Effects of Two Prey Species Combinations on Larval Development of the Predatory Ladybird Cheilomenes propinqua
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Experimental Design
2.2.1. General Methods
- First instar larva. Body is elongated, cylindrical, and tapered, with whitish yellow integument. Larval elements of armature are weakly sclerotized. Parascoli are short (about 0.06 mm) and thick.
- Second instar larva. Body and armature of the body surface are sclerotized, pigmented with whitish yellow areas of body. Parascoli are about 0.14 mm, well sclerotized, darker than those on the first instar.
- Third instar larva. Larvae are similar to the second instar in structure and color, body shape is cylindrical and slightly elongated; parascoli are about 0.2 mm, of the same color as the integument.
- Fourth instar larva. Tegument is dark brown with well-demarcated whitish yellow areas of body. Parascoli are brighter colored, larger (0.3–0.4 mm), and more sclerotized than those of the third instar.
2.2.2. The First Experiment (Mixed Diets)
2.2.3. The Second Experiment (Changing Diets)
2.3. Statistical Analysis
3. Results
3.1. The First Experiment (Mixed Diets)
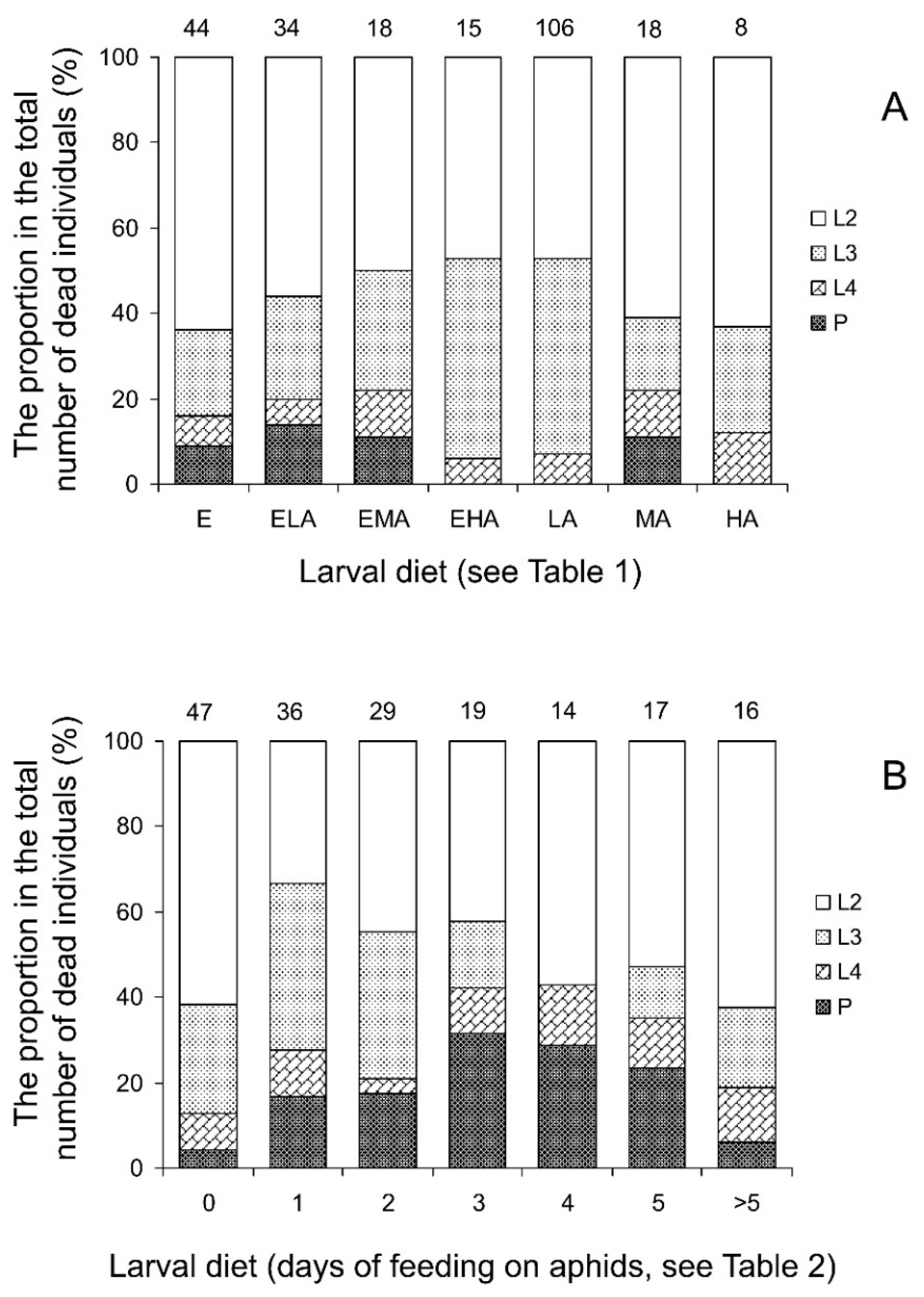
3.1.1. Pre-Adult Survival and Sex
3.1.2. Time of Development
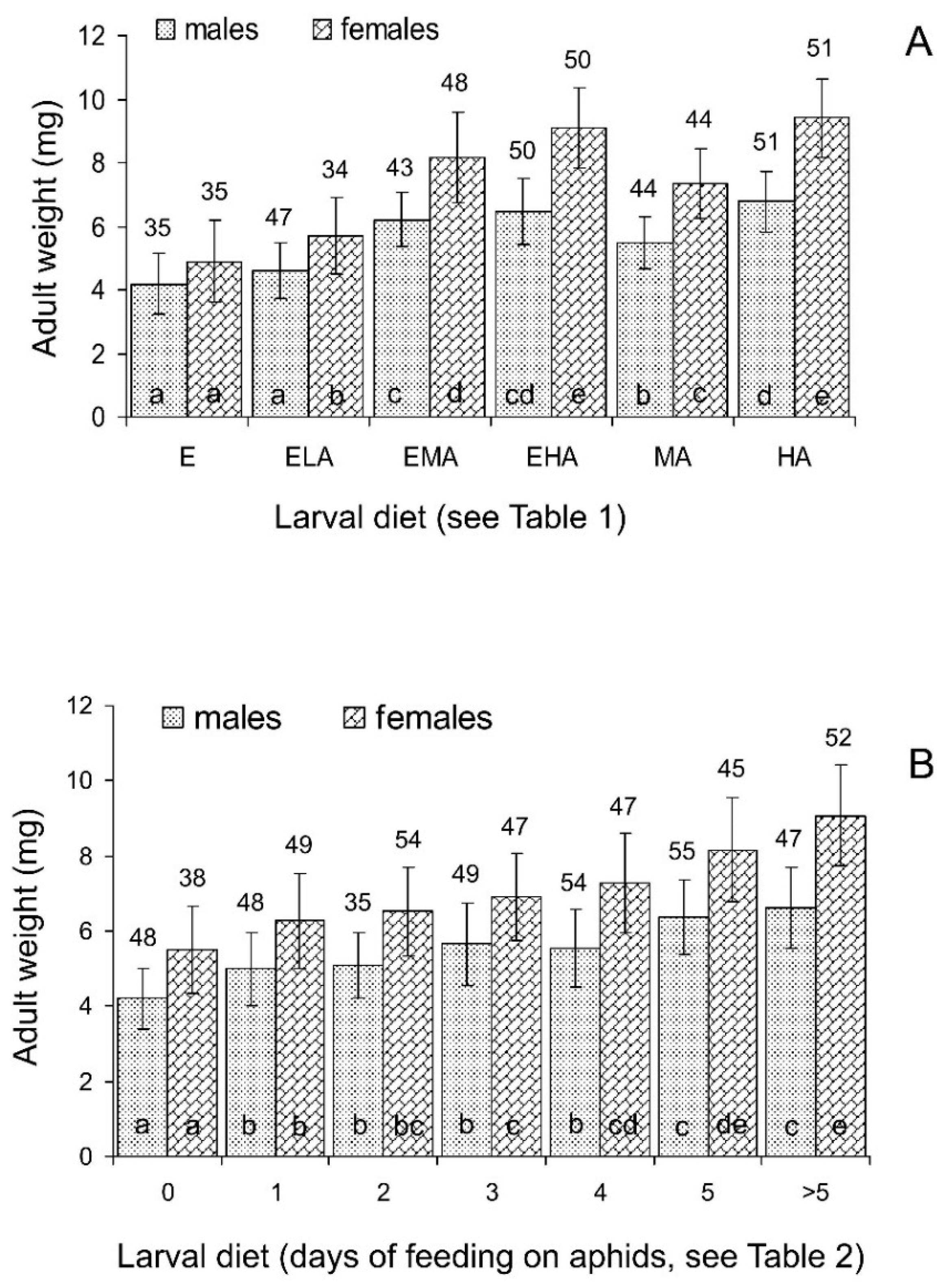
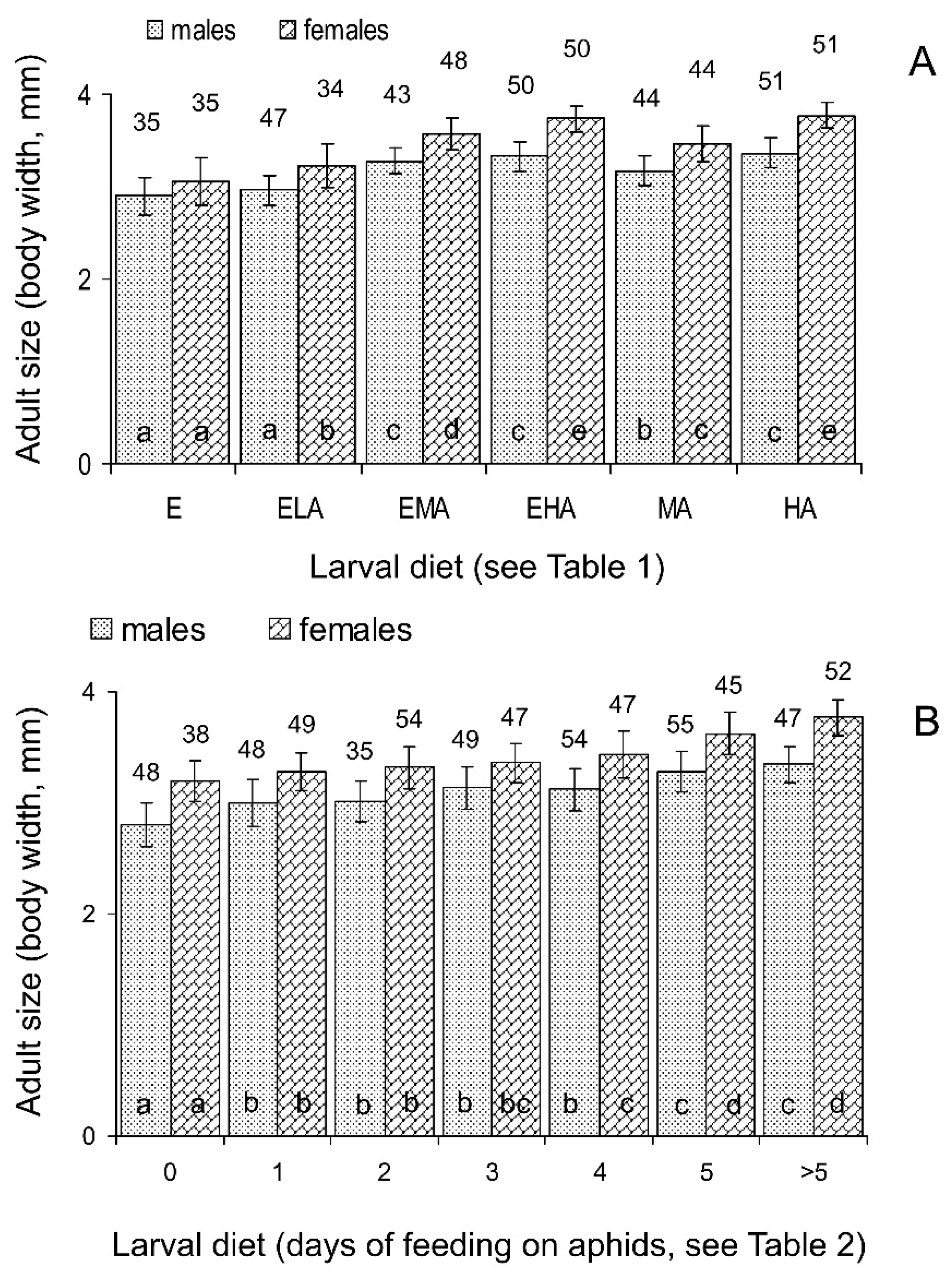
3.1.3. Size and Weight of Emerging Adults
3.2. The Second Experiment (Changing Diets)
3.2.1. Pre-Adult Survival and Sex
3.2.2. Time of Development
3.2.3. Size and Weight of Emerging Adults
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Lenteren, J.C. IOBC Internet Book of Biological Control, Version 6. 2012. Available online: https://www.iobc-global.org/publications_iobc_internet_book_of_biological_control.html (accessed on 25 May 2023).
- Van Lenteren, J.C.; Alomar, O.; Ravensberg, W.J.; Urbaneja, A. Biological control agents for control of pests in greenhouses. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer: Cham, Switzerland, 2020; pp. 409–439. [Google Scholar]
- Van Lenteren, J.C.; Bueno, V.H.; Klapwijk, J.N. Augmentative Biological Control. In Biological Control: Global Impacts, Challenges and Future Directions of Pest Management; Mason, P.G., Ed.; Csiro Publishing: Clayton, Australia, 2021; pp. 90–109. [Google Scholar]
- Riddick, E.W. Benefits and limitations of factitious prey and artificial diets on life parameters of predatory beetles, bugs, and lacewings: A mini-review. BioControl 2009, 54, 325–339. [Google Scholar] [CrossRef]
- Thompson, S.N. Nutrition and culture of entomophagous insects. Annu. Rev. Entomol. 1999, 44, 561–592. [Google Scholar] [CrossRef]
- Thomas, M. Heuristic price theory: A model of pluralistic price evaluations. Consum. Psychol. Rev. 2023, 6, 75–91. [Google Scholar] [CrossRef]
- Badrulhadza, A.; Velasco, L.R.I.; Medina, C. Suitability of non-natural prey during the larval growth of Heteroneda billardieri (Crotch), a potential predator of mango leafhopper. J. Trop. Agric. Food Sci. 2019, 47, 15–24. [Google Scholar]
- Berkvens, N.; Bonte, J.; Berkvens, D.; Deforce, K.; Tirry, L.; De Clercq, P. Pollen as an alternative food for Harmonia axyridis. BioControl 2008, 53, 201–210. [Google Scholar] [CrossRef]
