Diet Affects the Temperature–Size Relationship in the Blowfly Aldrichina grahami
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Stocks and Rearing
2.2. Measurement of Growth Curves
2.3. Curve Fitting for Weight and Growth Rate
2.4. Statistical Analysis
3. Results
3.1. The TSR for A. grahami
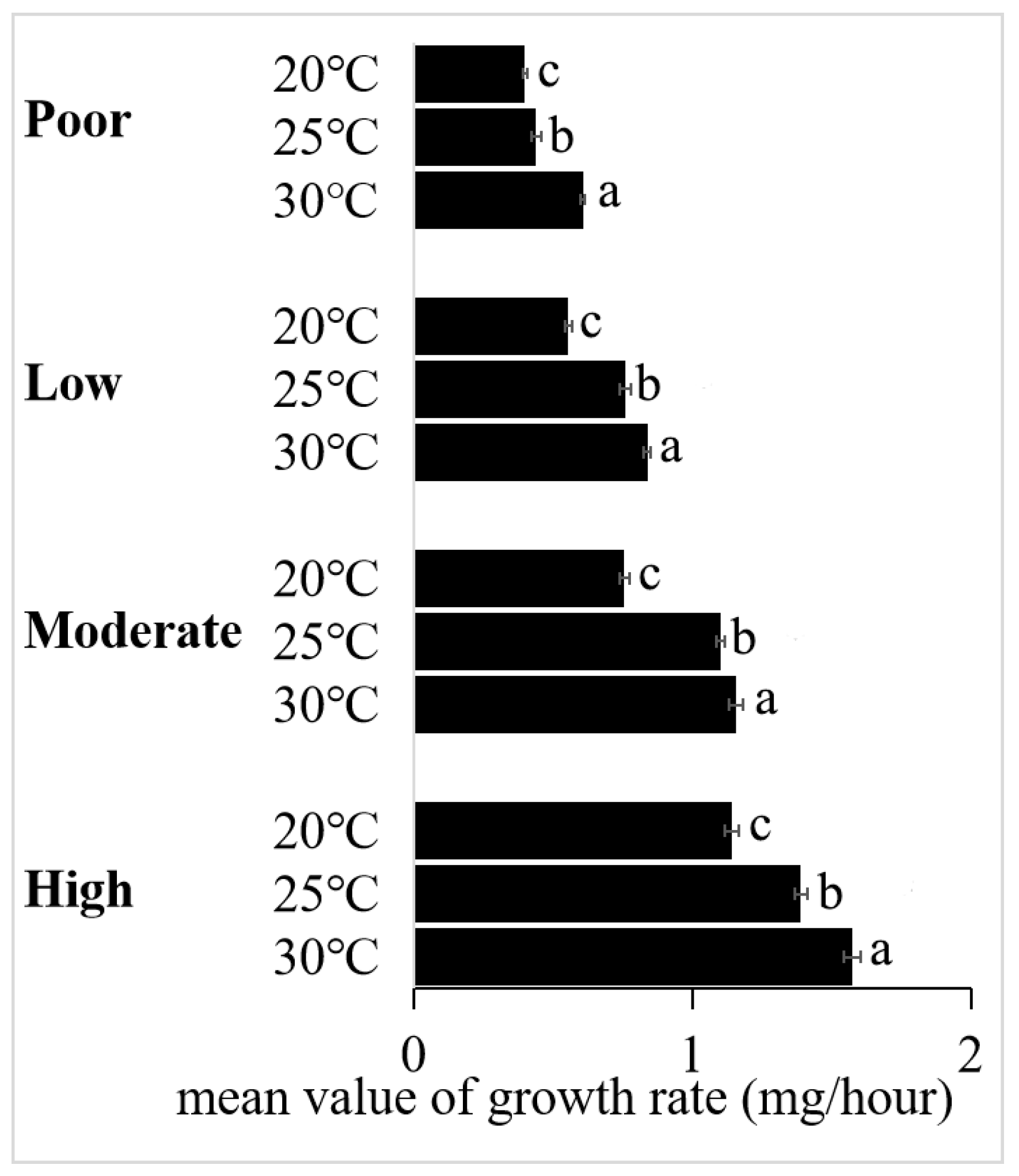
3.2. The Interaction between Temperature and Diet
3.3. Relationships between Weight and Growth Rate
4. Discussion
4.1. The Effects of Temperature on Body Size
4.2. The Potential Explanation of the Life-History Puzzle
4.3. Interconversion between Following and Not Following the TSR
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naganuma, K.H.; Roughgarden, J.D. Optimal body size in Lesser Antillean Anolis lizards–A mechanistic approach. Ecol. Monogr. 1990, 60, 239–256. [Google Scholar] [CrossRef]
- Roff, D. Evolution of Life Histories: Theory and Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Chown, S.L.; Gaston, K.J. Body size variation in insects: A macroecological perspective. Biol. Rev. 2010, 85, 139–169. [Google Scholar] [CrossRef] [PubMed]
- Baar, Y.; Friedman, A.L.L.; Meiri, S.; Scharf, I. Little effect of climate change on body size of herbivorous beetles. Insect Sci. 2018, 25, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, H.F. The control of body size in insects. Dev. Biol. 2003, 261, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mirth, C.; Shingleton, A. Integrating body and organ size in Drosophila: Recent advances and outstanding problems. Front. Endocrinol. 2012, 3, 49. [Google Scholar] [CrossRef]
- Koyama, T.; Mendes, C.; Mirth, C. Mechanisms regulating nutrition-dependent developmental plasticity through organ-specific effects in insects. Front. Physiol. 2013, 4, 60144. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, H.F.; Riddiford, L.M.; Mirth, C.; Shingleton, A.W.; Suzuki, Y.; Callier, V. The developmental control of size in insects. WIREs Dev. Biol. 2014, 3, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D. Effects of temperature on the size of aquatic ectotherms: Exceptions to the general rule. J. Therm. Biol. 1995, 20, 61–74. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and organism size—A Biological law for ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar]
- Atkinson, D.; Sibly, R.M. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 1997, 12, 235–239. [Google Scholar] [CrossRef]
- Kingsolver, J.G. The well-temperatured biologist. Am. Nat. 2009, 174, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.A.; Bickford, D. Shrinking body size as an ecological response to climate change. Nat. Clim Chang. 2011, 1, 401–406. [Google Scholar] [CrossRef]
