Bioinsecticides with Parasitoids for Enhanced Integrated Pest Management of Drosophila suzukii and Tuta absoluta
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Insecticides
2.3. Lethal and Sublethal Effects of Insecticides on Ganaspis kimorum
2.4. Lethal and Sublethal Effects of Insecticides on Necremnus tutae
2.5. Data Analysis
3. Results
3.1. Ganaspis kimorum Mortality during Residual Contact Exposure to Insecticides
3.2. Necremnus tutae Mortality during Residual Contact Exposure to Insecticides
3.3. Insecticide Effects on Ganaspis kimorum Parasitism
3.4. Insecticide Effects on Necremnus tutae Parasitism and Host Killing
3.5. Ganaspis kimorum Survival after Residual Contact Exposure to Insecticides
3.6. Necremnus tutae Survival after Residual Contact Exposure to Insecticides
3.7. Individual and Combined Insecticide-Ganaspis kimorum Impact on Pest Mortality
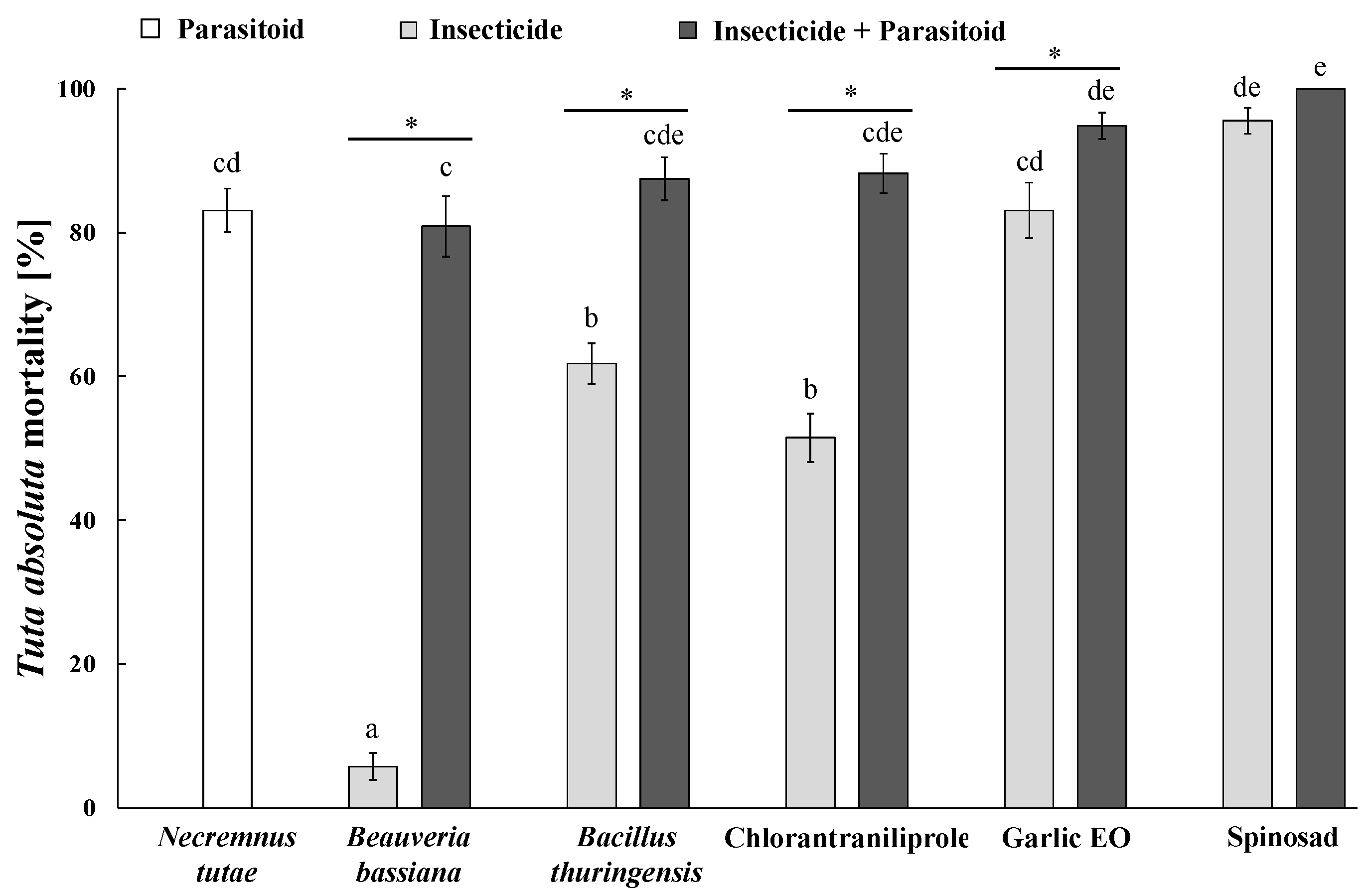
3.8. Individual and Combined Insecticide–Necremnus tutae Impact on Pest Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Gugliuzzo, A.; Biedermann, P.H.W.; Carrillo, D.; Castrillo, L.A.; Egonyu, J.P.; Gallego, D.; Haddi, K.; Hulcr, J.; Jactel, H.; Kajimura, H.; et al. Recent advances toward the sustainable management of invasive Xylosandrus ambrosia beetles. J. Pest Sci. 2021, 94, 615–637. [Google Scholar] [CrossRef]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.A.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2023, 43, 187–241. [Google Scholar] [CrossRef]
- Trombik, J.; Ward, S.F.; Norrbom, A.L.; Liebhold, A.M. Global drivers of historical true fruit fly (Diptera: Tephritidae) invasions. J. Pest Sci. 2023, 96, 345–357. [Google Scholar] [CrossRef]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; Kleunen, M.; Kühn, I.; et al. Projecting the continental accumulation of alien species through to 2050. Glob. Chang. Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Han, P.; Mansour, R.; Arnó, J.; Brévault, T.; Campos, M.R.; Chailleux, A.; Guedes, R.N.C.; Karimi, J.; Konan, K.A.J.; et al. Integrated pest management of Tuta absoluta: Practical implementations across different world regions. J. Pest Sci. 2022, 95, 17–39. [Google Scholar] [CrossRef]
- Song, Z.; Li, C.; Tan, Y.; Shen, S.; Gong, Y.; Wang, Y.; Wang, R.; Hernandez, Z.; Chen, J.; Zhang, Z. Chlorantraniliprole emulsified with botanical oils effectively controls invasive pest Spodoptera frugiperda larvae in corn plant. J. Pest Sci. 2023, 96, 1429–1440. [Google Scholar] [CrossRef]
- Tabet, D.H.; Visentin, E.; Bonadio, M.; Bjeljac, M.; Reyes-Domínguez, Y.; Gallmetzer, A.; Spitaler, U. Efficacy of insecticides against the invasive apricot aphid, Myzus mumecola. Insects 2023, 14, 746. [Google Scholar] [CrossRef]
