Ovicidal Toxicity and Morphological Changes in Housefly Eggs Induced by the Essential Oils of Star Anise and Lemongrass and Their Main Constituents
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils and Other Chemicals
2.2. The Treatments
2.3. Housefly Rearing
2.4. Ovicidal Bioassay
2.5. Safety Bioassay of Non-Target Aquatic Species
2.6. Morphological Changes in Housefly Eggs after Treatment
2.7. Ethics and Guidelines for Bioassays
2.8. Data Analysis
3. Results
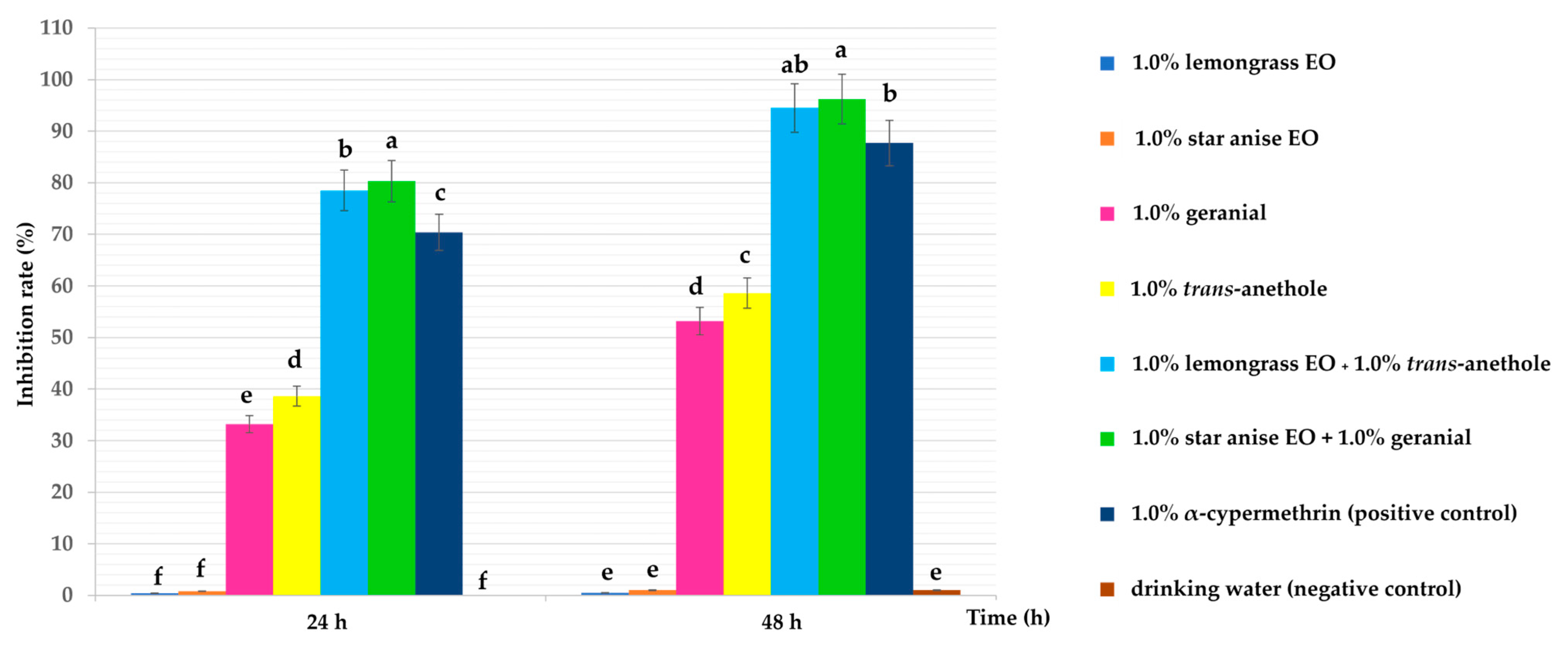
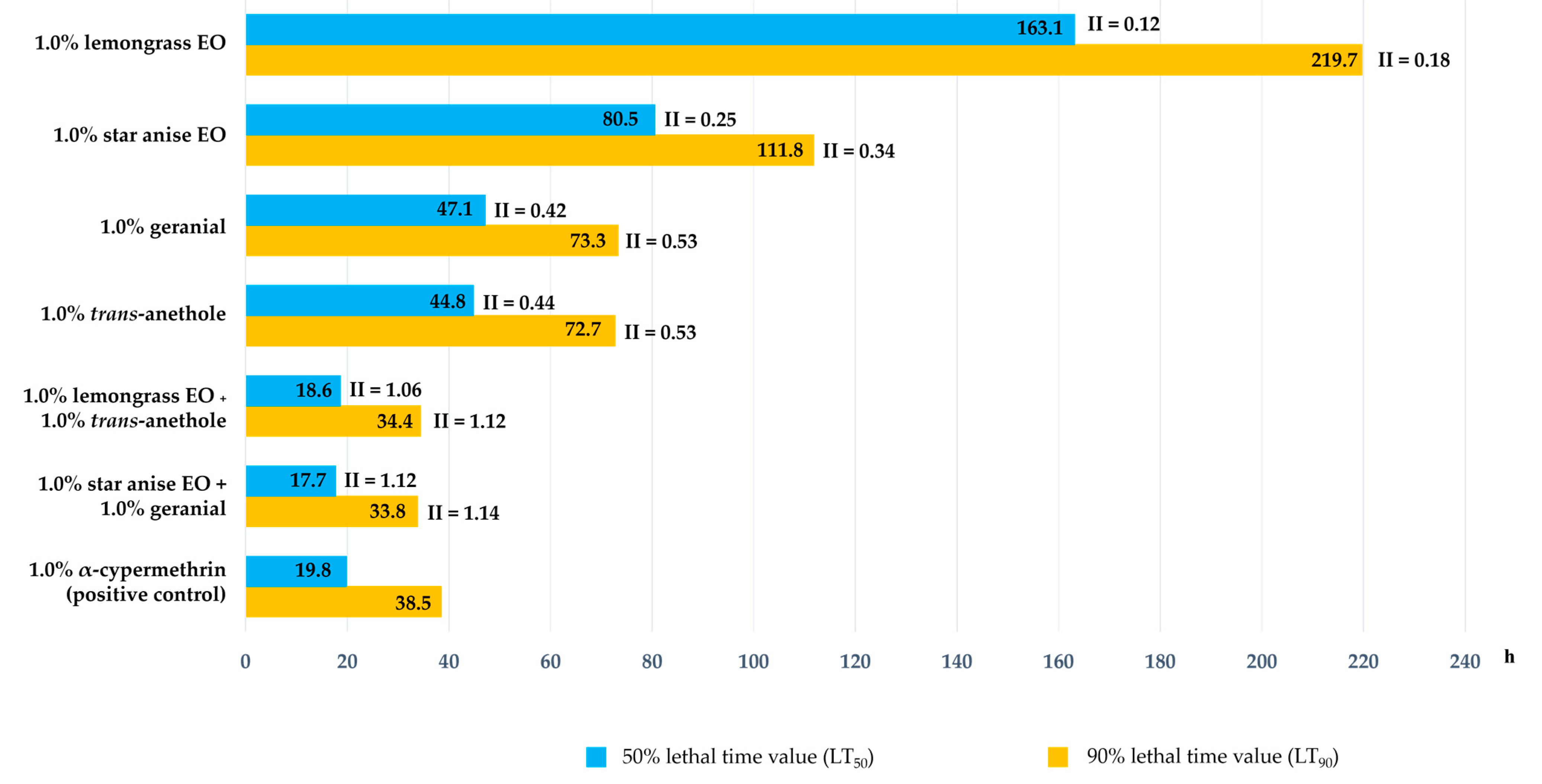
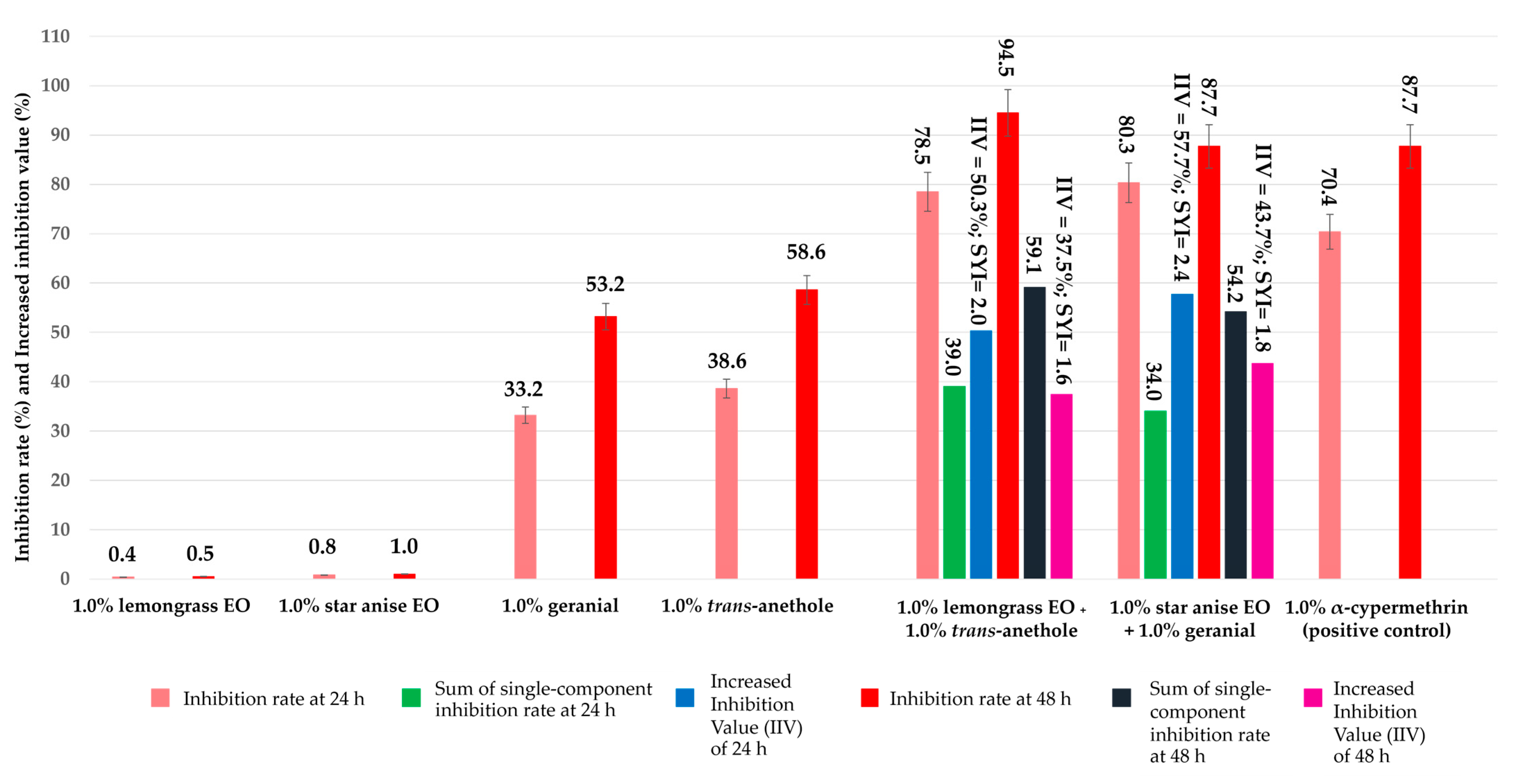
3.1. Ovicidal Activity
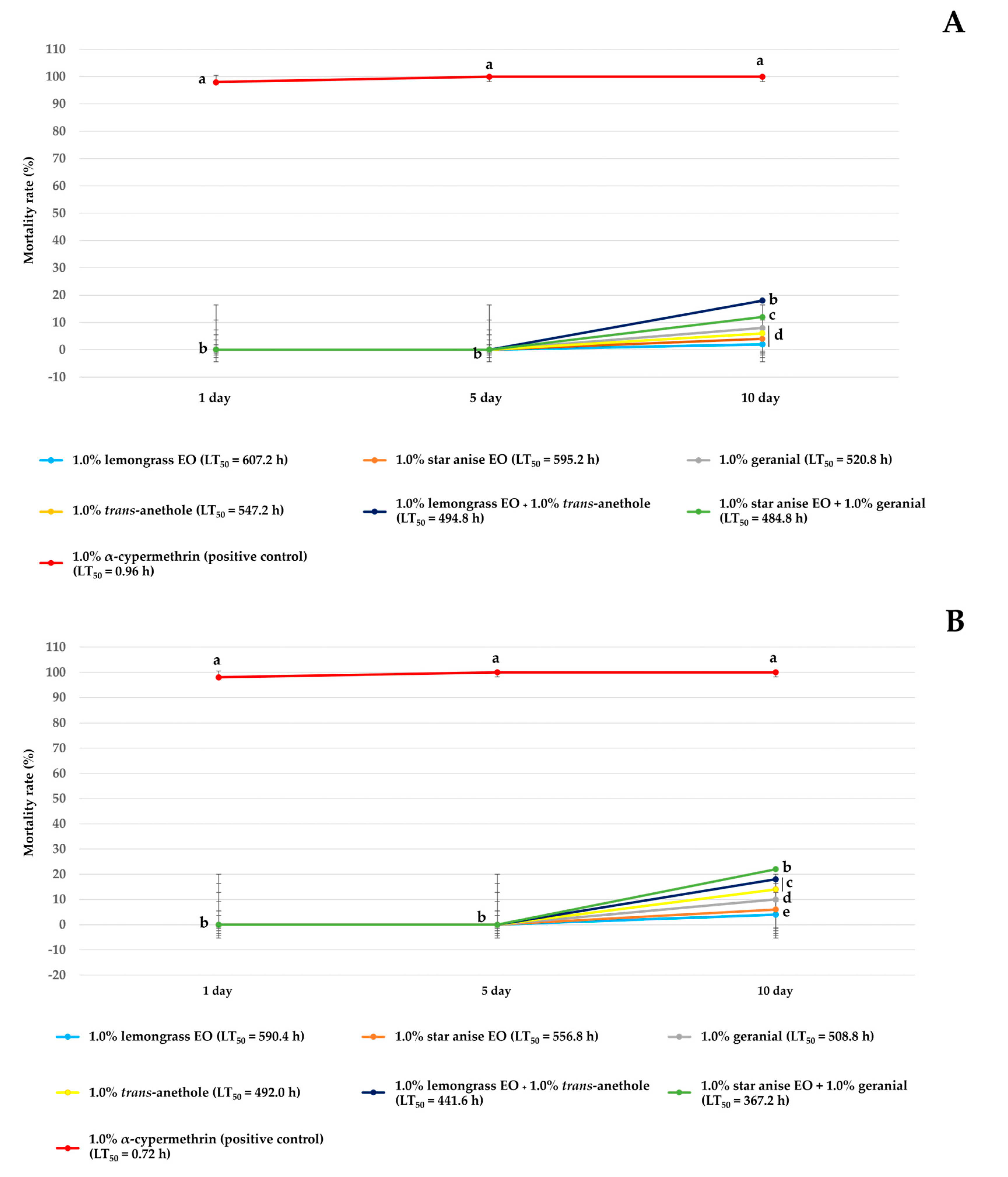
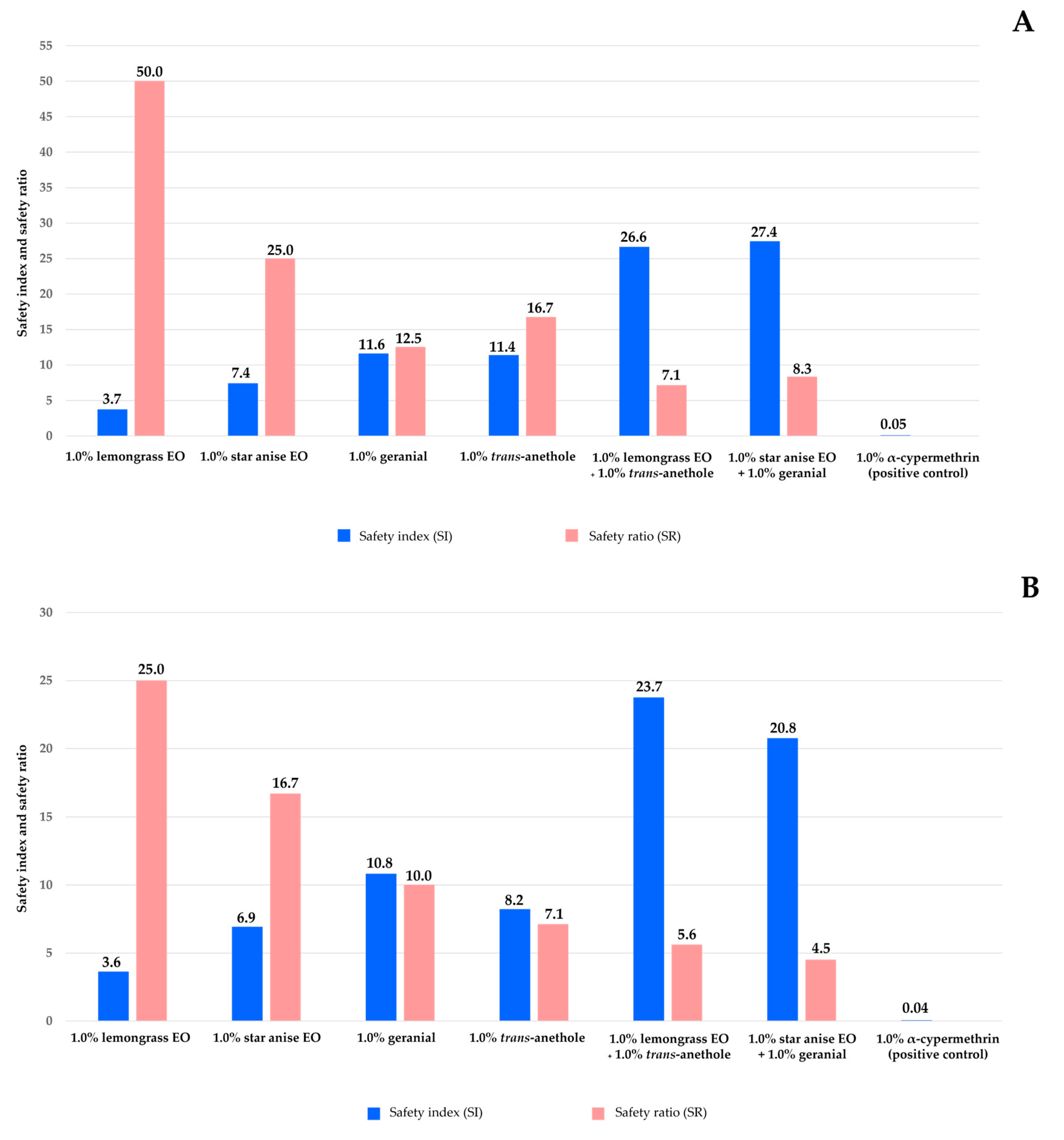
3.2. Toxicity to Two Non-Target Aquatic Species
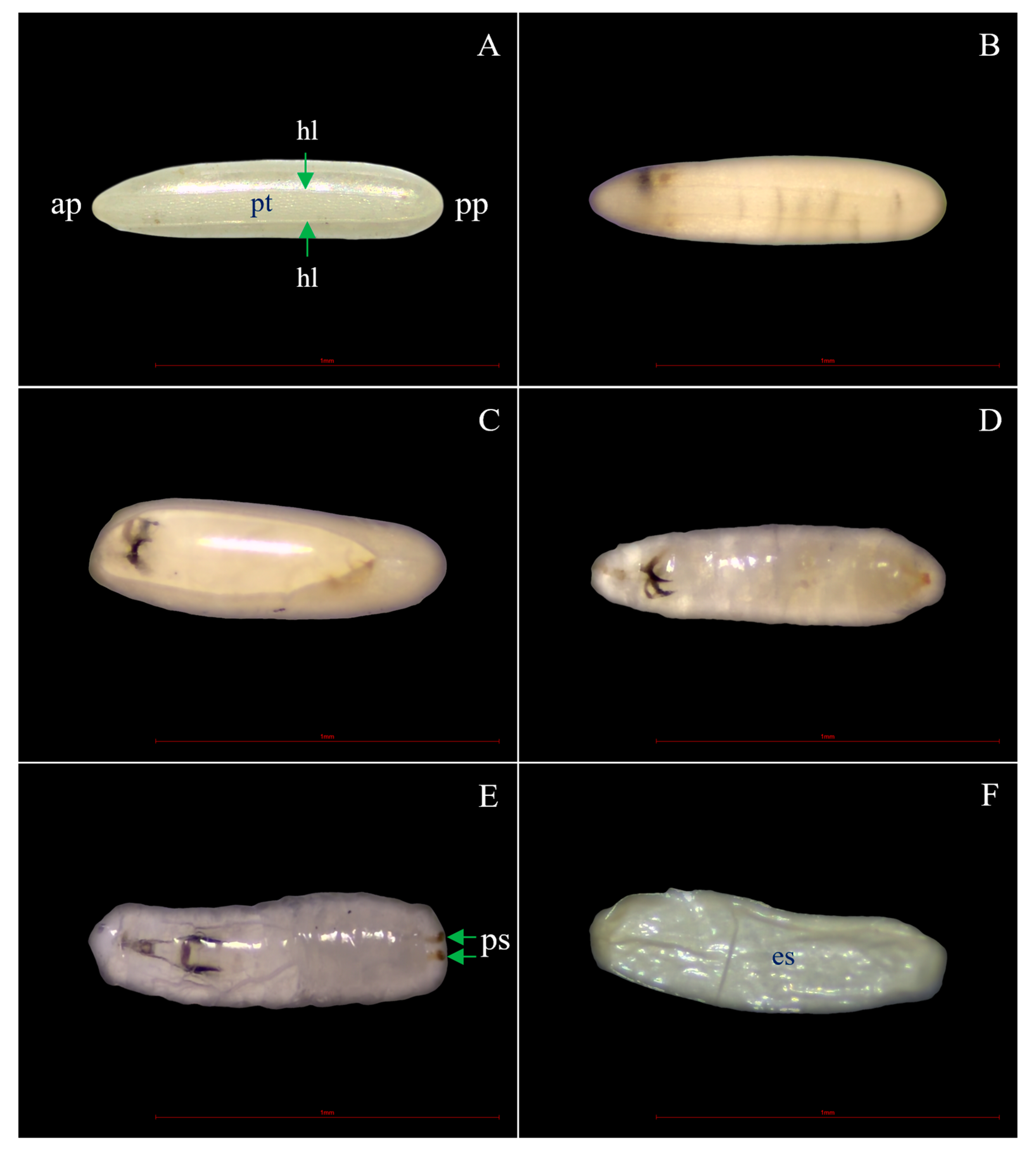
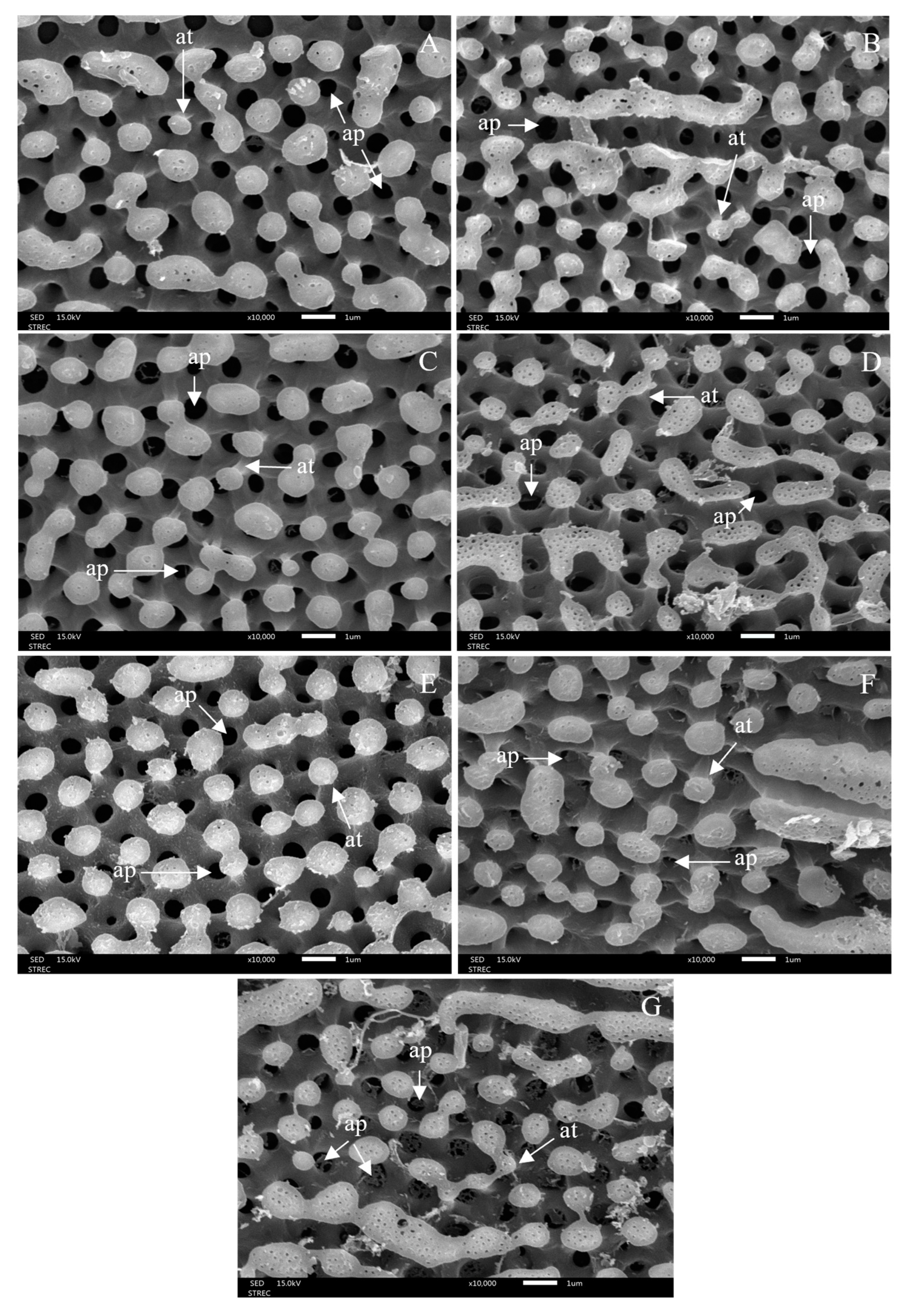
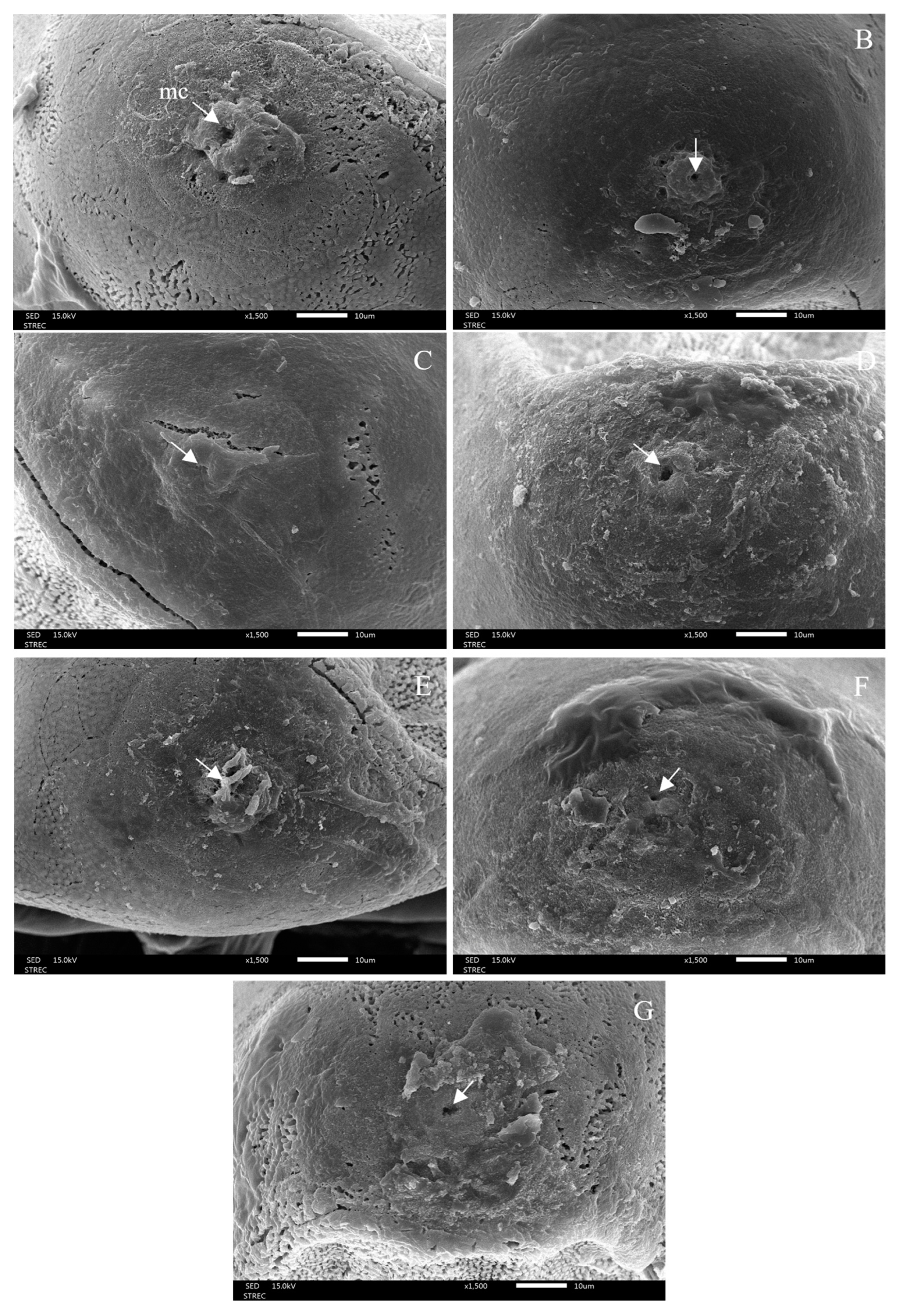
3.3. Morphological Changes after Ovicidal Bioassay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jiménez, A.J.M.; Santos, A.L.; Munguía, C.A.G.; González, J.A.T.; Munguía, A.M.G. Potential distribution of Musca domestica in Jesús María Municipality, Aguascalientes, Mexico, based on climate change scenarios. Rev. Mex. Cienc. Pecu. 2019, 10, 14–29. [Google Scholar] [CrossRef]
- Pu, J.; Wang, Z.; Chung, H. Climate change and the genetics of insecticide resistance. Pest Mang. Sci. 2020, 76, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Khamesipour, F.; Lankarani, K.B.; Hoharvar, B.; Kenti, T.E. A systemic review of human pathogens carried by the housefly (Musca domestica L.). BMC Public Health 2018, 18, 1049. [Google Scholar] [CrossRef] [PubMed]
