The Fumigation Toxicity of Three Benzoate Compounds against Phosphine-Susceptible and Phosphine-Resistant Strains of Rhyzopertha dominica and Sitophilus oryzae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Chemicals
2.3. PH3-Resistance Assessment
2.4. Fumigation Toxicity of Benzoate Compounds against Adults of R. dominica
2.5. Fumigation Toxicity of Benzoate Compounds against Adults of S. oryzae
2.6. Fumigation Toxicity of Methyl Benzoate against R. dominica and S. oryzae under Feeding Conditions
2.7. Statistical Analyses
3. Results
3.1. PH3 Resistance
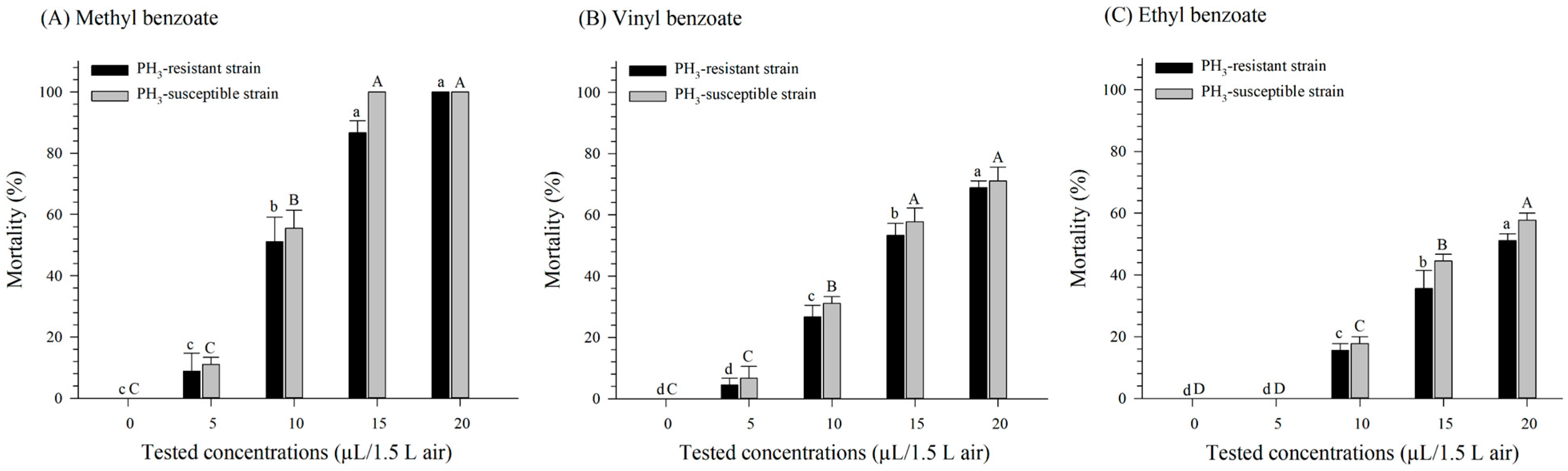
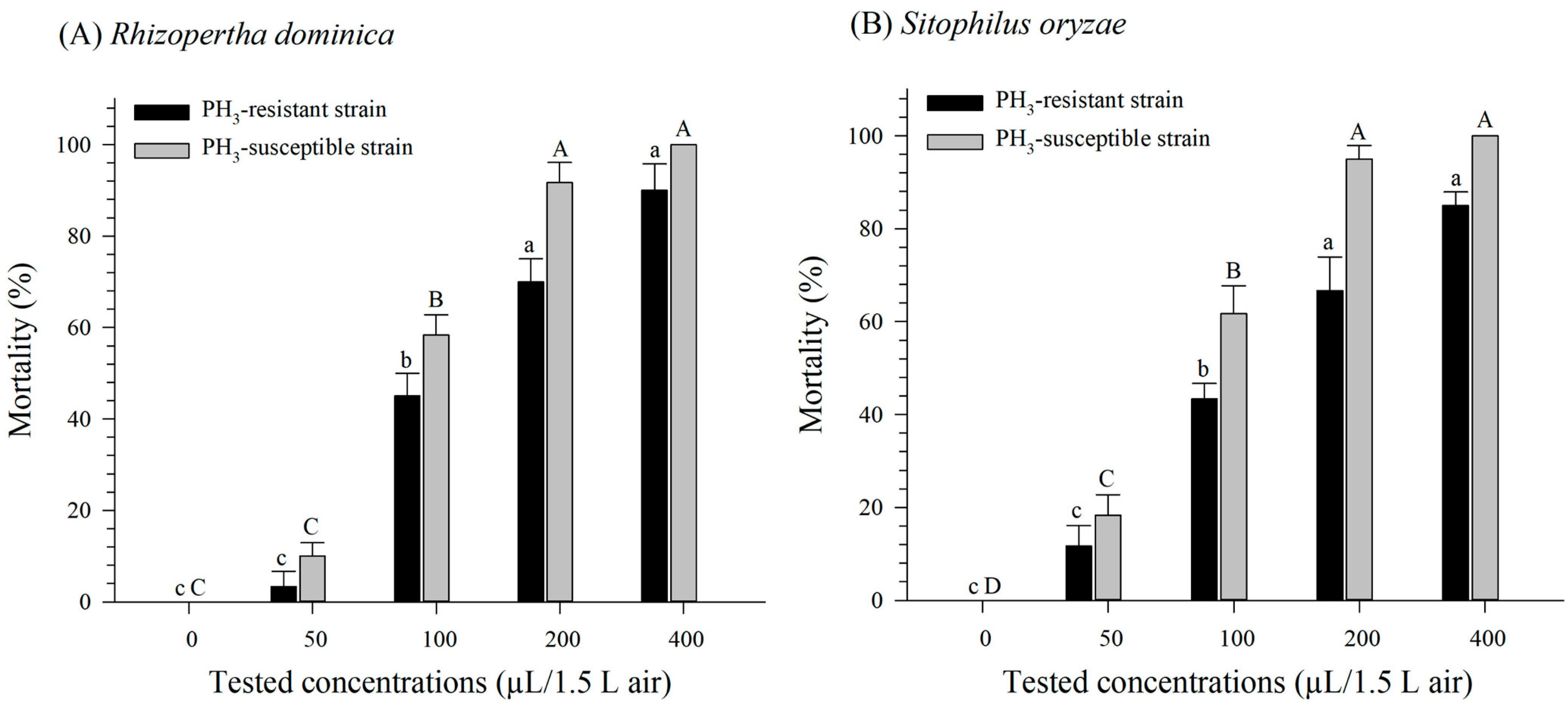
3.2. Fumigation Toxicity of Benzoate Compounds against PH3-Susceptible and -Resistant R. dominica Strains
3.3. Fumigation Toxicity of Benzoate Compounds against PH3-Susceptible and -Resistant S. oryzae Strains
3.4. Fumigation Toxicity of MBe against PH3-Susceptible and -Resistant R. dominica and S. oryzae under Feeding Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stathas, I.G.; Sakellaridis, A.C.; Papadelli, M.; Kapolos, J.; Papadimitriou, K.; Stathas, G.J. The effects of insect infestation on stored agricultural products and the quality of food. Foods 2023, 12, 2046. [Google Scholar] [CrossRef] [PubMed]
- Taddese, M.; Dibaba, K.; Bayissa, W.; Hunde, D.; Mendesil, E.; Kassie, M.; Mutungi, C.; Tefera, T. Assessment of quantitative and qualitative losses of stored grains due to insect infestation in Ethiopia. J. Stored Prod. Res. 2020, 89, 101689. [Google Scholar] [CrossRef]
- Stejskal, V.; Bostlova, M.; Nesvorna, M.; Volek, V.; Dolezal, V.; Hubert, J. Comparison of the resistance of mono-and multilayer packaging films to stored-product insects in a laboratory test. Food Control 2017, 73, 566–573. [Google Scholar] [CrossRef]
