Unveiling the Microbiome Diversity in Telenomus (Hymenoptera: Scelionidae) Parasitoid Wasps
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Gut Collection
2.2. Extraction of DNA
2.3. Bioinformatics Analysis
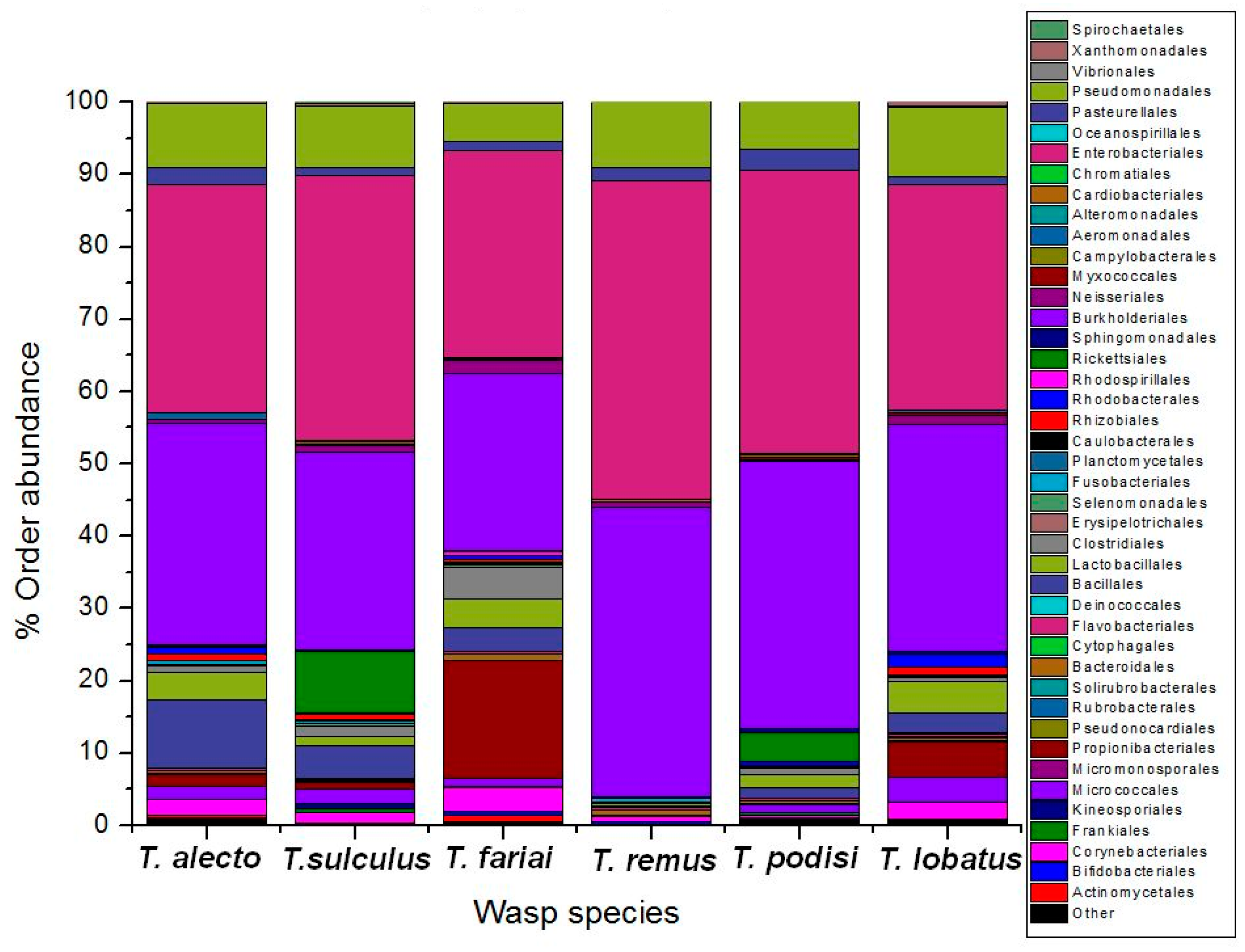
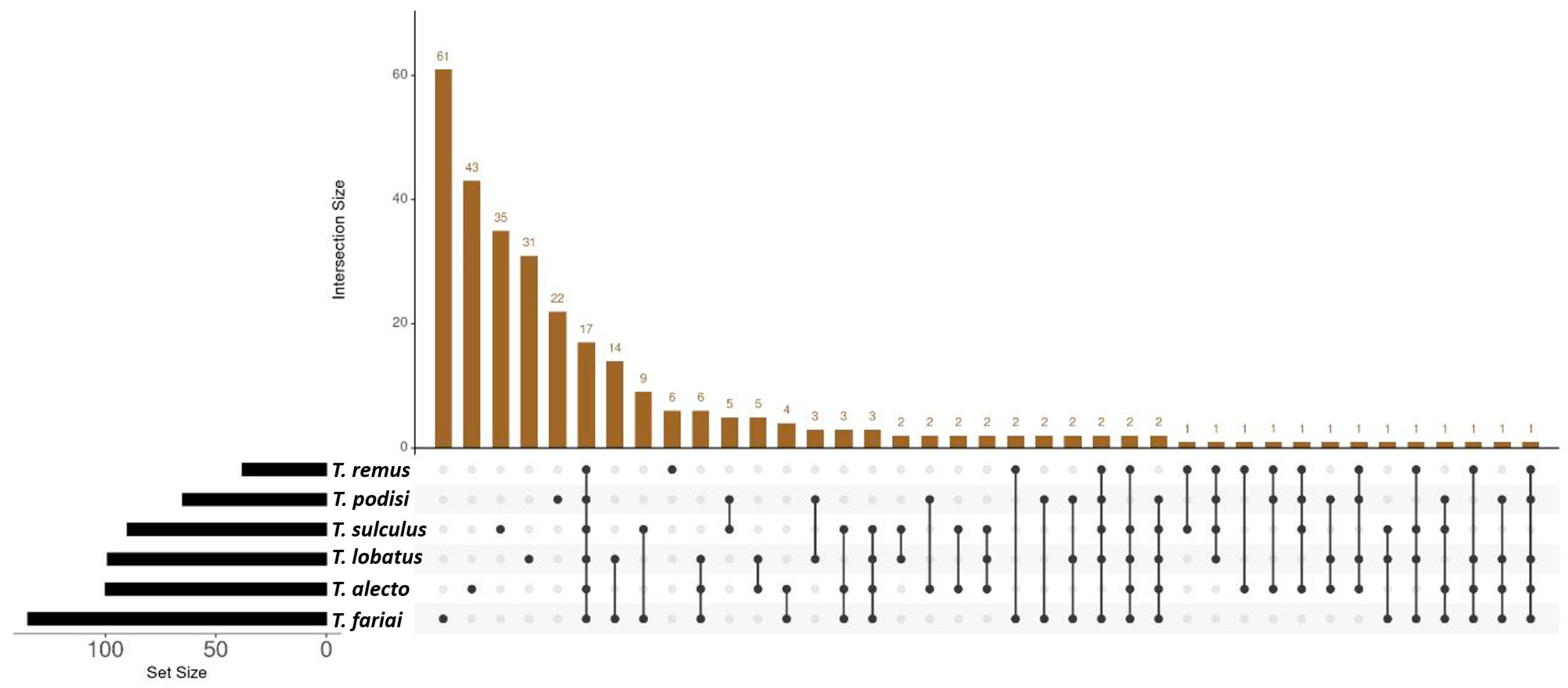
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masner, L. Revisionary notes and keys to world genera of Scelionidae (Hymenoptera: Proctotrupoidea). Mem. Entomol. Soc. Can. 1976, 108, 1–87. [Google Scholar] [CrossRef]
- Johnson, N.F. Systematics of Nearctic Telenomus: Classification and revisions of the podisi and phymatae species groups (Hymenoptera: Scelionidae). Bull. Ohio Biol. Surv. 1984, 6, 1–118. [Google Scholar]
- Bueno, R.C.O.D.F.; Carneiro, T.R.; Bueno, A.D.F.; Pratissoli, D.; Fernandes, O.A.; Vieira, S.S. Parasitism capacity of Telenomus remus Nixon (Hymenoptera: Scelionidae) on Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) eggs. Braz. Arch. Biol. Technol. 2010, 53, 133–139. [Google Scholar] [CrossRef]
- Ramírez-Ahuja, M.L.; Gómez-Govea, M.A.; Trujillo-Rodríguez, G.J.; Garza-González, E.; Rodriguez-Sanchez, I.P.; Talamas, E.J. Telenomus alecto (Crawford) (Hymenoptera: Scelionidae), parasitoid of Diatraea magnifactella Dyar (Lepidoptera: Crambidae) from Jalisco, Mexico: A study based on morphological and molecular evidence. Fla. Entomol. 2013, 105, 307–312. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects–diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Gloder, G.; Bourne, M.E.; Verreth, C.; Wilberts, L.; Bossaert, S.; Crauwels, S.; Dicke, M.; Poelman, E.H.; Jacquemyn, H.; Lievens, B. Parasitism by endoparasitoid wasps alters the internal but not the external microbiome in host caterpillars. Anim. Microbiome 2021, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R. The capacious hologenome. Zoology 2013, 116, 260–261. [Google Scholar]
- Colmenarez, Y.C.; Babendreier, D.; Ferrer Wurst, F.R.; Vásquez-Freytez, C.L.; de Freitas Bueno, A. The use of Telenomus remus (Nixon, 1937) (Hymenoptera: Scelionidae) in the management of Spodoptera spp.: Potential, challenges and major benefits. CABI Agric. Biosci. 2022, 3, 5. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Hu, H.; Wang, G.H. Nasonia–microbiome associations: A model for evolutionary hologenomics research. Trends Parasitol. 2022, 39, 101–112. [Google Scholar] [CrossRef]