- Nayduch, D.; Burrus, R.G. Flourishing in filth: House fly-microbe interactions across life history. Ann. Entomol. Soc. Am. 2017, 110, 6–18. [Google Scholar] [CrossRef]
- Barin, A.; Arabkhazaeli, F.; Rahbari, S.; Madani, S.A. The housefly, Musca domestica, as a possible mechanical vector of newcastle disease virus in the laboratory and field. Med. Vet. Entomol. 2010, 24, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Salamatian, I.; Moshaverinia, A.; Razmyar, J.; Ghaemi, M. In vitro acquisition and retention of low-pathogenic avian influenza H9N2 by Musca domestica (Diptera: Muscidae). J. Med. Entomol. 2020, 57, 563–567. [Google Scholar] [CrossRef]
- Seeger, R.M.; Hagerman, A.D.; Johnson, K.K.; Pendell, D.L.; Marsh, T.L. When poultry take a sick leave: Response costs for the 2014–2015 highly pathogenic avian influenza epidemic in the USA. Food Policy 2021, 102, 102068. [Google Scholar] [CrossRef]
- Geden, C.J.; Nayduch, D.; Scott, J.G.; Burgess IV, E.R.; Gerry, A.C.; Kaufman, P.E.; Thomson, J.; Pickens, V.; Machtinger, E.T. House fly (Diptera: Muscidae): Biology, pest status, current management prospects, and research needs. J. Integr. Pest Manag. 2021, 12, 39. [Google Scholar] [CrossRef]
- Hinkle, N.C.; Hogsettle, J.A. A review of alternative control for house flies. Insects 2021, 12, 1042. [Google Scholar] [CrossRef]
- Wang, J.N.; Hou, J.; Wu, Y.Y.; Guo, S.; Liu, Q.M.; Li, T.Q.; Gong, Z.Y. Resistance of house fly, Musca domestica L. (Diptera: Muscidae), to five insecticides in Zhenjiang Province, China: The situation in 2017. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 4851914. [Google Scholar] [CrossRef]