- Hubert, J.; Erban, T.; Nesvorna, M.; Stejskal, V. Emerging risk of infestation and contamination of dried fruits by mites in the Czech Republic. Food Addit. Contam. Part A Chem. 2011, 28, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- El Melki, M.N.; Al-Khayri, J.M.; Aldaej, M.I.; Almaghasla, M.I.; El Moueddeb, K.; Khlifi, S. Assessment of the effect of climate change on wheat storage in northwestern Tunisia: Control of Rhyzopertha dominica by aeration. Agronomy 2023, 13, 1773. [Google Scholar] [CrossRef]
- Singano, C.D.; Mvumi, B.M.; Stathers, T.E.; Machekano, H.; Nyamukondiwa, C. What does global warming mean for stored-grain protection? Options for Prostephanus truncatus (Horn) control at increased temperatures. J. Stored Prod. Res. 2020, 85, 101532. [Google Scholar] [CrossRef]
- Stejskal, V.; Vendl, T.; Kolar, V.; Zhihong, L.; Aulicky, R. First population quantification of the infestation of legumes by stored-product bruchids imported in freight containers into Europe. Bull. Insectol. 2020, 73, 233–239. [Google Scholar]
- Jian, F. Influences of stored product insect movements on integrated pest management decisions. Insects 2019, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Gerken, A.R.; Morrison III, W.R. Pest management in the postharvest agricultural supply chain under climate change. Front. Agron. 2022, 4, 918845. [Google Scholar] [CrossRef]
- Mbata, G.N.; Toews, M.D. Recent advances in postharvest pest biology and management. Insects 2021, 12, 543. [Google Scholar] [CrossRef]
- Zettler, J.L.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Budnik, L.T.; Kloth, S.; Velasco-Garrido, M.; Baur, X. Prostate cancer and toxicity from critical use exemptions of methyl bromide: Environmental protection helps protect against human health risks. Environ. Health 2012, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Nicewonger, M.; Saltzman, E.S.; Montzka, S. ENSO-driven fires cause large interannual variability in the naturally emitted, ozone-depleting trace gas CH3Br. Geophys. Res. Lett. 2022, 49, e2021GL094756. [Google Scholar] [CrossRef]