- Bonte, M.; Samih, M.A.; De Clercq, P. Development and reproduction of Adalia bipunctata on factitious and artificial foods. BioControl 2010, 55, 485–491. [Google Scholar] [CrossRef]
- De Clercq, P.; Bonte, M.; Van Speybroeck, K.; Bolckmans, K.; Deforce, K. Development and reproduction of Adalia bipunctata (Coleoptera: Coccinellidae) on eggs of Ephestia kuehniella (Lepidoptera: Phycitidae) and pollen. Pest Manag. Sci. 2005, 61, 1129–1132. [Google Scholar] [CrossRef]
- Evans, E.W. Egg production in response to combined alternative foods by the predator Coccinella transversalis. Entomol. Exp. Appl. 2000, 94, 141–147. [Google Scholar] [CrossRef]
- Evans, E.W.; Gunther, D.I. The link between food and reproduction in aphidophagous predators: A case study with Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 2005, 102, 423–430. Available online: https://www.eje.cz/pdfs/eje/2005/03/13.pdf (accessed on 15 May 2024). [CrossRef]
- Evans, E.W.; Stevenson, A.T.; Richards, D.R. Essential versus alternative foods of insect predators: Benefits of a mixed diet. Oecologia 1999, 121, 107–112. [Google Scholar] [CrossRef]
- Hassani, F.; Shirvani, A.; Rashki, M. Fitness evaluation of Oenopia conglobata contaminata (Menetries) (Col.: Coccinellidae) fed on different diets. Acta Biol. Slov. 2019, 62, 35–43. [Google Scholar] [CrossRef]
- Hauge, M.S.; Nielsen, F.N.; Toft, S. The influence of three cereal aphid species and mixed diet on larval survival, development and adult weight of Coccinella septempunctata. Entomol. Exp. Appl. 1998, 89, 319–322. [Google Scholar] [CrossRef]
- Li, D.; Chen, P.; Liu, J.; Chi, B.; Zhang, X.; Liu, Y. Artificial rearing of the ladybird Propylea japonica on a diet containing oriental armyworm, Mythimna separata. Entomol. Exp. Appl. 2021, 169, 472–479. [Google Scholar] [CrossRef]
- de Lima, M.S.; Pontes, W.J.T.; de Lucena Nóbrega, R. Pollen did not provide suitable nutrients for ovary development in a ladybird Brumoides foudrasii (Coleoptera: Coccinellidae). Divers. J. 2020, 5, 1486–1494. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Hauge, M.S.; Toft, S. The influence of mixed aphid diets on larval performance of Coccinella septempunctata (Col., Coccinellidae). J. Appl. Entomol. 2002, 126, 194–197. [Google Scholar] [CrossRef]
- Phoofolo, M.W.; Giles, K.L.; Elliott, N.C. Quantitative evaluation of suitability of the greenbug, Schizaphis graminum, and the bird cherry-oat aphid, Rhopalosiphum padi, as prey for Hippodamia convergens (Coleoptera: Coccinellidae). Biol. Control 2007, 41, 25–32. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Vaghina, N.P. Effects of photoperiod and diet on diapause tendency, maturation and fecundity in Harmonia axyridis (Coleoptera: Coccinellidae). J. Appl. Entomol. 2013, 137, 452–461. [Google Scholar] [CrossRef]
- Rojas, M.G.; Morales-Ramos, J.A.; Riddick, E.W. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) powder to enhance artificial diet formulations for Coleomegilla maculata (Coleoptera: Coccinellidae). Biol. Control 2016, 100, 70–78. [Google Scholar] [CrossRef]
- Sayed, S.M.; El Arnaouty, S.A. Effect of corn pollens, as supplemental food, on development and reproduction of the predatory species, Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae). Egypt. J. Biol. Pest Control 2016, 26, 457–461. [Google Scholar]