- Freeman, J.C.; Ross, D.H.; Scott, J.G. Insecticide resistance monitoring of house fly populations from the United States. Pest. Bio. Physiol. 2019, 158, 61–68. [Google Scholar] [CrossRef]
- Abbas, N.; Ijaz, M.; Shad, S.A.; Khan, H. Stability of field-selected resistance to conventional and newer chemistry insecticides in the house fly, Musca domestica L. (Diptera: Muscidae). Neotrop. Entomol. 2015, 44, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Levchenko, M.A.; Silivanova, E.A.; Balabanova, G.F.; Bikinyaeva, R.H. Insecticide susceptibility of house flies (Musca domestica) from a livestock farm in Tyumen region, Russia. Bulg. J. Vet. Med. 2019, 22, 213–219. [Google Scholar] [CrossRef]
- Hafez, A.M. First evaluation of field evolved resistance to commonly used insecticides in house fly populations from Sadui Arabian diary farms. Insects 2021, 12, 1120. [Google Scholar] [CrossRef]
- Chaiwong, T.; Srivoramas, T.; Sueabsamran, P.; Sukontason, K.; Sanford, M.R.; Sukontason, K.L. The blow fly, Chrysomya megacephala, and the house fly, Musca domestica, as mechanical vectors of pathogenic bacteria in Northeast Thailand. Trop. Biomed. 2014, 31, 336–346. [Google Scholar] [PubMed]
- Mužinić, V.; Želježić, D. Non-target toxicity of novel insecticides. Arh. Hig. Rada. Toksikol. 2018, 69, 86–102. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects-a review. Plant Protect. Sci. 2016, 4, 229–241. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Moungthipmalai, T.; Puwanard, C.; Aungtikun, J.; Sittichok, S.; Soonwera, M. Ovicidal toxicity of plant essential oils and their major constituents against two mosquito vectors and their non-target aquatic predators. Sci. Rep. 2023, 13, 2119. [Google Scholar] [CrossRef]