- Colautti, R.I.; Ricciardi, A.; Grigorovich, I.A.; MacIsaac, H.J. Is invasion success explained by the enemy release hypothesis? Ecol. Lett. 2004, 7, 721–733. [Google Scholar] [CrossRef]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A decade of research towards a sustainable integrated pest management program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef]
- de Albuquerque Melo Xavier, J.K.; de Jesus Alves Miranda, A.; dos Santos Soares Buna, S.; da Rocha, C.Q.; da Silva Lima, A. Neotropical flora’s contribution to the development of biorational products for Drosophila suzukii control. Neotrop. Entomol. 2024, 53, 400–414. [Google Scholar] [CrossRef]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and economic costs of nonindigenous species in the United States. BioScience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.-H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, K. Bioaccumulation of pesticides and its impact on biological systems. In Pesticides in Crop Production: Physiological and Biochemical Action; Srivastava, P.K., Singh, V.P., Singh, A., Tripathi, D.K., Singh, S., Prasad, S.M., Chauhan, D.K., Eds.; Wiley: New York, NY, USA, 2020; pp. 55–67. [Google Scholar]
- Siddiqui, J.A.; Fan, R.; Naz, H.; Bamisile, B.S.; Hafeez, M.; Ghani, M.I.; Wei, Y.; Xu, Y.; Chen, X. Insights into insecticide-resistance mechanisms in invasive species: Challenges and control strategies. Front. Physiol. 2023, 13, 1112278. [Google Scholar] [CrossRef]
- Copping, L.G.; Menn, J.J. Biopesticides: A review of their action, applications and efficacy. Pest Manag. Sci. 2000, 56, 651–676. [Google Scholar] [CrossRef]
- Campos, M.R.; Rodrigues, A.R.S.; Silva, W.M.; Silva, T.B.M.; Silva, V.R.F.; Guedes, R.N.C.; Siqueira, H.A.A. Spinosad and the tomato borer Tuta absoluta: A bioinsecticide, an invasive pest threat, and high insecticide resistance. PLoS ONE 2014, 9, e103235. [Google Scholar] [CrossRef] [PubMed]
- Gugliuzzo, A.; Giuliano, G.; Rizzo, R.; Tropea Garzia, G.; Biondi, A. Lethal and sublethal effects of synthetic and bioinsecticides toward the invasive ambrosia beetle Xylosandrus compactus. Pest Manag. Sci. 2023, 79, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Mantilla Afanador, J.G.; Araujo, S.H.C.; Teixeira, M.G.; Lopes, D.T.; Cerceau, C.I.; Andreazza, F.; Oliveira, D.C.; Bernardi, D.; Moura, W.S.; Aguiar, R.W.S.; et al. Novel lactone-based insecticides and Drosophila suzukii management: Synthesis, potential action mechanisms and selectivity for non-target parasitoids. Insects 2023, 14, 697. [Google Scholar] [CrossRef]
- Fanning, P.D.; Grieshop, M.J.; Isaacs, R. Efficacy of biopesticides on spotted wing Drosophila, Drosophila Suzukii Matsumura in fall red raspberries. J. Appl. Entomol. 2018, 142, 26–32. [Google Scholar] [CrossRef]