- Snyder, W.E.; Clevenger, G.M. Negative dietary effects of Colorado potato beetle eggs for the larvae of native and introduced ladybird beetles. Biol. Control 2004, 31, 353–361. [Google Scholar] [CrossRef]
- Soares, A.O.; Coderre, D.; Schanderl, H. Dietary self-selection behaviour by the adults of the aphidophagous ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae). J. Anim. Ecol. 2004, 73, 478–486. [Google Scholar] [CrossRef]
- Stowe, H.E.; Michaud, J.P.; Kim, T. The benefits of omnivory for reproduction and life history of a specialized aphid predator, Hippodamia convergens (Coleoptera: Coccinellidae). Environ. Entomol. 2021, 50, 69–75. [Google Scholar] [CrossRef]
- Vahmani, A.; Shirvani, A.; Rashki, M. Biological parameters of Oenopia conglobata (Linnaeus) (Coleoptera: Coccinellidae) feeding on different diets. Int. J. Trop. Insect Sci. 2022, 42, 2241–2247. [Google Scholar] [CrossRef]
- Zanuncio, J.C.; Molina-Rugama, A.J.; Serrao, J.; Pratissoli, D. Nymphal development and reproduction of Podisus nigrispinus (Heteroptera: Pentatomidae) fed with combinations of Tenebrio molitor (Coleoptera: Tenebrionidae) pupae and Musca domestica (Diptera: Muscidae) larvae. Biocontr. Sci. Technol. 2001, 11, 331–337. [Google Scholar] [CrossRef]
- Pazyuk, I.M.; Reznik, S.Y. On the prospects of rearing the predatory bug Orius laevigatus (Fieber)(Heteroptera, Anthocoridae) on cysts of Artemia salina Leach (Crustacea, Anostraca). Entomol. Rev. 2023, 103, 263–269. [Google Scholar] [CrossRef]
- Amzah, B.; Velasco, L.R.I.; Medina, C.D. Effect of diet switching on the reproduction of Heteroneda billardieri (Crotch)(Coleoptera: Coccinellidae). Acta Biol. Malaysiana 2012, 1, 55–61. [Google Scholar] [CrossRef]
- Badrulhadza, A.; Velasco, L.R.I.; Medina, C. Effect of diet switching on the larval growth of Heteroneda billardieri (Crotch)(Coleoptera: Coccinellidae), predator of mango leafhopper in the Philippines. J. Trop. Agric. Food Sci. 2018, 46, 137–148. [Google Scholar]
- Ponsonby, D.J.; Copland, M.J.W. Aspects of prey relations in the coccidophagous ladybird Chilocorus nigritus relevant to its use as a biological control agent of scale insects in temperate glasshouses. BioControl 2007, 52, 629–640. [Google Scholar] [CrossRef]
- Michaud, J.P. Coccinellids in biological control. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Hodek, I., van Emden, H.F., Honěk, A., Eds.; John Wiley & Sons: Chichester, UK, 2012; pp. 488–519. [Google Scholar]
- Parrella, M.P.; Lewis, E. Biological control in greenhouse and nursery production: Present status and future directions. Am. Entomol. 2017, 63, 237–250. [Google Scholar] [CrossRef]
- Pervez, A. Ecology and Behaviour of Predaceous Ladybird Beetles (Coleoptera: Coccinellidae). In Biodiversity, Environment and Ecosystem Services; Arya, M.K., Ed.; Discovery Publishing House: New Delhi, India, 2023; pp. 12–29. [Google Scholar]
- Hodek, I. Food Relationships. In Ecology of Coccinellidae; Hodek, I., Honěk, A., Eds.; Kluwer: Dordrecht, The Netherlands, 1996; pp. 143–238. [Google Scholar]
- Hodek, I.; Evans, E.W. Food relationships. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Hodek, I., van Emden, H.F., Honěk, A., Eds.; Wiley-Blackwell: Chichester, UK, 2012; pp. 141–274. [Google Scholar]
- Michaud, J.P. On the assessment of prey suitability in aphidophagous Coccinellidae. Eur. J. Entomol. 2005, 102, 385–390. [Google Scholar] [CrossRef]