- Munoz-Benavent, M.; Perez-Cobas, A.E.; Garcia-Ferris, C.; Moya, A.; Latorre, A. Insects’ potential: Understanding the functional role of their gut microbiome. J. Pharm. Biomed. Anal. 2021, 194, 113787. [Google Scholar] [CrossRef] [PubMed]
- Risely, A. Applying the core microbiome to understand host–microbe systems. J. Anim. Ecol. 2020, 89, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Moran, N.A.; Baumann, L. The evolution and genetics of aphid endosymbionts. Bioscience 1997, 47, 12–20. [Google Scholar] [CrossRef]
- Feldhaar, H.; Straka, J.; Krischke, M.; Berthold, K.; Stoll, S.; Mueller, M.J.; Gross, R. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol. 2007, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Iturbe-Ormaetxe, I.; Walker, T.; O’Neill, S.L. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011, 12, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Adachi-Hagimori, T.; Miura, K.; Stouthamer, R. A new cytogenetic mechanism for bacterial endosymbiont-induced parthenogenesis in Hymenoptera. Proc. Roy. Soc. B-Biol. Sci. 2008, 275, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Gerth, M.; Saeed, A.; White, J.A.; Bleidorn, C. Extensive screen for bacterial endosymbionts reveals taxon-specific distribution patterns among bees (Hymenoptera, Anthophila). FEMS Microbiol. Ecol. 2015, 91, fiv047. [Google Scholar] [CrossRef] [PubMed]
- Jeyaprakash, A.; Hoy, M.A.; Allsopp, M.H. Bacterial diversity in worker adults of Apis mellifera capensis and Apis mellifera scutellata (Insecta: Hymenoptera) assessed using 16S rRNA sequences. J. Invertebr. Pathol. 2003, 84, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Martinson, V.G.; Danforth, B.N.; Minckley, R.L.; Rueppell, O.; Tingek, S.; Moran, N.A. A simple and distinctive microbiota associated with honey bees and bumble bees. Mol. Ecol. 2011, 20, 619–628. [Google Scholar] [CrossRef]
- Martinson, V.G.; Moy, J.; Moran, N.A. Establishment of characteristic gut bacteria during development of the honeybee worker. Appl. Environ. Microbiol. 2012, 78, 2830–2840. [Google Scholar] [CrossRef]
- Graystock, P.; Rehan, S.M.; McFrederick, Q.S. Hunting for healthy microbiomes: Determining the core microbiomes of Ceratina, Megalopta, and Apis bees and how they associate with microbes in bee collected pollen. Conserv. Genet. 2017, 18, 701–711. [Google Scholar] [CrossRef]
- Koch, H.; Schmid-Hempel, P. Bacterial communities in central european bumblebees: Low diversity and high specificity. Microb. Ecol. 2011, 62, 121–133. [Google Scholar] [CrossRef]