- Alzahrani, S.M.; Ebert, P.R. Pesticidal toxicity of phosphine and its interaction with other pest control treatments. Curr. Issues Mol. Biol. 2023, 45, 2461–2473. [Google Scholar] [CrossRef]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef]
- Ranabhat, S.; Zhu, K.Y.; Bingham, G.V.; Morrison, W.R., III. Mobility of phosphine-susceptible and -resistant Rhyzopertha dominica (Coleoptera: Bostrichidae) and Tribolium castaneum (Coleoptera: Tenebrionidae) after exposure to controlled release materials with existing and novel active ingredients. J. Econ. Entomol. 2022, 115, 888–903. [Google Scholar] [CrossRef]
- Agrafioti, P.; Athanassiou, C.; Nayak, M. Detection of phosphine resistance in major stored-product insects in Greece and evaluation of a field resistance test kit. J. Stored Prod. Res. 2019, 82, 40–47. [Google Scholar] [CrossRef]
- Danso, J.K.; Opit, G.P.; Noden, B.H.; Giles, K.L. Estimating discriminating doses of phosphine for adults of eight species of psocids of genera Liposcelis (Psocodea: Liposcelididae) and Lepinotus (Psocodea: Trogiidae). J. Stored Prod. Res. 2022, 99, 102025. [Google Scholar] [CrossRef]
- Barakat, D.A.; Flingelli, C.; Reichmuth, C. Lethal effect of sulfuryl fluoride on eggs of different age of the Indian meal moth Plodia interpunctelia (Hübner)-demonstration of the no constancy of the ct product for control. J. Kult. 2011, 63, 323–332. [Google Scholar]
- Haritos, V.S.; Damcevski, K.A.; Dojchinov, G. Improved efficacy of ethyl formate against stored grain insects by combination with carbon dioxide in a ‘dynamic’application. Pest Manag. Sci. 2006, 62, 325–333. [Google Scholar] [CrossRef]
- Rambeau, M.; Benitez, D.; Dupuis, S.; Ducom, P. Hydrogen Cyanide as an Immediate Alternative to Methyl Bromide for Structural Fumigations. In Proceedings of the International Conference on Controlled Atmosphere and Fumigation in Stored Products; Executive Printing Services: Clovis, CA, USA, 2001; pp. 101–110. Available online: http://ftic.co.il/caf2000-en.php (accessed on 18 April 2024).