- Nedvěd, O.; Honěk, A. Life History and Development. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Hodek, I., van Emden, H.F., Honěk, A., Eds.; Wiley-Blackwell: Chichester, UK, 2012; pp. 54–109. [Google Scholar]
- Chen, J.; Qin, Q.J.; Liu, S.; He, Y.Z. Effect of six diets on development and fecundity of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Afr. Entomol. 2012, 20, 76–84. [Google Scholar] [CrossRef]
- De Castro-Guedes, C.F.; de Almeida, L.M.; do Rocio Chiarello Penteado, S.; Moura, M.O. Effect of different diets on biology, reproductive variables and life and fertility tables of Harmonia axyridis (Pallas) (Coleoptera, Coccinellidae). Rev. Bras. Entomol. 2016, 60, 260–266. [Google Scholar] [CrossRef]
- Honěk, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- Mirhosseini, M.A.; Hosseini, M.R.; Jalali, M.A. Effects of diet on development and reproductive fitness of two predatory coccinellids (Coleoptera: Coccinellidae). Eur. J. Entomol. 2015, 112, 446–452. [Google Scholar] [CrossRef]
- Reznik, S.; Ovchinnikov, A.; Ovchinnikova, A.; Bezman-Moseyko, O.; Belyakova, N. Photoperiodic, thermal and trophic responses of a predatory ladybird Cheilomenes propinqua. J. Appl. Entomol. 2021, 145, 134–144. [Google Scholar] [CrossRef]
- Abdel-Salam, A.H.; Abdel-Baky, N.F. Life table and biological studies of Harmonia axyridis Pallas (Col., Coccinellidae) reared on the grain moth eggs of Sitotroga cerealella Olivier (Lep., Gelechiidae). J. Appl. Entomol. 2001, 125, 455–462. [Google Scholar] [CrossRef]
- Kovář, I. Family Coccinellidae Latreille, 1807. In Catalogue of Palaearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2007; Volume 4, pp. 568–631. [Google Scholar]
- Youssif, M.A.I. Coccinellid species and their insect parasitoids in pear orchards at El-Khattara district, Sharkia Governorate, Egypt. J. Entomol. Zool. Stud. 2019, 7, 780–790. [Google Scholar]
- Samways, M.J. Interrelationship between an entomogenous fungus and two ant-homopteran (Hymenoptera: Formicidae-Hemiptera: Pseudococcidae & Aphididae) mutualisms on guava trees. Bull. Entomol. Res. 1983, 73, 321–331. [Google Scholar]
- Borkakati, R.N.; Venkatesh, M.R.; Saikia, D.K. Insect pests of Brinjal and their natural enemies. J. Entomol. Zool. Stud. 2019, 7, 932–937. [Google Scholar]
- Catling, H.D. The bionomics of the South African citrus psylla Trioza erytreae (Del Guercio) (Homoptera: Psyllidae) 4. The influence of predators. J. Entomol. Soc. S. Afr. 1970, 3, 341–348. [Google Scholar]
- Adly, D.; Fadl, H.A.; Mousa, S.F.M. Survey and seasonal abundance of mealybug species, their parasitoids and associated predators on guava trees in Egypt. Egypt J. Biol. Pest Control 2016, 26, 657–664. [Google Scholar]
- Spodek, M.; Ben-Dov, Y.; Mondaca, L.; Protasov, A.; Erel, E.; Mendel, Z. The cotton mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) in Israel: Pest status, host plants and natural enemies. Phytoparasitica 2018, 46, 45–55. [Google Scholar] [CrossRef]
- Magina, F.L.; Kilambo, D.L.; Maerere, A.P.; Teri, J.M. Innovative strategies for control of coffee insect pests in Tanzania: A review. Huria J. Open Univ. Tanzan. 2016, 22, 63–72. [Google Scholar]