- Gurung, K.; Wertheim, B.; Falcao Salles, J. The microbiome of pest insects: It is not just bacteria. Entomol. Exp. Appl. 2019, 167, 156–170. [Google Scholar] [CrossRef]
- Gómez-Govea, M.A.; Ramírez-Ahuja, M.D.L.; Contreras-Perera, Y.; Jiménez-Camacho, A.J.; Ruiz-Ayma, G.; Villanueva-Segura, O.K.; Trujillo-Rodriguez, G.D.J.; Delgado-Enciso, I.; Martínez-Fierro, M.L.; Manrique-Saide, P.; et al. Suppression of Midgut Microbiota impact pyrethroid susceptibility in Aedes aegypti. Front. Microbiol. 2022, 13, 761459. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Huttley, G.A. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Dicke, M.; Cusumano, A.; Poelman, E.H. Microbial symbionts of parasitoids. Annu. Rev. Entomol. 2020, 65, 171–190. [Google Scholar] [CrossRef]
- Ramírez-Ahuja, M.L.; Gómez-Govea, M.A.; Lugo-Trampe, A.; Borrego-Soto, G.; Delgado-Enciso, I.; Ponce-Garcia, G.; Martínez-Fierro, M.L.; Ramírez-Valles, E.G.; Treviño, V.; Flores-Suarez, A.E.; et al. Microbiota of Telenomus tridentatus (Platygastroidea: Scelionidae): An unwanted parasitoid. J. Appl. Entomol. 2019, 143, 834–841. [Google Scholar] [CrossRef]
- Bright, M.; Bulgheresi, S. A complex journey: Transmission of microbial symbionts. Nat. Rev. Microbiol. 2010, 8, 218–230. [Google Scholar] [CrossRef]
- Bhat, S.V.; Maughan, H.; Cameron, A.D.; Yost, C.K. Phylogenomic analysis of the genus Delftia reveals distinct major lineages with ecological specializations. Microb. Genom. 2022, 8, 000864. [Google Scholar] [CrossRef]
- Tehara, S.K.; Keasling, J.D. Gene cloning, purification, and characterization of a phosphodiesterase from Delftia acidovorans. AEM 2003, 69, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Hail, D.; Lauzìere, I.; Dowd, S.E.; Bextine, B. Culture independent survey of the microbiota of the glassy-winged sharpshooter (Homalodisca vitripennis) using 454 pyrosequencing. Environ. Entomol. 2011, 40, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Deming, C.; Cassidy, S.K.B.; Harrison, O.J.; Ng, W.-I.; Conlan, S.; NISC Comparative Sequencing Program; Belkaid, Y.; Segre, J.A.; Kong, H.H. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 2017, 9, eaal4651. [Google Scholar] [PubMed]