- Sinthusiri, J.; Soonwera, M. Oviposition deterrent and ovicidal activities of seven herbal essential oils against female adults of housefly, Musca domestica. Parasiotol. Res. 2014, 113, 3015–3022. [Google Scholar] [CrossRef]
- Khan, H.A.A. Toxicity, repellent and oviposition deterrent effects of select essential oils against the house fly Musca domestica. J. Asian. Pac. Entomol. 2021, 24, 15–20. [Google Scholar] [CrossRef]
- Soonwera, M. Larvicidal and ovicidal deterrent activities of essential oils against housefly (Musca domestica L.; Diptera: Musci dae). Int. J. Agric. Technol. 2015, 11, 657–667. [Google Scholar]
- Chantawee, A.; Soonwera, M. Larvicidal, pupicidal and oviposition deterrent activities of essential oils from Umbelliferae plants against house fly Musca domestica. Asian Pac. J. Trop. Med. 2018, 11, 612–629. [Google Scholar] [CrossRef]
- Priestley, C.M.; Burgess, I.F.; Williamson, E.M. Lethality of essential oil constituents towards the human louse, Pediculus humanus, and its eggs. Fitoterapia 2006, 77, 303–309. [Google Scholar] [CrossRef]
- Soonwera, M.; Wongnet, O.; Sittichok, S. Ovicidal effect of essential oils from Zingiberaceae plants and Eucalytus globulus on eggs of head lice, Pediculus humanus capitis De Geer. Phytomedicine 2018, 47, 93–104. [Google Scholar] [CrossRef]
- Soonwera, M.; Moungthipmalai, T.; Takawirapat, W.; Sittichok, S. Ovicidal and repellent activities of several plant essential oils against Periplneta americana L. and enhanced activities from their combined formulation. Sci. Rep. 2022, 12, 12070. [Google Scholar] [CrossRef]