- Badary, H. Ecological aspects of Sassetia spp. (Coccidae: Coccoidae: Hemiptera) and thier natural enemies in Egypt. Egypt. Acad. J. Biol. Sci. A Entomol. 2011, 4, 163–174. [Google Scholar] [CrossRef]
- Atuncha, H.; Ateka, E.; Amata, R.; Mwirichia, R.; Kasina, M.; Mbevi, B.; Wakoli, E. Evaluation of predation potential of coccinellids on cassava whiteflies. J. Entomol. Nematol. 2013, 5, 84–87. [Google Scholar] [CrossRef]
- Ghanim, A.A.; Abou-El-naga, A.A.; El-Serafi, H.A.; Jabbar, A.S. Studies on the population densities of certain insect pests attacking grapevine trees and their associated predators in Mansoura district. J. Plant Prot. Pathol. 2015, 6, 1089–1104. [Google Scholar] [CrossRef][Green Version]
- Bayoumy, M.H.; Michaud, J.P. Cannibalism in two subtropical lady beetles (Coleoptera: Coccinellidae) as a function of density, life stage, and food supply. J. Insect Behav. 2015, 28, 387–402. [Google Scholar] [CrossRef]
- Abdel-Salam, A.H.; El-Serafi, H.A.; Bayoumy, M.H.; Abdel-Hady, A.A. Effect of temperature and aphid-host plant variety on performance and thermal requirements of Coccinella undecimpunctata L. and Cheilomenes propinqua isis (Mulsant). J. Plant Prot. Pathol. 2018, 9, 375–380. [Google Scholar]
- Ramadan, M.M.; Hassan, M.A.; Afifi, M. Ecological studies on the Peach green aphid, Myzus persicae and its natural enemies. J. Plant Prot. Pathol. 2022, 13, 29–35. [Google Scholar] [CrossRef]
- Mahmoud, A.K.; Yousif, N.E.; Satti, A.A. Effect of three aphid species on the life tables data of the coccinellid predator, Cheilomenes propinqua vicina (Mulsant). Environ. Nat. Resour. Int. J. 2016, 1, 49–55. [Google Scholar]
- Sæthre, M.G.; Godonou, I.; Hofsvang, T.; Tepa-Yotto, G.T.; James, B. Aphids and their natural enemies in vegetable agroecosystems in Benin. Int. J. Trop. Insect Sci. 2011, 31, 103–117. [Google Scholar] [CrossRef]
- Nordey, T.; Boni, S.B.; Agbodzavu, M.K.; Mwashimaha, R.; Mlowe, N.; Ramasamy, S.; Deletre, E. Comparison of biological methods to control Aphis fabae Scopoli (Hemiptera: Aphididae) on kalanchoe crops in East Africa. Crop Prot. 2021, 142, 105520. [Google Scholar] [CrossRef]
- El-Heneidy, A.H.; El-Serafy, H.A.; Mohamed, N.E.; Elbanna, E.H.E. Biological characteristics of two coccinellid predators when reared on Aphis gossypii Glover and an artificial diet under laboratory condition. J. Plant Prot. Pathol. 2021, 12, 575–578. [Google Scholar] [CrossRef]
- Yarpuzlu, F.; Uygun, N. Effects of different temperatures on development and fecundity of a predatory beetle, Cheilomenes propinqua (Mulsant) (Coleoptera: Coccinellidae). Türkiye Biyol. Mücadele Derg. 2010, 1, 97–107. [Google Scholar]
- El-Batran, L.A.; Ghanim, A.A.; Shanab, L.M.; Ramadan, M.M. Thermal requirements for development of Aphis nerii Boyer de Fonscolombe and its predator Cydonia vicina isis Muls. J. Plant Prot. Path. 2015, 6, 825–837. [Google Scholar] [CrossRef][Green Version]
- Reznik, S.Y.; Ovchinnikov, A.N.; Bezman-Moseyko, O.S.; Samartsev, K.G.; Belyakova, N.A. Storage potential of the predatory ladybird Cheilomenes propinqua in relation to temperature, humidity, and factitious food. Insects 2022, 13, 613. [Google Scholar] [CrossRef]