- Sial, A.A.; Roubos, C.R.; Gautam, B.K.; Fanning, P.D.; Van Timmeren, S.; Spies, J.; Petran, A.; Rogers, M.A.; Liburd, O.E.; Little, B.A.; et al. Evaluation of organic insecticides for management of Spotted-Wing Drosophila (Drosophila suzukii) in berry crops. J. Appl. Entomol. 2019, 143, 593–608. [Google Scholar] [CrossRef]
- Boughdad, A.; Haddi, K.; El Bouazzati, A.; Nassiri, A.; Tahiri, A.; El Anbri, C.; Eddaya, T.; Zaid, A.; Biondi, A. First record of the invasive spotted wing Drosophila infesting berry crops in Africa. J. Pest Sci. 2021, 94, 261–271. [Google Scholar] [CrossRef]
- Wang, M.H.; Ismoilov, K.; Liu, W.X.; Bai, M.; Bai, X.; Chen, B.; Chen, H.; Chen, H.; Dong, Y.; Fang, K.; et al. Tuta absoluta management in China: Progress and prospects. Entomol. Gen. 2024, 44, 269–278. [Google Scholar] [CrossRef]
- Hamby, K.A.; Bellamy, D.E.; Chiu, J.C.; Lee, J.C.; Walton, V.M.; Wiman, N.G.; York, R.M.; Biondi, A. Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii. J. Pest Sci. 2016, 89, 605–619. [Google Scholar] [CrossRef]
- Caruso, A.G.; Tortorici, S.; Davino, S.; Bertacca, S.; Ragona, A.; Lo Verde, G.; Biondi, A.; Noris, E.; Rizzo, R.; Panno, S. The invasive tomato pest Tuta absoluta can transmit the emergent tomato brown rugose fruit virus. Entomol. Gen. 2024, 44, 289–296. [Google Scholar] [CrossRef]
- Bernardi, D.; Ribeiro, L.; Andreazza, F.; Neitzke, C.; Oliveira, E.E.; Botton, M.; Nava, D.E.; Vendramim, J.D. Potential use of annona by products to control Drosophila suzukii and toxicity to its parasitoid Trichopria anastrephae. Ind. Crops Prod. 2017, 110, 30–35. [Google Scholar] [CrossRef]
- Lisi, F.; Mansour, R.; Cavallaro, C.; Alınç, T.; Porcu, E.; Ricupero, M.; Zappalà, L.; Desneux, N.; Biondi, A. Sublethal effects of nine insecticides on Drosophila suzukii and its major pupal parasitoid Trichopria drosophilae. Pest Manag. Sci. 2023, 79, 5003–5014. [Google Scholar] [CrossRef]
- Ricupero, M.; Biondi, A.; Cincotta, F.; Condurso, C.; Palmeri, V.; Verzera, A.; Zappalà, L.; Campolo, O. Bioactivity and physico-chemistry of garlic essential oil nanoemulsion in tomato. Entomol. Gen. 2022, 42, 921–930. [Google Scholar] [CrossRef]
- de Paiva Silva, G.T.; Figueiredo, K.G.; Alves, D.S.; de Oliveira, D.F.; Silva, G.H.; de Souza e Silva, G.T.; de Oliveira, M.S.; Biondi, A.; Carvalho, G.A. Survival and demography of the tomato borer (Tuta absoluta) exposed to citrus essential oils and major compounds. Agriculture 2023, 13, 538. [Google Scholar] [CrossRef]
- de Figueiredo, K.G.; de Paiva Silva, G.T.; Passos, L.C.; Alves, D.S.; Biondi, A.; Carvalho, G.A. Toxicity of Cinnamomum spp. essential oil to Tuta absoluta and to predatory mirid. J. Pest Sci. 2023, 2023, 1–17. [Google Scholar] [CrossRef]