- Tejman-Yarden, N.; Robinson, A.; Davidov, Y.; Shulman, A.; Varvak, A.; Reyes, F.; Rahav, G.; Nissan, I. Delftibactin-A, a Non-ribosomal Peptide with Broad Antimicrobial Activity. Front. Microbiol. 2019, 10, 2377. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, T.; Matsumoto, Y.; Cho, O.; Ogasawara, Y.; Sugita, T. Delftia acidovorans secretes substances that inhibit the growth of Staphylococcus epidermidis through TCA cycle-triggered ROS production. PLoS ONE 2021, 16, e0253618. [Google Scholar] [CrossRef] [PubMed]
- Dee Tan, I.Y.; Bautista, M.A.M. Bacterial Survey in the Guts of Domestic Silkworms, Bombyx mori L. Insects 2022, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.E.; Emche, S.; Shao, J.; Simpkins, A.; Summers, R.M.; Mock, M.B.; Ebert, D.; Infante, F.; Aoki, S.; Maul, J.E. Cultivation and genome sequencing of bacteria isolated from the coffee berry borer (Hypothenemus hampei), with emphasis on the role of caffeine degradation. Front. Microbiol. 2021, 12, 644768. [Google Scholar] [CrossRef]
- Almeida, L.G.D.; Moraes, L.A.B.D.; Trigo, J.R.; Omoto, C.; Consoli, F.L. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation. PLoS ONE 2017, 12, e0174754. [Google Scholar] [CrossRef]
- Kuncham, R.; Sivaprakasam, T.; Kumar, R.P.; Sreenath, P.; Nayak, R.; Thayumanavan, T.; Reddy, G.V.S. Bacterial fauna associating with chironomid larvae from lakes of Bengaluru city, India-A 16s rRNA gene based identification. Genom. Data. 2017, 12, 44–48. [Google Scholar] [CrossRef]
- Rossi, J.P.; Rasplus, J.Y. Climate change and the potential distribution of the glassy-winged sharpshooter (Homalodisca vitripennis), an insect vector of Xylella fastidiosa. Sci. Total Environ. 2023, 860, 160375. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Delalibera, I., Jr.; Handelsman, J.; Klepzig, K.D.; Schloss, P.D.; Raffa, K.F. Characterization of gut-associated bacteria in larvae and adults of the southern pine beetle, Dendroctonus frontalis Zimmermann. Environ. Entomol. 2006, 35, 1710–1717. [Google Scholar] [CrossRef]
- Geiger, A.; Fardeau, M.L.; Grebaut, P.; Vatunga, G.; Josénando, T.; Herder, S.; Cuny, G.; Truc, P.; Ollivier, B. First isolation of Enterobacter, Enterococcus, and Acinetobacter spp. as inhabitants of the tsetse fly (Glossina palpalis palpalis) midgut. Infect. Genet. Evol. 2009, 9, 1364–1370. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Kyritsis, G.A.; Papadopoulos, N.T.; Abd-Alla, A.M.; Cáceres, C.; Bourtzis, K. Exploitation of the medfly gut microbiota for the enhancement of sterile insect technique: Use of Enterobacter sp. in larval diet-based probiotic applications. PLoS ONE 2015, 10, e0136459. [Google Scholar] [CrossRef]