- Liu, Y.-B. Nitric oxide as a potent fumigant for postharvest pest control. J. Econ. Entomol. 2013, 106, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Mühle, J.; Huang, J.; Weiss, R.; Prinn, R.; Miller, B.; Salameh, P.; Harth, C.; Fraser, P.; Porter, L.; Greally, B. Sulfuryl fluoride in the global atmosphere. J. Geophys. Res. Atmos. 2009, 114, D05306. [Google Scholar] [CrossRef]
- Duke, S.O.; Cantrell, C.L.; Meepagala, K.M.; Wedge, D.E.; Tabanca, N.; Schrader, K.K. Natural toxins for use in pest management. Toxins 2010, 2, 1943–1962. [Google Scholar] [CrossRef] [PubMed]
- Giunti, G.; Benelli, G.; Palmeri, V.; Laudani, F.; Ricupero, M.; Ricciardi, R.; Maggi, F.; Lucchi, A.; Guedes, R.N.C.; Desneux, N. Non-target effects of essential oil-based biopesticides for crop protection: Impact on natural enemies, pollinators, and soil invertebrates. Biol. Control 2022, 176, 105071. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.; Zhang, A. Commercially available natural benzyl esters and their synthetic analogs exhibit different toxicities against insect pests. Sci. Rep. 2018, 8, 7902. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, A. A floral fragrance, methyl benzoate, is an efficient green pesticide. Sci. Rep. 2017, 7, 42168. [Google Scholar] [CrossRef]
- Larson, N.R.; Zhang, A.; Feldlaufer, M.F. Fumigation activities of methyl benzoate and its derivatives against the common bed bug (Hemiptera: Cimicidae). J. Med. Entomol. 2019, 57, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Ryu, J.; Akintola, A.A.; Choi, K.S.; Hwang, U.W.; Hassan, E.; Lee, K.-Y. Larvicidal activity of methyl benzoate, a volatile organic compound, against the mosquitoes Aedes albopictus and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2022, 59, 788–794. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.B.; Feng, Y.; Zhang, A. Methyl benzoate fumigation for control of post-harvest pests and its effects on apple quality. J. Appl. Entomol. 2020, 144, 191–200. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Güncan, A.; Lee, K.-Y. Evaluation of lethal and sublethal effects of methyl benzoate on the generalist predator Orius laevigatus (Fieber). J. Econ. Entomol. 2022, 115, 1911–1920. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Lee, K.-Y. Methyl benzoate as a promising, environmentally safe insecticide: Current status and future perspectives. Agriculture 2022, 12, 378. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Insecticidal efficacy of three benzoate derivatives against Aphis gossypii and its predator Chrysoperla carnea. Ecotoxicol. Environ. Saf. 2019, 184, 109653. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Lethal and sublethal effects of methyl benzoate on the predatory bug Nesidiocoris tenuis. Insects 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-C.; Wang, Y.; Portilla, M.; Parys, K.; Li, W. Risk and toxicity assessment of a potential natural insecticide, methyl benzoate, in honey bees (Apis mellifera L.). Insects 2019, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Larson, N.L.; Brabec, D.; Zhang, A. Methyl benzoate as a putative alternative, environmentally friendly fumigant for the control of stored product insects. J. Econ. Entomol. 