- Gonthier, J.; Arnó, J.; Romeis, J.; Collatz, J. Few indirect effects of baculovirus on parasitoids demonstrate high compatibility of biocontrol methods against Tuta absoluta. Pest Manag. Sci. 2023, 79, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Koller, J.; Gonthier, J.; Norgrove, L.; Arnó, J.; Sutter, L.; Collatz, J. A parasitoid wasp allied with an entomopathogenic virus to control Tuta absoluta. Crop Prot. 2024, 179, 106617. [Google Scholar] [CrossRef]
- Biondi, A.; Mommaerts, V.; Smagghe, G.; Viñuela, E.; Zappalà, L.; Desneux, N. The non-target impact of spinosyns on beneficial arthropods. Pest Manag. Sci. 2012, 68, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Giunti, G.; Benelli, G.; Palmeri, V.; Laudani, F.; Ricupero, M.; Ricciardi, R.; Maggi, F.; Lucchi, A.; Guedes, R.N.C.; Desneux, N.; et al. Non-target effects of essential oil-based biopesticides for crop protection: Impact on natural enemies, pollinators, and soil invertebrates. Biol. Control 2022, 176, 105071. [Google Scholar] [CrossRef]
- Schlesener, D.C.H.; Wollmann, J.; Pazini, J.D.B.; Padilha, A.C.; Grützmacher, A.D.; Garcia, F.R.M. Insecticide toxicity to Drosophila suzukii (Diptera: Drosophilidae) parasitoids: Trichopria anastrephae (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae). J. Econ. Entomol. 2019, 112, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Zappalà, L.; Stark, J.D.; Desneux, N. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 2013, 8, e76548. [Google Scholar] [CrossRef]
- Mansour, R.; Biondi, A. Releasing natural enemies and applying microbial and botanical pesticides for managing Tuta absoluta in the MENA region. Phytoparasitica 2021, 49, 179–194. [Google Scholar] [CrossRef]
- Passos, L.C.; Ricupero, M.; Gugliuzzo, A.; Soares, M.A.; Desneux, N.; Campolo, O.; Carvalho, G.A.; Biondi, A.; Zappalá, L. Sublethal effects of plant essential oils toward the zoophytophagous mirid Nesidiocoris tenuis. J. Pest Sci. 2022, 95, 1609–1619. [Google Scholar] [CrossRef]
- Seehausen, M.L.; Ris, N.; Driss, L.; Racca, A.; Girod, P.; Warot, S.; Borowiec, N.; Toševski, I.; Kenis, M. Evidence for a cryptic parasitoid species reveals its suitability as a biological control agent. Sci. Rep. 2020, 10, 19096. [Google Scholar] [CrossRef]
- Daane, K.M.; Wang, X.; Hogg, B.N.; Biondi, A. Potential host ranges of three Asian larval parasitoids of Drosophila suzukii. J. Pest Sci. 2021, 94, 1171–1182. [Google Scholar] [CrossRef]
- Lisi, F.; Biondi, A.; Cavallaro, C.; Zappalà, L.; Campo, G.; Roversi, P.F.; Sabbatini Peverieri, G.; Giovannini, L.; Tavella, L.; Tortorici, F.; et al. Current status of Drosophila suzukii classical biological control in Italy. Acta Hortic. 2022, 1354, 193–200. [Google Scholar] [CrossRef]