- Ovchinnikov, A.; Reznik, S.; Bezman-Moseyko, O.; Belyakova, N. Walking activity of a predatory ladybird, Cheilomenes propinqua: Impacts of photoperiod, temperature, and starvation. BioControl 2022, 67, 513–522. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bennison, J.; Alomar, O.; Ingegno, B.L.; Tavella, L.; Shipp, L.; Palevsky, E.; Wäckers, F.L. Approaches to conserving natural enemy populations in greenhouse crops: Current methods and future prospects. BioControl 2014, 59, 377–393. [Google Scholar] [CrossRef]
- Brenard, N.; Sluydts, V.; De Bruyn, L.; Leirs, H.; Moerkens, R. Food supplementation to optimize inoculative release of the predatory bug Macrolophus pygmaeus in sweet pepper. Entomol. Exp. Appl. 2018, 166, 574–582. [Google Scholar] [CrossRef]
- Brenard, N.; Sluydts, V.; Christianen, E.; Bosmans, L.; De Bruyn, L.; Moerkens, R.; Leirs, H. Biweekly supplementation with Artemia spp. cysts allows efficient population establishment by Macrolophus pygmaeus in sweet pepper. Entomol. Exp. Appl. 2019, 167, 406–414. [Google Scholar] [CrossRef]
- Kozlova, E.G.; Krasavina, L.P.; Orlova, G.S. Experience of biocontrol use in cucumber protection from the complex of greenhouse pests. Zashchita Karantin Rastenii 2019, 11, 39–42. (in Russian). [Google Scholar]
- Sade, A.; Steinberg, S.; Salinger-Bubnov, M.; Gilboa, E.; Klempert, G.; Roitman, N.; Grosman, A. Improved western flower thrips control through artemia-based early introduction of Orius laevigatus in commercial pepper greenhouses. IOBC/WPRS Bull. 2019, 147, 39–46. [Google Scholar]
- Dader, B.; Colomer, I.; Adan, A.; Medina, P.; Vinuela, E. Compatibility of early natural enemy introductions in commercial pepper and tomato greenhouses with repeated pesticide applications. Insect Sci. 2020, 27, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Kruidhof, H.M.; Elmer, W.H. Cultural Methods for Greenhouse Pest and Disease Management. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer: Cham, Switzerland, 2020; pp. 285–330. [Google Scholar]
- Benson, C.M.; Labbe, R.M. Exploring the role of supplemental foods for improved greenhouse biological control. Ann. Entomol. Soc. Am. 2021, 114, 302–321. [Google Scholar] [CrossRef]
- Woelke, J.B.; Bouw, M.; Cusumano, A.; Messelink, G.J. Lygus rugulipennis on chrysanthemum: Supplemental prey effects and an evaluation of trap plants. J. Appl. Entomol. 2023, 147, 157–166. [Google Scholar] [CrossRef]
- Toft, S. The quality of aphids as food for generalist predators: Implications for natural control of aphids. Eur. J. Entomol. 2005, 102, 371–383. [Google Scholar] [CrossRef]
- Zaslavskiy, V.A.; Semyanov, V.P.; Vaghina, N.P. Food as a cue factor controlling adult diapause in the lady beetle Harmonia sedecimnotata (Coleoptera, Coccinellidae). Entomol. Rev. 1998, 78, 774–779. [Google Scholar]
- Ovchinnikov, A.N.; Ovchinnikova, A.A.; Reznik, S.Y.; Belyakova, N.A. Signal and nutritional effects of mixed diets on reproduction of a predatory ladybird, Cheilomenes propinqua. Insects 2023, 14, 587. [Google Scholar] [CrossRef]
- Ünlü, A.G.; Obrycki, J.J.; Bucher, R. Comparison of native and non-native predator consumption rates and prey avoidance behavior in North America and Europe. Ecol. Evol. 2020, 10, 13334–13344. [Google Scholar] [CrossRef]
- Stellwag, L.; Losey, J.E. Sexual dimorphism in North American Coccinellids: Sexing methods for species of Coccinella L. (Coleoptera: Coccinellidae) and implications for conservation research. Coleopt. Bull. 2014, 68, 271–281. [Google Scholar] [CrossRef]