- Ramkumar, G.; Karthi, S.; Shivakumar, M.S.; Kweka, E.J. Culex quinquefasciatus egg membrane alteration and ovicidal activity of Cipadessa baccifera (Roth) plant extracts compared to synthetic insect growth regulators. Res. Rep. Trop. Med. 2019, 10, 145–151. [Google Scholar] [CrossRef]
- Souza, L.M.; Venturini, F.P.; Inada, N.M.; Iermak, I.; Garbuio, M.; Mezzacappo, N.F.; Oliveira, K.T.; Bagnato, V.S. Curcumin in formulation against Aedes aegypti: Mode of action, photolarvicidal and ovicidal activity. Photodiagnosis Photodyn. Ther. 2020, 31, 101840. [Google Scholar] [CrossRef]
- Alves, R.R.; Soares, T.; Bento, E.F.; Roldan-Filho, R.S.; Souza, B.S.; Lima, M.K.; Nascimento, J.S.; Coelho, L.C.; Sá, R.A.; Lima, T.A.; et al. Ovicidal lectins from Moringa oleifera and Myracrodruon cause alterations in chorionic surface and penetrate the embryos of Aedes aegypti eggs. Pest Manag. Sci. 2020, 76, 730–736. [Google Scholar] [CrossRef]
- Hong, T.K.; Perumalsamy, H.; Jang, K.H.; Na, E.S.; Ahn, Y.J. Ovicidal and larvicidal activity and possible mode of action of phe-nylpropanoids and ketone identified in Syzygium aromaticum bud against Bradysia procera. Pestic. Biochem. Physiol. 2018, 145, 29–48. [Google Scholar] [CrossRef]
- Sukontason, K.; Sukontason, K.; Boonchu, N.; Piangjai, S. Some ultrastructural superficial changes in house fly (Diptera: Muscidae) and blow fly (Diptera: Calliphoridae) larvae induced by eucalyptol oil. Rev. Inst. Med. Trop. Sao. Paulo. 2004, 46, 263–267. [Google Scholar] [CrossRef]
- Pinto, Z.T.; Sánchez, F.F.; Santos, A.R.d.; Amaral, A.C.F.; Ferreira, J.L.P.; Escalona-Arranz, J.C.; Queiroz, M.M.d.C. Chemical composition and insecticidal activity of Cymbopogon citratus essential oil from Cuba and Brazil against housefly. Rev. Bras. Par-aitol. Vet. 2015, 24, 36–44. [Google Scholar] [CrossRef]