- Fellin, L.; Grassi, A.; Puppato, S.; Saddi, A.; Anfora, G.; Ioriatti, C.; Rossi-Stacconi, M.V. First report on classical biological control releases of the larval parasitoid Ganaspis brasiliensis against Drosophila suzukii in Northern Italy. BioControl 2023, 68, 1–12. [Google Scholar] [CrossRef]
- Sosa-Calvo, J.; Forshage, M.; Buffington, M.L. Circumscription of the Ganaspis brasiliensis (Ihering, 1905) species complex (Hymenoptera, Figitidae), and the description of two new species parasitizing the spotted wing drosophila, Drosophila suzukii Matsumura, 1931 (Diptera, Drosophilidae). J. Hymenopt. Res. 2024, 97, 441–470. [Google Scholar] [CrossRef]
- Salas Gervassio, N.G.; Aquino, D.; Vallina, C.; Biondi, A.; Luna, M.G. A re-examination of Tuta absoluta parasitoids in South America for optimized biological control. J. Pest Sci. 2019, 92, 1343–1357. [Google Scholar] [CrossRef]
- Bodino, N.; Ferracini, C.; Tavella, L. Functional response and age-specific foraging behaviour of Necremnus tutae and N. cosmopterix, native natural enemies of the invasive pest Tuta absoluta in Mediterranean Area. J. Pest Sci. 2019, 92, 1467–1478. [Google Scholar]
- Zhang, Y.; Tian, X.; Wang, H.; Castañé, C.; Arnó, J.; Wu, S.; Xian, X.; Liu, W.; Desneux, N.; Wan, F.; et al. Nonreproductive effects are more important than reproductive effects in a host feeding parasitoid. Sci. Rep. 2022, 12, 11475. [Google Scholar] [CrossRef] [PubMed]
- Crisol-Martínez, E.; van der Blom, J. Necremnus tutae (Hymenoptera, Eulophidae) is widespread and efficiently controls Tuta absoluta in tomato greenhouses in SE Spain. IOBC/WPRS Bull. 2019, 147, 22–29. [Google Scholar]
- Denis, C.; Riudavets, J.; Alomar, O.; Agustí, N.; Gonzalez-Valero, H.; Cubí, M.; Matas, M.; Rodríguez, D.; van Achterberg, K.; Arnó, J. Dolichogenidea gelechiidivoris Marsh (Hymenoptera: Braconidae), a new biological control agent of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in the Mediterranean basin. IOBC/WPRS Bull. 2023, 167, 37–38. [Google Scholar]
- Dalton, D.T.; Walton, V.M.; Shearer, P.W.; Walsh, D.B.; Caprile, J.; Isaacs, R. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag. Sci. 2011, 67, 1368–1374. [Google Scholar] [CrossRef]
- Girod, P.; Lierhmann, O.; Urvois, T.; Turlings, T.C.J.; Kenis, M.; Haye, T. Host specificity of asian parasitoids for potential classical biological control of Drosophila suzukii. J. Pest Sci. 2018, 91, 1241–1250. [Google Scholar] [CrossRef]
- Rossi-Stacconi, M.V.; Wang, X.; Stout, A.; Fellin, L.; Daane, K.M.; Biondi, A.; Stahl, J.M.; Buffington, M.L.; Anfora, G.; Hoelmer, K.A. Methods for rearing the parasitoid Ganaspis brasiliensis, a promising biological control agent for the invasive Drosophila suzukii. JoVE 2022, 184, e63898. [Google Scholar]