2019, 112, 2458–2468. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Hassan, E.; Acharya, R.; Shim, J.-K.; Lee, K.-Y. Methyl benzoate is superior to other natural fumigants for controlling the Indian meal moth (Plodia interpunctella). Insects 2020, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, G.R.; Mosallam, E.M.; Phillips, T.W. Methyl benzoate and its derivative, acetophenone, as fumigants to control stored product insects. J. Stored Prod. Res. 2024, 105, 102248. [Google Scholar] [CrossRef]
- Hagstrum, D.; Subramanyam, B. Fundamentals of Stored-Product Entomology; AACC International: St. Paul, MN, USA, 2006; Volume 323. [Google Scholar]
- Selvapandian, U.; Nallusamy, S.; Singh, S.K.; Mannu, J.; Shanmugam, V.; Ravikumar, C.; Subbarayalu, M. Transcriptome profiling and in silico docking analysis of phosphine resistance in rice weevil, Sitophilus oryzae (Coleoptera: Curculionidae). J. Insect Sci. 2023, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Park, J.S.; Lee, H.; Kwon, M.; Kim, G.-H.; Kim, J. Identification of a phosphine resistance mechanism in Rhyzopertha dominica based on transcriptome analysis. J. Asia. Pac. Entomol. 2018, 21, 1450–1456. [Google Scholar] [CrossRef]
- Kim, B.; Song, J.-E.; Park, J.S.; Park, Y.; Shin, E.-M.; Yang, J. Insecticidal effects of fumigants (EF, MB, and PH3) towards phosphine-susceptible and-resistant Sitophilus oryzae (Coleoptera: Curculionidae). Insects 2019, 10, 327. [Google Scholar] [CrossRef]
- Bond, E.J.; Monro, H.A.U. Manual of Fumigation for Insect Control; FAO: Rome, Italy, 1984; Volume 54. [Google Scholar]
- Yang, Y.; Isman, M.B.; Tak, J.-H. Insecticidal activity of 28 essential oils and a commercial product containing Cinnamomum cassia bark essential oil against Sitophilus zeamais Motschulsky. Insects 2020, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Plague, G.R.; Voltaire, G.; Walsh, B.E.; Dougherty, K.M. Rice weevils and maize weevils (Coleoptera: Curculionidae) respond differently to disturbance of stored grain. Ann. Entomol. Soc. Am. 2010, 103, 683–687. [Google Scholar] [CrossRef]
- SAS Institute Inc. Base SAS 9.4 Procedures Guide: High-Performance Procedures; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Alnajim, I.; Agarwal, M.; Liu, T.; Li, B.; Du, X.; Ren, Y. Preliminary study on the differences in hydrocarbons between phosphine-susceptible and -resistant strains of Rhyzopertha dominica (Fabricius) and Tribolium castaneum (Herbst) using direct immersion solid-phase microextraction coupled with GC-MS. Molecules 2020, 25, 1565. [Google Scholar] [CrossRef] [PubMed]
- Pratt, S.J. A new measure of uptake: Desorption of unreacted phosphine from susceptible and resistant strains of Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2003, 39, 507–520. [Google Scholar] [CrossRef]
- Isikber, A.A.; Athanassiou, C.G. The use of ozone gas for the control of insects and micro-organisms in stored products. J. Stored Prod. Res. 2015, 64, 139–145. [Google Scholar] [CrossRef]