- Zou, Q.; Huang, Y.; Zhang, W.; Lu, C.; Yuan, J. A comprehensive review of the pharmacology, chemistry, traditional uses and quality control of star anise (Illicium verum Hook.F.): An aromatic medicinal plant. Molecules 2023, 28, 7378. [Google Scholar] [CrossRef]
- Soonwera, M.; Moungthipmalai, T.; Puwanard, C.; Sittichok, S.; Sinthusiri, J.; Passara, H. Adulticidal synergy of two plant essential oils and their major constituents against the housefly Musca domestica and bioassay on non-target species. Heliyon 2024, 10, e26910. [Google Scholar] [CrossRef]
- Soonwera, M.; Sinthusiri, J.; Passara, H.; Moungthipmalai, T.; Puwanard, C.; Sittichok, S.; Murata, K. Combinations of lemongrass and star anise essential oils and their main constituent: Synergistic housefly repellency and safety against non-target organisms. Insects 2024, 15, 210. [Google Scholar] [CrossRef]
- Aungtikun, J.; Soonwera, M.; Sittichok, S. Insecticidal synergy of essential oils from Cymbopogon citratus (Stapf.), Myristica fragrans (Houtt.), and Illicium verun Hook.f. and their major active constitutes. Ind. Crops Prod. 2021, 164, 113386. [Google Scholar] [CrossRef]
- Soonwera, M.; Moungthipmalai, T.; Aungtikun, J.; Sittichok, S. Combinations of plant essential oils and their major compositions inducing mortality and morphological abnormality of Aedes aegypti and Aedes albopictus. Heliyon 2022, 8, e09346. [Google Scholar] [CrossRef]
- The National Research Council of Thailand. Ethical Principles and Guidelines for the Use of Animals. 2023. Available online: https://labanimals.nrct.go.th./ACT/ (accessed on 25 January 2024).