- Wang, X.G.; Nance, A.H.; Jones, J.M.; Hoelmer, K.A.; Daane, K.M. Aspects of the biology and reproductive strategy of two Asian larval parasitoids evaluated for classical biological control of Drosophila suzukii. Biol. Control 2018, 121, 58–65. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Survival probabilities (the Kaplan-Meier method). BMJ 1998, 317, 1572. [Google Scholar] [CrossRef] [PubMed]
- Bodino, N.; Ferracini, C.; Tavella, L. Is host selection influenced by natal and adult experience in the parasitoid Necremnus tutae (Hymenoptera: Eulophidae)? Anim. Behav. 2016, 112, 221–228. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Wang, H.; Casteňé, C.; Arnó, J.; Collatz, J.; Romeis, J.; Wu, S.; Xian, X.; Liu, W.; et al. Host selection behavior of the host-feeding parasitoid Necremnus tutae on Tuta absoluta. Entomol. Gen. 2021, 42, 445–456. [Google Scholar] [CrossRef]
- Cahenzli, F.; Strack, T.; Daniel, C. Screening of 25 different natural crop protection products against Drosophila suzukii. J. Appl. Entomol. 2018, 142, 563–577. [Google Scholar] [CrossRef]
- Babin, A.; Lemauf, S.; Rebuf, C.; Poirié, M.; Gatti, J.-L. Effects of Bacillus thuringiensis kurstaki bioinsecticide on two non-target Drosophila larval endoparasitoid wasps. Entomol. Gen. 2022, 42, 611–620. [Google Scholar] [CrossRef]
- Klieber, J.; Reineke, A. The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J. Appl. Entomol. 2016, 140, 580–589. [Google Scholar] [CrossRef]
- Zimmermann, G. Review on Safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Techn. 2007, 17, 553–596. [Google Scholar] [CrossRef]
- Thungrabeab, M.; Tongma, S. Effect of entomopathogenic fungi, Beauveria bassiana (Balsam) and Metarhizium anisopliae (Metsch) on non-target insects. Curr. Appl. Sci. Technol. 2007, 7, 8–12. [Google Scholar]
- De Bortoli, S.A.; Vacari, A.M.; Polanczyk, R.A.; Pires Veiga, A.C.; Marchi Goulart, R. Effect of Bacillus thuringiensis on parasitoids and predators. In Bacillus Thuringiensis and Lysinibacillus Sphaericus: Characterization and Use in the Field of Biocontrol; Fiuza, L.M., Polanczyk, R.A., Crickmore, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 67–77. [Google Scholar]
- Alsaedi, G.; Ashouri, A.; Talaei-Hassanloui, R. Assessment of two Trichogramma species with Bacillus thuringiensis var. krustaki for the control of the tomato leafminer Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) in Iran. Open J. Ecol. 2017, 7, 112–124. [Google Scholar]
- Khidr, A.E.A.A.; Gaffar, S.A.; Nada, M.S.; Mohammad, A.M. New approaches for controlling tomato leafminer, Tuta absoluta (Meyrick) in tomato fields in Egypt. Egypt. J. Agric. Res. 2013, 91, 335–348. [Google Scholar] [CrossRef]
- Pérez-Guerrero, S.; Mateus, C. Field evaluation of commercial plant extracts against Drosophila suzukii (Diptera: Drosophlidae) in raspberry. Int. J. Pest Manag. 2019, 65, 53–58. [Google Scholar] [CrossRef]