- Arthur, F.H. Impact of accumulated food on survival of Tribolium castaneum on concrete treated with cyfluthrin wettable powder. J. Stored Prod. Res. 2000, 36, 15–23. [Google Scholar] [CrossRef]
- Opit, G.P.; Thoms, E.; Phillips, T.W.; Payton, M.E. Effectiveness of sulfuryl fluoride fumigation for the control of phosphine-resistant grain insects infesting stored wheat. J. Econ. Entomol. 2016, 109, 930–941. [Google Scholar] [CrossRef]
- Sakka, M.K.; Gatzali, F.; Karathanos, V.T.; Athanassiou, C.G. Effect of nitrogen on phosphine-susceptible and-resistant populations of stored product insects. Insects 2020, 11, 885. [Google Scholar] [CrossRef]
- Fulcher, A.; Farooq, M.; Richardson, A.G.; Smith, M.L.; Scott, J.M.; Gaines, M.K.; Xue, R.-D. Characteristics and efficacy of three commercial handheld thermal foggers with pyrethroid insecticides against three species of mosquitoes. J. Am. Mosq. Control Assoc. 2016, 32, 44–50. [Google Scholar] [CrossRef]
- Arthur, F.; Campbell, J.; Fontenot, E.; Toews, M. Assessing effects of esfenvalerate aerosol applications on resident populations of Tribolium castaneum (Herbst), the red flour beetle, through direct and indirect sampling. J. Stored Prod. Res. 2013, 53, 1–6. [Google Scholar] [CrossRef]
- Arthur, F.H. Aerosol distribution and efficacy in a commercial food warehouse. Insect Sci. 2008, 15, 133–140. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Jhan, P.K.; Shim, J.-K.; Lee, K.-Y. Methyl benzoate exhibits insecticidal and repellent activities against Bemisia tabaci (Gennadius)(Hemiptera: Aleyrodidae). PLoS ONE 2018, 13, e0208552. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Shim, J.K.; Hwang, H.S.; Bunch, H.; Lee, K.Y. Acaricidal effects of methyl benzoate against Tetranychus urticae Koch (Acari: Tetranychidae) on common crop plants. Pest Manag. Sci. 2020, 76, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Kravets-Bekker, A.; Ivanova, O. Toxicological characteristics of methyl benzoate and potassium benzoate. Int. J. Toxicol. 1970, 75, 125–129. [Google Scholar]
- Opdyke, D.L. Monographs on fragrance raw materials. Food Cosmet. Toxicol. 1979, 17, 509–533. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Alam, M.B.; Chi, H.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Effects of sublethal doses of methyl benzoate on the life history traits and acetylcholinesterase (AChE) activity of Aphis gossypii. Agronomy 2020, 10, 1313. [Google Scholar] [CrossRef]
- Abdel-Baki, A.-A.S.; Ibrahium, S.M.; Aboelhadid, S.M.; Hassan, A.O.; Al-Quraishy, S.; Abdel-Tawab, H. Benzyl alcohol, benzyl benzoate and methyl benzoate as bio-insecticides against dried bean beetle Acanthoscelides obtectus (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2024, 105, 102246. [Google Scholar] [CrossRef]
- Kamel, A.A.; Aboelhadid, S.M.; Abdel-Baki, A.-A.S.; Ibrahium, S.M.; Al-Quraishy, S.; Hassan, A.O.; El-Kareem, A.; Shams, G.; Gadelhaq, S.M. Benzoate derivatives toxicity to Musca domestica results in severe muscle relaxation and body distortion. Neotrop. Entomol. 2024, 1–12. [Google Scholar] [CrossRef]