- ARRIVE. The Animal Research: Reporting of In Vivo Experiment Guidelines. 2023. Available online: https://arriveguidelines.org/arrive-guidelines (accessed on 15 January 2024).
- Wheeler, M.W.; Park, R.M.; Bailer, A.J. Comparing median lethal concentration values using confidence interval overlap or ratio test. Environ. Toxicol. Chem. 2006, 25, 1441–1444. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The evolutionary origins of pesticide resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef]
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What can we learn from commercial insecticides? efficacy, toxicity, environmental impacts, and future developments. Environ. Pollut. 2022, 300, 118983. [Google Scholar] [CrossRef]
- Hu, Z. What socio-economic and political factors lead to global pesticide dependence? A critical review from a social science perspective. Int. J. Environ. Res. Public Health 2020, 17, 8119. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Ann. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides in the twenty-first century-fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Atkovska, K.; Kuvendziev, S.; Mustafa, E.; Marinkovski, M.; Ghaffari, P.; Lisichkov, K. Essential oils as green repellents against mosquito vectors. Qual. Life 2021, 12, 51–60. [Google Scholar] [CrossRef]
- Chintalchere, J.M.; Dar, M.A.; Raut, K.D.; Pandit, R.S. Bioefficacy of lemongrass and tea tree essential oils against house fly, Musca domestica. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Chintalchere, J.M.; Dar, M.A.; Pandit, R.S. Biocontrol efficacy of bay essential oil against housefly, Musca domestica (Diptera: Muscidae). J. Basic Appl. Zool. 2020, 81, 6. [Google Scholar] [CrossRef]
- Soonwera, M.; Sittichok, S. Adulticidal activities of Cymbopogon citratus (Stapf.) and Eucalyptus globulus (Labill.) essential oils and their synergistic combination against Aedes aegypti (L.), Aedes albopictus (Skuse), and Musca domestica (L.). Environ. Sci. Pollut. Res. 2020, 27, 20201–20214. [Google Scholar] [CrossRef]
- Moungthipmalai, T.; Soonwera, M. Adulticidal activity against housefly (Musca domestica L.; Muscidae: Diptera) of combinations of Cymbopogon citratus and Eucalyptus globulus essential oils and their major constituents. Int. J. Agric. Technol. 2023, 19, 1127–1134. [Google Scholar]
- Pavela, R. Acute and synergistic effects of some monoterpenoid essential oil compound on the housefly (Musca domestica). J. Es-sent. Oil-Bear. Plants 2008, 11, 451–459. [Google Scholar] [CrossRef]
- Gad, H.A.; Ramadan, G.R.M.; El-Bakry, A.M.; El-Sabrout, A.M.; Abdelgaleil, S.A.M. Monoterpenes: Promising natural products for public health insect control- a review. Int. J. Trop. Insect Sci. 2022, 42, 1059–1075. [Google Scholar] [CrossRef]
- Tak, J.H.; Jovel, E.; Isman, M.B. Synergistic interactions among the major constituents of lemongrass essential oil against larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni. J. Pest Sci. 2017, 90, 735–744. [Google Scholar] [CrossRef]
- Cotchakaew, N.; Soonwera, M. Toxicity of several botanical essential oils and their combinations against females of Aedes albopictus (Skuse) and Anopheles minimus (Theobald): Oviposition deterrent, ovicidal and adulticidal efficacies. Asian Pac. J. Trop. Biomed. 2019, 9, 29–39. [Google Scholar] [CrossRef]
- Arafa, W.M.; Aboelhadid, S.M.; Moawad, A.; Shokeir, K.M.; Ahmed, O. Toxicity, repellency and anti-cholinesterase activities of thymol-eucalyptus combinations against phenotypically resistant Rhipicephalus annulatus ticks. Exp. Appl. Acarol. 2020, 81, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Scalerandi, E.; Flores, G.A.; Palacio, M.; Defagó, M.T.; Carpinella, M.C.; Valladares, G.; Bertoni, A.; Palacios, S.M. Understanding synergistic toxicity of terpenene as insecticides: Contribution of metabolic detoxification in Musca domestica. Front. Plant Sci. 2018, 9, 1579. [Google Scholar] [CrossRef]
- Puwanard, C.; Sonwera, M. Ovicidal and adulticidal activities of Cymbopogon citratus (DC.) Stapf and Illicium verum Hook.f. against Aedes aegypti (Linn.). Int. J. Agric. Technol. 2022, 18, 319–328. [Google Scholar]
- Pushpanathan, T.; Jebanesan, A.; Govindarajan, M. Larvicidal, ovicidal and repellent activities of Cymbopogan citratus Stapf (Graminae) essential oil against the filarial mosquito Culex quinquefasciatus (Say) (Diptera: Culicidae). Trop. Biomed. 2006, 23, 208–212. [Google Scholar] [PubMed]
- Phasomkusolsil, S.; Soonwera, M. The effects of herbal essential oils on the oviposition deterrent and ovicidal activities of Aedes aegypti (Linn.), Anopheles dirus (Peyton and Harrison) and Culex quinquefasciatus (Say). Trop. Biomed. 2012, 29, 138–150. [Google Scholar]
- Tunç, L.; Berger, B.M.; Erler, F.; Dağlı, F. Ovicidal activity of essential oils from five plants against two stored-product insects. J. Stored Prod. Res. 2000, 36, 161–168. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Housefly (Musca domestica L.) control potential of Cymbopogon citratus Stapf. (Poales: Poaceae) essential oil and monoterpenes (citral and 1,8-cineole). Parasitol. Res. 2013, 112, 69–76. [Google Scholar] [CrossRef]