- Beers, E.H.; Van Steenwyk, R.A.; Shearer, P.W.; Coates, W.W.; Grant, J.A. Developing Drosophila suzukii management programs for sweet cherry in the western United States. Pest Manag. Sci. 2011, 67, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, F.; Bernardi, D.; Baronio, C.A.; Pasinato, J.; Nava, D.E.; Botton, M. Toxicities and effects of insecticidal toxic baits to control Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae). Pest Manag. Sci. 2017, 73, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Shawer, R.; Tonina, L.; Tirello, P.; Duso, C.; Mori, N. Laboratory and field trials to identify effective chemical control strategies for integrated management of Drosophila suzukii in european cherry orchards. Crop Prot. 2018, 103, 73–80. [Google Scholar] [CrossRef]
- Tiwari, S.; Stelinski, L.L. Effects of cyantraniliprole, a novel anthranilic diamide insecticide, against asian citrus psyllid under laboratory and field conditions. Pest Manag. Sci. 2013, 69, 1066–1072. [Google Scholar] [CrossRef]
- Lisi, F.; Cavallaro, C.; Fellin, L.; Gugliuzzo, A.; Desneux, N.; Anfora, G.; Rossi Stacconi, M.V.; Biondi, A. Non-target effects of neurotoxic insecticides on Ganaspis cf. brasiliensis, a classical biological control agent of the spotted wing Drosophila. CABI A B 2024, 5, 48. [Google Scholar]
- Brugger, K.E.; Cole, P.G.; Newman, I.C.; Parker, N.; Scholz, B.; Suvagia, P.; Walker, G.; Hammond, T.G. Selectivity of chlorantraniliprole to parasitoid wasps. Pest Manag. Sci. 2010, 66, 1075–1081. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Ricupero, M.; Harwood, J.D.; Liang, Y.; Zang, L.-S.; Wang, S. Hormesis effects of chlorantraniliprole on a key egg parasitoid used for management of rice lepidopterans. Entomol. Gen. 2022, 42, 941–948. [Google Scholar] [CrossRef]
- De Sousa Pereira, K.; Chediak, M.; Zanuncio, J.C.; Guedes, R.N.C. Chlorantraniliprole impact on survival and progeny quality of the pupa of the parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae). Can. Entomol. 2019, 151, 94–100. [Google Scholar] [CrossRef]
- Wise, J.C.; Vanderpoppen, R.; Vandervoort, C.; O’Donnell, C.; Isaacs, R. Curative activity contributes to control of Spotted-Wing Drosophila (Diptera: Drosophilidae) and blueberry maggot (Diptera: Tephritidae) in highbush blueberry. Can. Entomol. 2015, 147, 109–117. [Google Scholar] [CrossRef]
- Van Timmeren, S.; Isaacs, R. Control of Spotted Wing Drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection programs. Crop Prot. 2013, 54, 126–133. [Google Scholar] [CrossRef]
- Cossentine, J.E.; Ayyanath, M.-M. Limited protection of the parasitoid Pachycrepoideus vindemiae from Drosophila suzukii host-directed spinosad suppression. Entomol. Exp. Appl. 2017, 164, 78–86. [Google Scholar] [CrossRef]