- McCornack, B.P.; Koch, R.L.; Ragsdale, D.W. A simple method for in-field sex determination of the multicolored Asian lady beetle Harmonia axyridis. J. Insect Sci. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.E.; Jackson, H.B.; Eikenbary, R.D.; Starks, K.J. Sex determination in Propylea 14-punctata (Coleoptera: Coccinellidae), an imported predator of aphids. Ann. Entomol. Soc. Am. 1971, 64, 957–959. [Google Scholar] [CrossRef]
- Celli, N.G.R.; Almeida, L.M.; Basílio, D.S.; Castro, C.F. The way to maturity: Taxonomic study on immatures of Southern Brazilian Coccinellini (Coleoptera: Coccinellidae) species important in biological control. Zoologia 2021, 38, e64154. [Google Scholar] [CrossRef]
- LeSage, L. Coccinellidae (Cucujoidea). In Immature Insects; Stehr, F.W., Ed.; Kendall/Hunt: Dubuque, IA, USA, 1991; Volume 2, pp. 485–494. [Google Scholar]
- Gage, J.H. The larvae of Coccinellidae. Ill. Biol. Monogr. 1920, 6, 23–294. [Google Scholar]
- Bista, M.; Omkar. Effects of body size and prey quality on the reproductive attributes of two aphidophagous Coccinellidae (Coleoptera) species. Can. Entomol. 2013, 145, 566–576. [Google Scholar] [CrossRef]
- Kajita, Y.; Evans, E.W. Relationships of body size, fecundity, and invasion success among predatory lady beetles (Coleoptera: Coccinellidae) inhabiting alfalfa fields. Ann. Entomol. Soc. Am. 2010, 103, 750–756. [Google Scholar] [CrossRef]
- Khan, A.A. Stage-specific functional response of predaceous ladybird beetle, Harmonia eucharis (Mulsant)(Coleoptera: Coccinellidae) to green apple aphid, Aphis pomi De Geer (Hemiptera: Aphididae). J. Biol. Control 2010, 24, 222–226. [Google Scholar]
- Kundoo, A.A.; Khan, A.A. Coccinellids as biological control agents of soft bodied insects: A review. J. Entomol. Zool. Stud. 2017, 5, 1362–1373. [Google Scholar]



| Time of the Experiment 1 | Diets (Full Names and Abbreviations Used in the Present Paper) | ||||||
|---|---|---|---|---|---|---|---|
| Eggs (E) | Eggs and Low Quantity of Aphids (ELA) | Eggs and Medium Quantity of Aphids (EMA) | Eggs and High Quantity of Aphids (EHA) | Low Quantity of Aphids (LA) | Medium Quantity of Aphids (MA) | High Quantity of Aphids (HA) | |
| 1st day | Eggs in excess | Eggs + 1 aphid larva | Eggs + 2 adult aphids | Eggs + aphids in excess | 1 aphid larva | 2 adult aphids | Aphids in excess |
| 2nd day | Eggs in excess | Eggs + 1 aphid larva | Eggs + 4 adult aphids | Eggs + aphids in excess | 1 aphid larva | 4 adult aphids | Aphids in excess |
| 3rd day | Eggs in excess | Eggs + 1 aphid larva | Eggs + 6 adult aphids | Eggs + aphids in excess | 1 aphid larva | 6 adult aphids | Aphids in excess |
| 4th day | Eggs in excess | Eggs + 1 adult aphid | Eggs + 8 adult aphids | Eggs + aphids in excess | 1 adult aphid | 8 adult aphids | Aphids in excess |
| 5th day | Eggs in excess | Eggs + 1 adult aphid | Eggs + 10 adult aphids | Eggs + aphids in excess | 1 adult aphid | 10 adult aphids | Aphids in excess |
| 6th and subsequent days | Eggs in excess | Eggs + 1 adult aphid | Eggs + 14 adult aphids | Eggs + aphids in excess | 1 adult aphid | 14 adult aphids | Aphids in excess |
| Time of the Experiment 1 | Diets (The Number of Days When the Larvae Fed on Aphids) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | >5 | |