- Stathopoulou, P.M.; Asimakis, E.; Tsiamis, G. Enterobacter: One bacterium multiple functions. In Area-Wide Integrated Pest Management. Development and Field Application; CRC Press: Boca Raton, FL, USA, 2021; pp. 917–945. [Google Scholar]
- Kyritsis, G.A.; Augustinos, A.A.; Ntougias, S.; Papadopoulos, N.T.; Bourtzis, K.; Cáceres, C. Enterobacter sp. AA26 gut symbiont as a protein source for Mediterranean fruit fly mass-rearing and sterile insect technique applications. BMC Microbiol. 2019, 19, 288. [Google Scholar] [CrossRef] [PubMed]
- Cavichiolli de Oliveira, N.; Cônsoli, F.L. Beyond host regulation: Changes in gut microbiome of permissive and non-permissive hosts following parasitization by the wasp Cotesia flavipes. FEMS Microbiol. Ecol. 2020, 96, fiz206. [Google Scholar] [CrossRef] [PubMed]
- Freitak, D.; Schmidtberg, H.; Dickel, F.; Lochnit, G.; Vogel, H.; Vilcinskas, A. The maternal transfer of bacteria can mediate trans-generational immune priming in insects. Virulence 2014, 5, 547–554. [Google Scholar] [CrossRef]
- Arakaki, N.; Noda, H.; Yamagishi, K. Wolbachia-induced parthenogenesis in the egg parasitoid Telenomus nawai. Entomol. Exp. Appl. 2000, 96, 177–184. [Google Scholar] [CrossRef]
- Jeong, G.; Stouthamer, R. Genetics of female functional virginity in the parthenogenesis-Wolbachia infected parasitoid wasp Telenomus nawai (Hymenoptera: Scelionidae). Heredity 2005, 94, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Saati-Santamaría, Z.; Rivas, R.; Kolařik, M.; García-Fraile, P. A new perspective of Pseudomonas—Host interactions: Distribution and potential ecological functions of the genus Pseudomonas within the Bark Beetle Holobiont. Biology 2021, 10, 164. [Google Scholar] [CrossRef]
- Clark, L.L.; Dajcs, J.J.; McLean, C.H.; Bartell, J.G.; Stroman, D.W. Pseudomonas otitidis sp. nov., isolated from patients with otic infections. Int. J. Syst. Evol. Microbiol. 2006, 56, 709–714. [Google Scholar] [CrossRef]
- Perry, A.L.; Lambert, P.A. Propionibacterium acnes. Lett. Appl. Microbiol. 2006, 42, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Kim, H.H.; Yoo, J.Y.; Lim, S.K.; Yoon, S.S.; Cho, I.S. Isolation and identification of Moraxella cuniculi from a rabbit with keratoconjunctivitis. KJVR 2017, 57, 201–204. [Google Scholar] [CrossRef]