- Gong, X.; Ren, Y. Larvicidal and ovicidal activity of carvacrol, p-cymene, and γ-terpinene from Origanum vulgare essential oil against the cotton bollworm, Helicoverpa armigera (Hübner). Environ. Sci. Pollut. Res. 2020, 27, 18708–18716. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Morales, R.M.; Serrano, S.O.; Villamizar, A.L.R.; Mendez-Sanchez, S.C.; Duque, J.E. Impact of Cymbopogon flexuosus (Poaceae) essential oil and primary components on the eclosion and larval development of Aedes aegypti. Sci. Rep. 2021, 11, 24291. [Google Scholar] [CrossRef] [PubMed]
- Radwan, W.A.; Helmy, N.; Salem, D.A.M.; Bakr, R.F.A.; Guneidy, N.A.; Mohammed, S.S. Ultrastructure changes induced by chitin synthesis inhibitor and extract of rice bran in embryogenesis of the house fly Musca domestica Linnaeus. Egypt. J. Exp. Biol. Zool. 2012, 8, 363–375. [Google Scholar]
- Suresh, M.; Devi, K.P.; Kalaiarasi, J.M.V. Biological studies on the housefly, Musca domestica L. treated with essential oils. Int. J. Entomol. Res. 2018, 3, 72–80. [Google Scholar]
- Madi, Y.F.; Choucry, M.A.; Meselhy, M.R.; El-Kashoury, E.S.A. Essential oil of Cymbopogon citratus cultivated in Egypt: Seasonal variation in chemical composition and anticholinesterase activity. Nat. Prod. Res. 2021, 35, 4063–4067. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, M.K. Insecticidal activities of Anethum graveolens L. and Illicium verum Hook. f. essential oils against Sitophilus zeamais Motschulsky. Rev. Cienc. Agric. 2021, 38, 38–49. [Google Scholar] [CrossRef]
- Shahriari, M.; Zibaee, A.; Sahebzadeh, M.; Shamakhi, L. Effects of α-pinene, trans-anethole, and thymol as the essential oil constituents on antioxidant system and acetylcholine esterase of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2018, 150, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Silvério, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant natural products for the control of Aedes aegypti: The main vector of important arboviruses. Molecules 2020, 25, 3484. [Google Scholar] [CrossRef] [PubMed]
- AlSalhi, M.S.; Elumalai, K.; Devanesan, S.; Govindarajan, M.; Krishnappa, K.; Maggi, F. The aromatic ginger Kaempferia galanga L. (Zingiberaceae) essential oil and its main compounds are effective larvicidal agents against Aedes vittatus and Anopheles maculatus without toxicity on the non-target aquatic fauna. Ind. Crops Prod. 2020, 158, 113012. [Google Scholar] [CrossRef]
- Bullangpoti, V.; Mujchariyakul, W.; Laksanavilat, N.; Junhirun, P. Acute toxicity of essential oil compound (thymol and 1,8-cineole) to insectivorous guppy, Poecilia reticulata Peters, 1859. Agric. Nat. Resour. 2018, 52, 190–194. [Google Scholar] [CrossRef]
- Serrão, J.E.; Plata-Rueda, A.; Martín, L.C.; Zanuncio, J.C. Side-effects of pesticides on non-target insects in agriculture: A mini-review. Sci. Nat. 2022, 109, 17. [Google Scholar] [CrossRef]
- Salako, A.F.; Amaeze, N.H.; Shobajo, H.M.; Osuala, F.I. Comparative acute toxicity of three pyrethroids (Deltamethrin, cypermethrin and lambda-cyhalothrin) on guppy fish (Poecilia reticulata peters, 1859). Sci. Afr. 2020, 9, e00504. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bilal, R.M.; Gewida, A.G.A.; Dhama, K.; Abdel-Latif, H.M.R.; Amer, M.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Binnaser, Y.S.; et al. An overview on the potential hazards of pyrethroid insecticides in fish, with special emphasis on cypermethrin toxicity. Animals 2021, 11, 1880. [Google Scholar] [CrossRef]
- Shilpakar, O.; Karki, B. Cypermethrin poisoning manifesting with prolonged bradycardia: A case report. Toxicol. Rep. 2020, 8, 10–12. [Google Scholar] [CrossRef]
- Asif, M.; Yehya, A.H.S.; Al-Mansoub, M.A.; Revadigar, V.; Ezzat, M.O.; Ahamed, M.B.K.; Oon, C.E.; Murugaiyah, V.; Majid, A.S.A.; Majid, A.M.S.A. Anticancer attributes of Illicium verum essential oils against colon cancer. S. Afr. J. Bot. 2016, 103, 156–161. [Google Scholar] [CrossRef]
- Lulekal, E.; Tesfaye, S.; Gebrechristos, S.; Dires, K.; Zenebe, T.; Zegeye, N.; Feleke, G.; Kassahun, A.; Shiferaw, Y.; Monkonnen, A. Phytochemical analysis and evaluation of skin irritation, acute and sub acute toxicity of Cymbopogon citratus essential oil in mice and rabbits. Toxicol. Rep. 2019, 6, 1289–1294. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passara, H.; Sittichok, S.; Sinthusiri, J.; Moungthipmalai, T.; Puwanard, C.; Murata, K.; Soonwera, M. Ovicidal Toxicity and Morphological Changes in Housefly Eggs Induced by the Essential Oils of Star Anise and Lemongrass and Their Main Constituents. Insects 2024, 15, 481. https://doi.org/10.3390/insects15070481
Passara H, Sittichok S, Sinthusiri J, Moungthipmalai T, Puwanard C, Murata K, Soonwera M. Ovicidal Toxicity and Morphological Changes in Housefly Eggs Induced by the Essential Oils of Star Anise and Lemongrass and Their Main Constituents. Insects. 2024; 15(7):481. https://doi.org/10.3390/insects15070481
Chicago/Turabian StylePassara, Hataichanok, Sirawut Sittichok, Jirisuda Sinthusiri, Tanapoom Moungthipmalai, Cheepchanok Puwanard, Kouhei Murata, and Mayura Soonwera. 2024. "Ovicidal Toxicity and Morphological Changes in Housefly Eggs Induced by the Essential Oils of Star Anise and Lemongrass and Their Main Constituents" Insects 15, no. 7: 481. https://doi.org/10.3390/insects15070481