| 1st day | Eggs | Aphids | Aphids | Aphids | Aphids | Aphids | Aphids |
| 2nd day | Eggs | Eggs | Aphids | Aphids | Aphids | Aphids | Aphids |
| 3rd day | Eggs | Eggs | Eggs | Aphids | Aphids | Aphids | Aphids |
| 4th day | Eggs | Eggs | Eggs | Eggs | Aphids | Aphids | Aphids |
| 5th day | Eggs | Eggs | Eggs | Eggs | Eggs | Aphids | Aphids |
| 6th and subsequent days | Eggs | Eggs | Eggs | Eggs | Eggs | Eggs | Aphids |
| Factor or Interaction of Factors and the Number of Degrees of Freedom | Biological Parameters | ||
|---|---|---|---|
| Time of Development from the Second Instar to the Adult Stage | Adult Size | Adult Weight | |
| Female diet, df = 1 | F = 17.5, p < 0.001 | F = 2.3, p = 0.134 | F = 1.5, p = 0.217 |
| Larval diet, df = 5 | F = 68.7, p < 0.001 | F = 152.8, p < 0.001 | F = 151.7, p < 0.001 |
| Sex, df = 1 | F = 2.7, p = 0.098 | F = 433.1, p < 0.001 | F = 357.7, p < 0.001 |
| Female diet × larval diet, df = 5 | F = 1.8, p = 0.101 | F = 0.8, p = 0.576 | F = 0.4, p = 0.851 |
| Female diet × sex, df = 1 | F = 0.7, p = 0.415 | F = 0.0, p = 0.841 | F = 0.7, p = 0.395 |
| Larval diet × sex, df = 5 | F = 0.9, p = 0.503 | F = 7.3, p < 0.001 | F = 7.8, p < 0.001 |
| Female diet × larval diet × sex, df = 5 | F = 0.6, p = 0.722 | F = 1.3, p = 0.255 | F = 1.6, p = 0.153 |
| Factor or Interaction of Factors and the Number of Degrees of Freedom | Biological Parameters | ||
|---|---|---|---|
| Time of Development from the Second Instar to the Adult Stage | Adult Size | Adult Weight | |
| Female diet, df = 1 | F = 1.74, p = 0.187 | F = 0.7, p = 0.437 | F = 0.3, p = 0.580 |
| Larval diet, df = 6 | F = 58.2, p < 0.001 | F = 85.9, p < 0.001 | F = 75.8, p < 0.001 |
| Sex, df = 1 | F = 7.3, p = 0.007 | F = 426.3, p < 0.001 | F = 323.6, p < 0.001 |
| Female diet × larval diet, df = 6 | F = 1.1, p = 0.346 | F = 2.3, p = 0.036 | F = 2.2, p = 0.042 |
| Female diet × sex, df = 1 | F = 0.1, p = 0.893 | F = 0.7, p = 0.419 | F = 0.6, p = 0.450 |
| Larval diet × sex, df = 6 | F = 1.3, p = 0.264 | F = 1.0, p = 0.432 | F = 0.9, p = 0.461 |
| Female diet × larval diet × sex, df = 6 | F = 0.8, p = 0.523 | F = 0.6, p = 0.760 | F = 0.4, p = 0.833 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovchinnikov, A.N.; Ovchinnikova, A.A.; Reznik, S.Y.; Belyakova, N.A. Effects of Two Prey Species Combinations on Larval Development of the Predatory Ladybird Cheilomenes propinqua. Insects 2024, 15, 484. https://doi.org/10.3390/insects15070484
Ovchinnikov AN, Ovchinnikova AA, Reznik SY, Belyakova NA. Effects of Two Prey Species Combinations on Larval Development of the Predatory Ladybird Cheilomenes propinqua. Insects. 2024; 15(7):484. https://doi.org/10.3390/insects15070484
Chicago/Turabian StyleOvchinnikov, Andrey N., Antonina A. Ovchinnikova, Sergey Y. Reznik, and Natalia A. Belyakova. 2024. "Effects of Two Prey Species Combinations on Larval Development of the Predatory Ladybird Cheilomenes propinqua" Insects 15, no. 7: 484. https://doi.org/10.3390/insects15070484
APA StyleOvchinnikov, A. N., Ovchinnikova, A. A., Reznik, S. Y., & Belyakova, N. A. (2024). Effects of Two Prey Species Combinations on Larval Development of the Predatory Ladybird Cheilomenes propinqua. Insects, 15(7), 484. https://doi.org/10.3390/insects15070484