- Salem, H.; Florez, L.; Gerardo, N.; Kaltenpoth, M. An out-of-body experience: The extracellular dimension for the transmission of mutualistic bacteria in insects. Proc. R. Soc. Lond. B Biol. Sci. 2015, 282, 20142957. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Janzen, D.H.; Hallwachs, W.; Jaffe, S.P.; Fierer, N. Caterpillars lack a resident gut microbiome. Proc. Natl. Acad. Sci. USA 2017, 114, 9641–9646. [Google Scholar] [CrossRef] [PubMed]
- Paniagua Voirol, L.R.; Frago, E.; Kaltenpoth, M.; Hilker, M.; Fatouros, N.E. Bacterial symbionts in Lepidoptera: Their diversity, transmission, and impact on the host. Front. Microbiol. 2018, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Carmo, E.D.; Bueno, A.; Bueno, R.C.O.F. Pesticide selectivity for the insect egg parasitoid Telenomus remus. BioControl 2010, 55, 455–464. [Google Scholar] [CrossRef]
- Parra, J.R.P. Mass rearing of egg parasitoids for biological control programs. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma, Progress in Biological Control (PIBC); Springer: Berlin/Heidelberg, Germany, 2010; pp. 267–292. [Google Scholar]
- Cave, R.D. Biology, ecology and use in pest management of Telenomus remus. Biocontrol News Inf. 2000, 21, 21N–26N. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Govea, M.A.; Peña-Carillo, K.I.; Ruiz-Ayma, G.; Guzmán-Velasco, A.; Flores, A.E.; Ramírez-Ahuja, M.d.L.; Rodríguez-Sánchez, I.P. Unveiling the Microbiome Diversity in Telenomus (Hymenoptera: Scelionidae) Parasitoid Wasps. Insects 2024, 15, 468. https://doi.org/10.3390/insects15070468
Gómez-Govea MA, Peña-Carillo KI, Ruiz-Ayma G, Guzmán-Velasco A, Flores AE, Ramírez-Ahuja MdL, Rodríguez-Sánchez IP. Unveiling the Microbiome Diversity in Telenomus (Hymenoptera: Scelionidae) Parasitoid Wasps. Insects. 2024; 15(7):468. https://doi.org/10.3390/insects15070468
Chicago/Turabian StyleGómez-Govea, Mayra A., Kenzy I. Peña-Carillo, Gabriel Ruiz-Ayma, Antonio Guzmán-Velasco, Adriana E. Flores, María de Lourdes Ramírez-Ahuja, and Iram Pablo Rodríguez-Sánchez. 2024. "Unveiling the Microbiome Diversity in Telenomus (Hymenoptera: Scelionidae) Parasitoid Wasps" Insects 15, no. 7: 468. https://doi.org/10.3390/insects15070468
APA StyleGómez-Govea, M. A., Peña-Carillo, K. I., Ruiz-Ayma, G., Guzmán-Velasco, A., Flores, A. E., Ramírez-Ahuja, M. d. L., & Rodríguez-Sánchez, I. P. (2024). Unveiling the Microbiome Diversity in Telenomus (Hymenoptera: Scelionidae) Parasitoid Wasps. Insects, 15(7), 468. https://doi.org/10.3390/insects15070468