- De Oliveira, B.M.S.; Melo, C.R.; Alves, P.B.; Santos, A.A.; Santos, A.C.C.; Santana, A.D.S.; Araújo, A.P.A.; Nascimento, P.E.S.; Blank, A.F.; Bacci, L. Essential oil of Aristolochia trilobata: Synthesis, routes of exposure, acute toxicity, binary mixtures and behavioral effects on leaf-cutting ants. Molecules 2017, 22, 335. [Google Scholar] [CrossRef]

| Species | Benzoate Compounds | Strains † | LC50 (µL/1.5 L Air) | 95% CI (Lower–Upper) | Slope (±SEM) | X2 (df) |
|---|---|---|---|---|---|---|
| Rhyzopertha dominica | Methyl benzoate | PH3-S | 8.42 | - | 6.52 (1.52) | 16.68 (2) |
| PH3-R | 9.29 | (6.33–12.11) | 5.67 (0.77) | 5.82 (2) | ||
| Vinyl benzoate | PH3-S | 13.72 | (12.60–15.03) | 3.73 (0.38) | 0.44 (2) | |
| PH3-R | 14.45 | (13.17–16.06) | 3.41 (0.37) | 0.52 (2) | ||
| Ethyl benzoate | PH3-S | 17.03 | (15.69–18.84) | 4.01 (0.51) | 2.65 (2) | |
| PH3-R | 18.97 | (17.23–21.67) | 4.01 (0.52) | 1.85 (2) |
| Species | Benzoate Compounds | Strains † | LC50 (µL/1.5 L Air) | 95% CI (Lower–Upper) | Slope (±SEM) | X2 (df) |
|---|---|---|---|---|---|---|
| Sitophilus oryzae | Methyl benzoate | PH3-S | 6.04 | (5.64–6.46) | 7.65 (0.78) | 0.15 (2) |
| PH3-R | 7.60 | (7.04–8.15) | 6.00 (0.49) | 1.26 (2) | ||
| Vinyl benzoate | PH3-S | 9.30 | (8.55–10.04) | 4.52 (0.37) | 3.36 (2) | |
| PH3-R | 10.16 | (6.62–13.88) | 4.39 (0.63) | 5.64 (2) | ||
| Ethyl benzoate | PH3-S | 20.14 | (18.53–22.77) | 5.30 (0.76) | 4.09 (2) | |
| PH3-R | 21.01 | - | 6.29 (1.75) | 6.51 (2) |
| Tested Compound | Species | Strains † | LC50 (µL/1.5 L Air) | 95% CI (Lower–Upper) | Slope (±SEM) | X2 (df) |
|---|---|---|---|---|---|---|
| Methyl benzoate | Rhyzopertha dominica | PH3-S | 93.29 | (85.16–101.91) | 4.52 (0.39) | 1.08 (2) |
| PH3-R | 134.96 | (47.98–319.46) | 3.09 (0.58) | 10.19 (2) | ||
| Sitophilus oryzae | PH3-S | 83.11 | (75.37–91.14) | 4.25 (0.38) | 0.38 (2) | |
| PH3-R | 134.84 | (117.66–154.09) | 2.38 (0.22) | 2.64 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafiz, M.M.; Hwang, H.-S.; Kim, J.-R.; Kim, B.-S.; Lee, K.-Y. The Fumigation Toxicity of Three Benzoate Compounds against Phosphine-Susceptible and Phosphine-Resistant Strains of Rhyzopertha dominica and Sitophilus oryzae. Insects 2024, 15, 477. https://doi.org/10.3390/insects15070477
Mostafiz MM, Hwang H-S, Kim J-R, Kim B-S, Lee K-Y. The Fumigation Toxicity of Three Benzoate Compounds against Phosphine-Susceptible and Phosphine-Resistant Strains of Rhyzopertha dominica and Sitophilus oryzae. Insects. 2024; 15(7):477. https://doi.org/10.3390/insects15070477
Chicago/Turabian StyleMostafiz, Md Munir, Hwal-Su Hwang, Jun-Ran Kim, Bong-Su Kim, and Kyeong-Yeoll Lee. 2024. "The Fumigation Toxicity of Three Benzoate Compounds against Phosphine-Susceptible and Phosphine-Resistant Strains of Rhyzopertha dominica and Sitophilus oryzae" Insects 15, no. 7: 477. https://doi.org/10.3390/insects15070477
APA StyleMostafiz, M. M., Hwang, H.-S., Kim, J.-R., Kim, B.-S., & Lee, K.-Y. (2024). The Fumigation Toxicity of Three Benzoate Compounds against Phosphine-Susceptible and Phosphine-Resistant Strains of Rhyzopertha dominica and Sitophilus oryzae. Insects, 15(7), 477. https://doi.org/10.3390/insects15070477