- Osman, M.A.M.; Mandour, N.S.; Abd El-Hady, M.A.; Sarhan, A.A. Susceptibility of four trichogrammatid parasitoids to some bio-rational insecticides used to control tomato leaf miner Tuta absoluta (Lepidoptera, Gelechiidae). J. Appl. Plant Prot. 2014, 2, 31–38. [Google Scholar]
- Nozad-Bonab, Z.; Hejazi, M.J.; Iranipour, S.; Arzanlou, M.; Biondi, A. Lethal and sublethal effects of synthetic and bio-insecticides on Trichogramma brassicae parasitizing Tuta absoluta. PLoS ONE 2021, 16, e024334. [Google Scholar] [CrossRef] [PubMed]
- Abbes, K.; Biondi, A.; Kurtulus, A.; Ricupero, M.; Russo, A.; Siscaro, G.; Chermiti, B.; Zappalà, L. Combined non-target effects of insecticide and high temperature on the parasitoid Bracon nigricans. PLoS ONE 2015, 10, e0138411. [Google Scholar] [CrossRef]
- Rizk, A.M. Effectiveness of different bio-techniques for controlling the pin worm, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Egypt. J. Biol. Pest Control 2016, 26, 797–802. [Google Scholar]

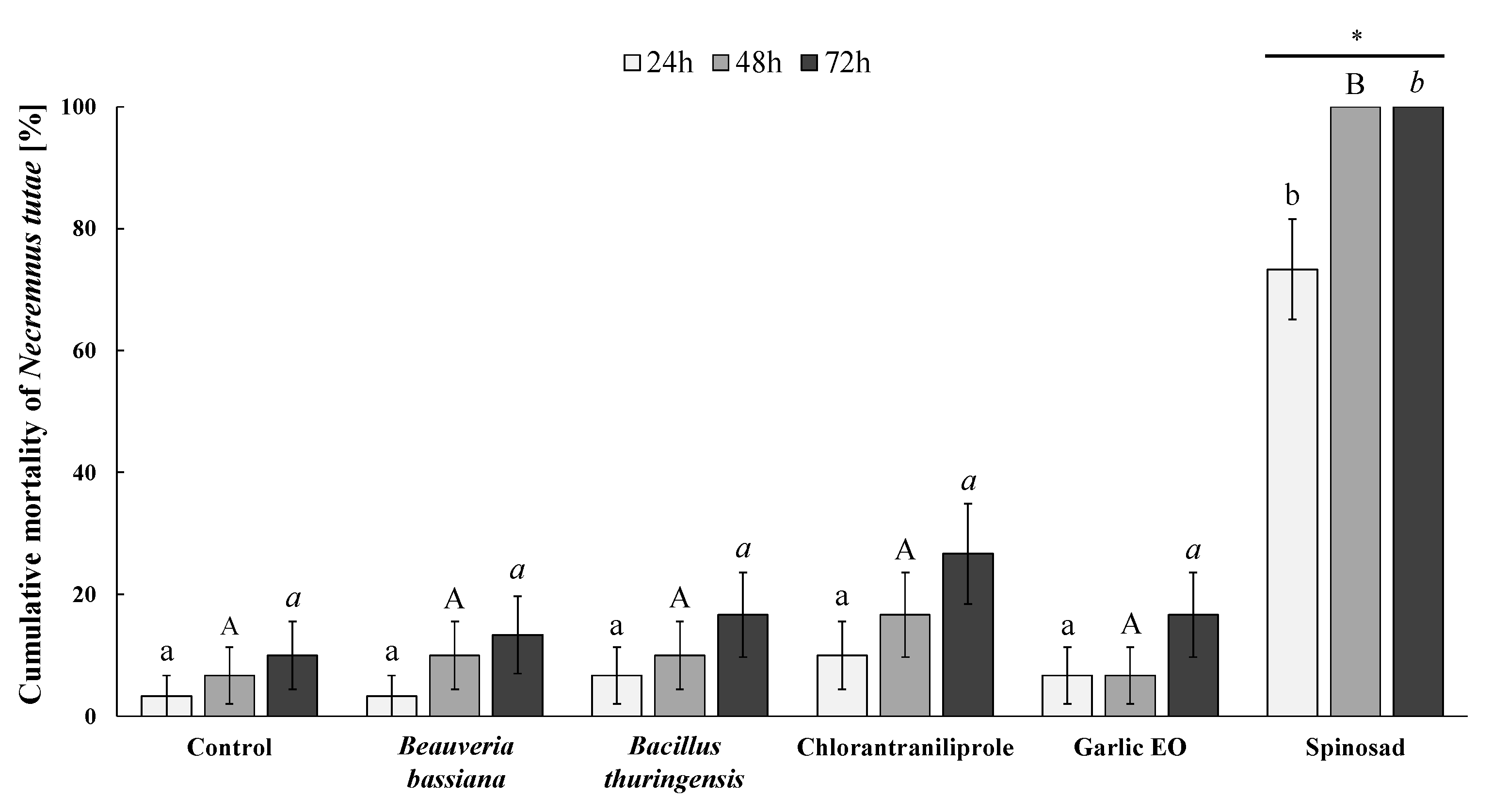
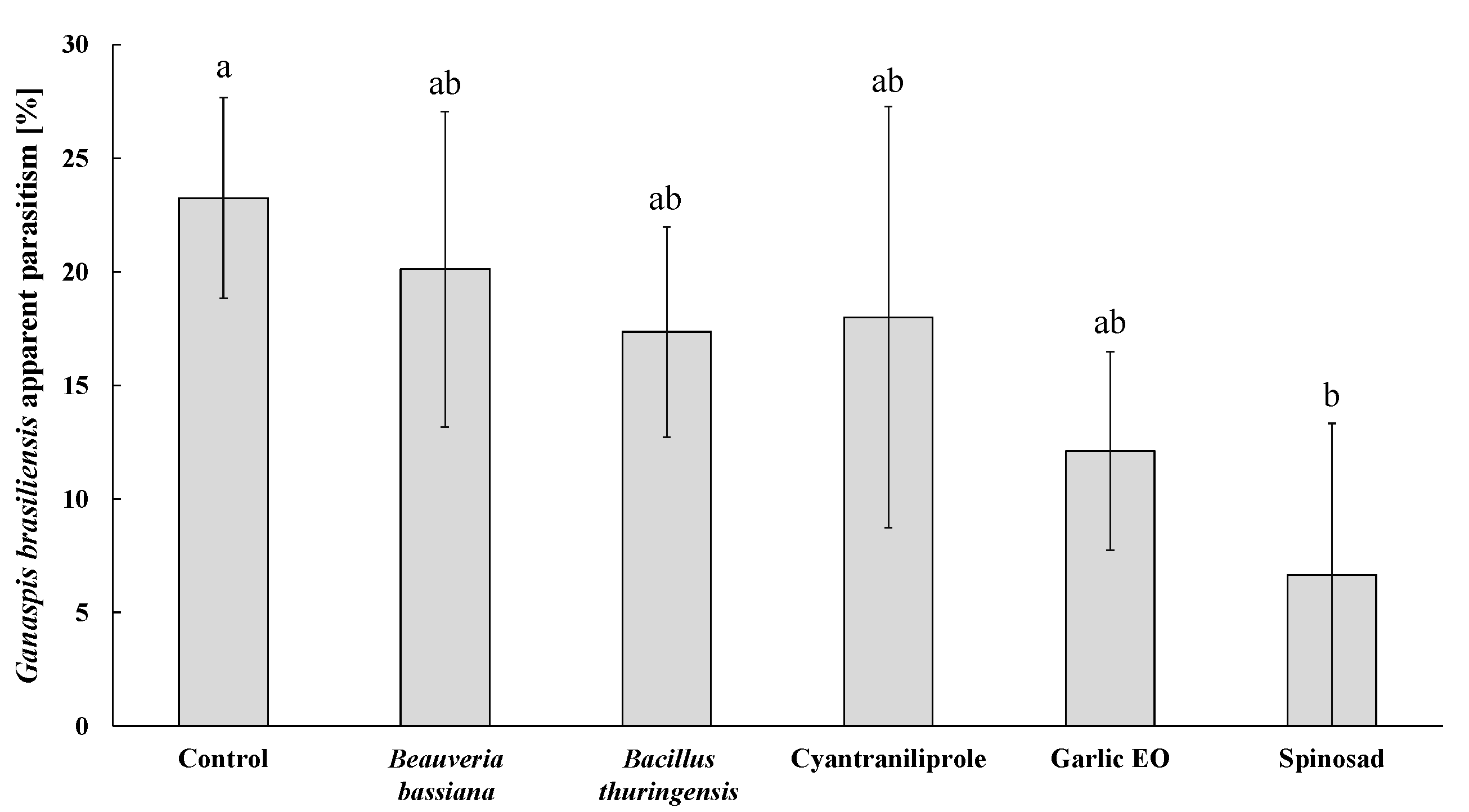
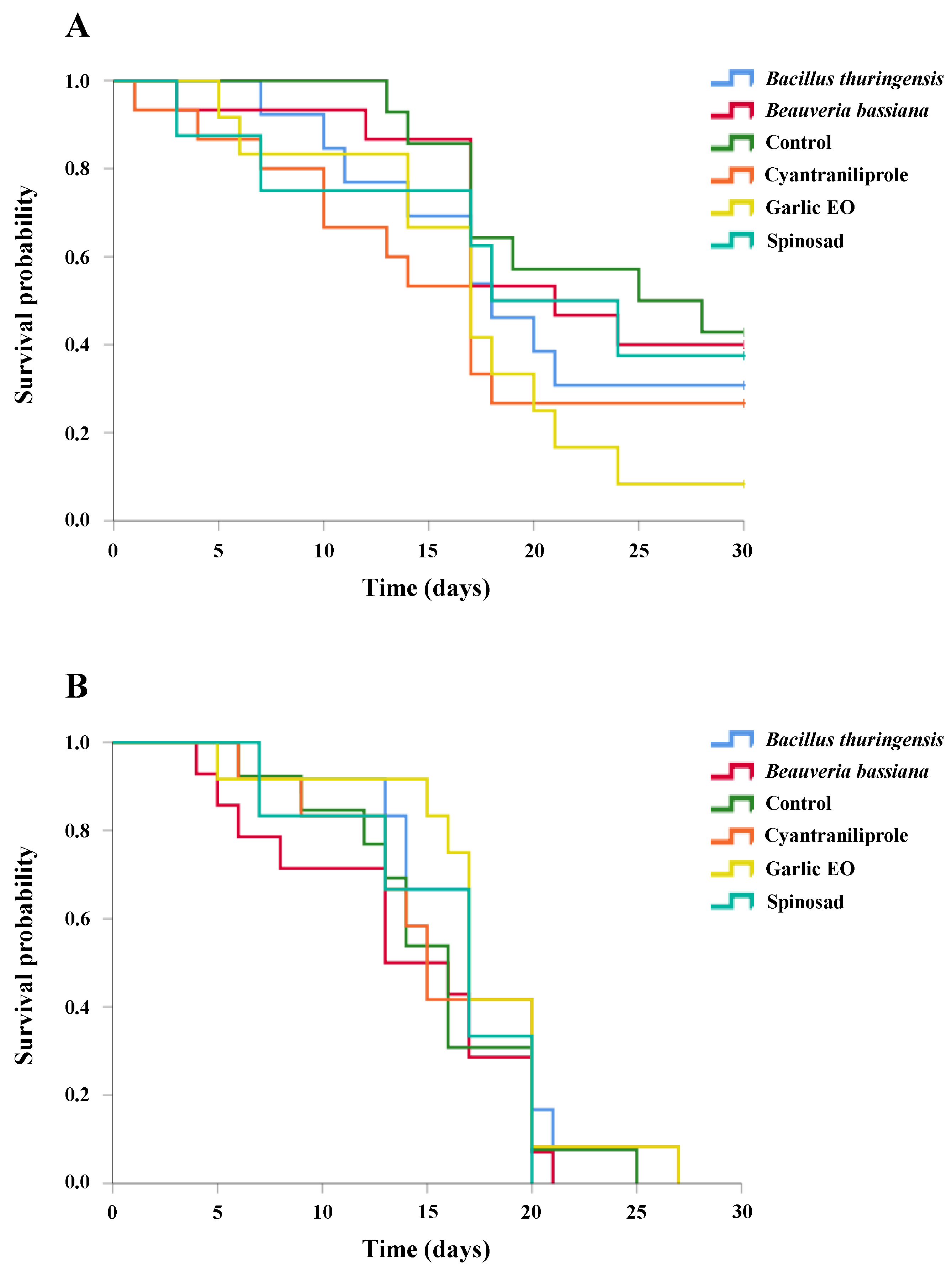

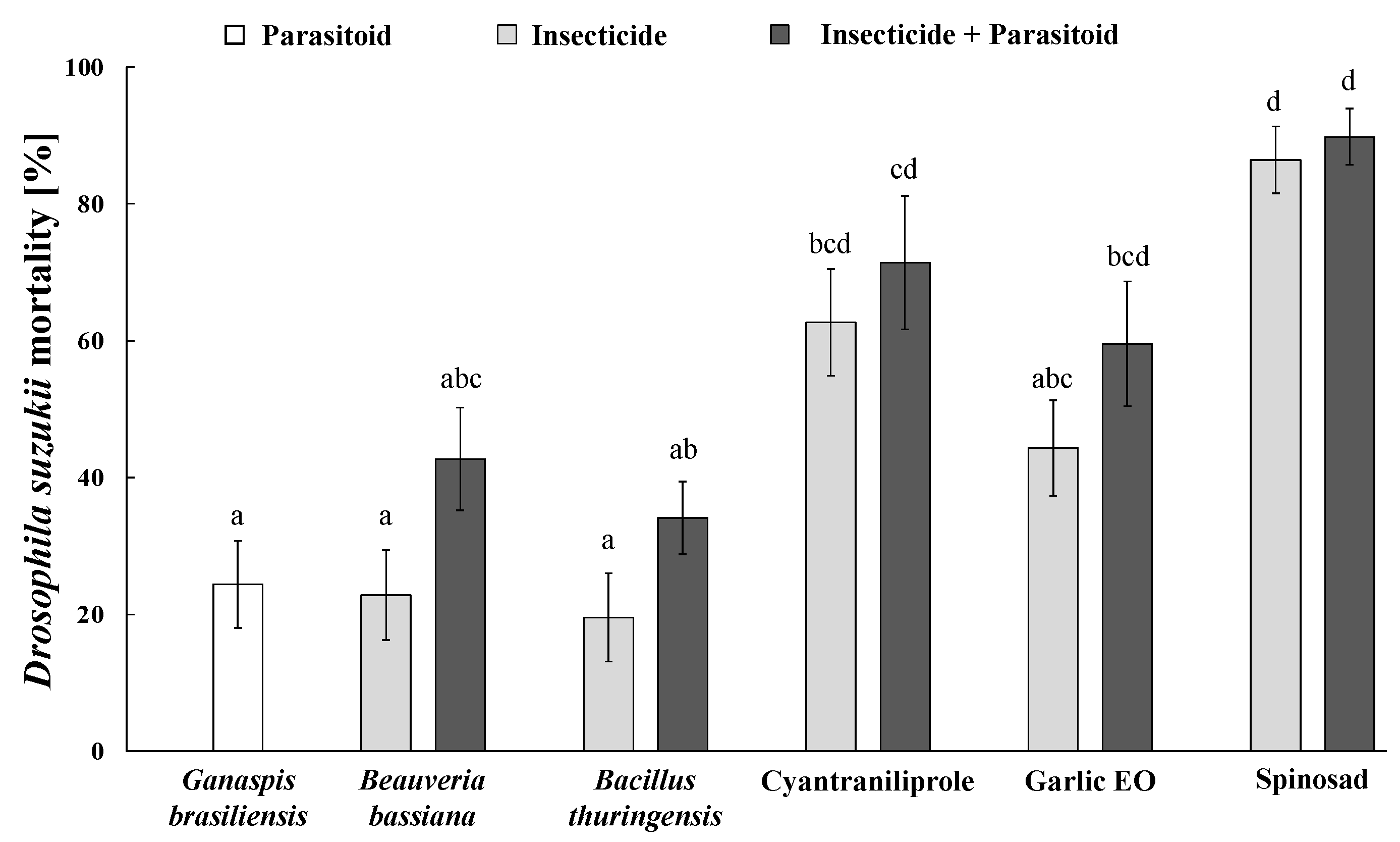
| Treatment | Non-Reproductive Mortality | Reproductive Mortality | Parasitoid Juvenile Survival |
|---|---|---|---|
| Control (water) | 46.00 ± 3.06 a | 36.00 ± 3.88 a | 60.89 ± 7.79 a |
| Beauveria bassiana | 36.00 ± 3.49 a | 34.00 ± 3.75 ab | 61.56 ± 7.89 a |
| Bacillus thuringiensis | 38.67 ± 3.76 a | 29.33 ± 4.52 ab | 42.56 ± 9.76 ab |
| Chlorantraniliprole | 16.67 ± 3.98 b | 23.33 ± 3.86 b | 36.67 ± 8.94 b |
| Garlic EO | 7.33 ± 2.48 bc | 9.33 ± 2.67 c | 36.67 ± 11.52 b |
| Spinosad | 2.67 ± 1.18 c | 4.00 ± 1.90 c | 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisi, F.; Cavallaro, C.; Pitruzzello, M.F.; Arnó, J.; Desneux, N.; Han, P.; Wang, X.; Zappalà, L.; Biondi, A.; Gugliuzzo, A. Compatibility of Bioinsecticides with Parasitoids for Enhanced Integrated Pest Management of Drosophila suzukii and Tuta absoluta. Insects 2024, 15, 467. https://doi.org/10.3390/insects15070467
Lisi F, Cavallaro C, Pitruzzello MF, Arnó J, Desneux N, Han P, Wang X, Zappalà L, Biondi A, Gugliuzzo A. Compatibility of Bioinsecticides with Parasitoids for Enhanced Integrated Pest Management of Drosophila suzukii and Tuta absoluta. Insects. 2024; 15(7):467. https://doi.org/10.3390/insects15070467
Chicago/Turabian StyleLisi, Fabrizio, Carmelo Cavallaro, Maria Flavia Pitruzzello, Judit Arnó, Nicolas Desneux, Peng Han, Xingeng Wang, Lucia Zappalà, Antonio Biondi, and Antonio Gugliuzzo. 2024. "Compatibility of Bioinsecticides with Parasitoids for Enhanced Integrated Pest Management of Drosophila suzukii and Tuta absoluta" Insects 15, no. 7: 467. https://doi.org/10.3390/insects15070467