- Tseng, M.; Kaur, K.M.; Pari, S.S.; Sarai, K.; Chan, D.; Yao, C.H.; Porto, P.; Toor, A.; Toor, H.S.; Fograscher, K. Decreases in beetle body size linked to climate change and warming temperatures. J. Anim. Ecol. 2018, 87, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Größe; Vandenhoeck und Ruprecht: Göttingen, Germany, 1848. [Google Scholar]
- Classen, A.; Steffan-Dewenter, I.; Kindeketa, W.J.; Peters, M.K. Integrating intraspecific variation in community ecology unifies theories on body size shifts along climatic gradients. Funct. Ecol. 2017, 31, 768–777. [Google Scholar] [CrossRef]
- Henry, E.; Santini, L.; Huijbregts, M.A.J.; Benítez-López, A. Unveiling the environmental drivers of intraspecific body size variation in terrestrial vertebrates. Glob. Ecol. Biogeogr. 2023, 32, 267–280. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef] [PubMed]
- Ohlberger, J. Climate warming and ectotherm body size–from individual physiology to community ecology. Funct. Ecol. 2013, 27, 991–1001. [Google Scholar] [CrossRef]
- Diamond, S.E.; Kingsolver, J.G. Environmental dependence of thermal reaction norms: Host plant quality can reverse the temperature-size rule. Am. Nat. 2010, 175, 1–10. [Google Scholar] [CrossRef]
- Ismail, M.; Brooks, M.; van Baaren, J.; Albittar, L. Synergistic effects of temperature and plant quality, on development time, size and lipid in Eccritotarsus eichhorniae. J. Appl. Entomol. 2021, 145, 239–249. [Google Scholar] [CrossRef]
- Yasuda, N.; Miyamoto, N.; Fujiwara, Y.; Yamamoto, T.; Yusa, Y. Effects of food availability on growth and reproduction of the deep-sea pedunculate barnacle Heteralepas canci. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 108, 53–57. [Google Scholar] [CrossRef]
- Huey, R.B.; Kingsolver, J.G. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol. Evol. 1989, 4, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagel, K.N.; de Vries, E.H.J.; Jongejans, E.; Verberk, W.C.E.P. The temperature-size rule in Daphnia magna across different genetic lines and ontogenetic stages: Multiple patterns and mechanisms. Ecol. Evol. 2018, 8, 3828–3841. [Google Scholar] [CrossRef]
- Perrin, N. About Berrigan and Charnov’s life-history puzzle. Oikos 1995, 73, 137–139. [Google Scholar] [CrossRef]
- Xia, Q.W.; Chen, C.; Tang, J.J.; He, H.M.; Xue, F.S. A reverse temperature-size rule associated with a negative relationship between larval development time and pupal weight in a tropical population of Ostrinia furnacalis. Physiol. Entomol. 2019, 44, 209–214. [Google Scholar] [CrossRef]
- Clissold, F.J.; Coggan, N.; Simpson, S.J. Insect herbivores can choose microclimates to achieve nutritional homeostasis. J. Exp. Biol. 2013, 216, 2089–2096. [Google Scholar] [CrossRef]
- Lee, K.P.; Jang, T.; Ravzanaadii, N.; Rho, M.S. Macronutrient balance modulates the temperature-size rule in an ectotherm. Am. Nat. 2015, 186, 212–222. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Gwen Shlichta, J.; Ragland, G.J.; Massie, K.R. Thermal reaction norms for caterpillar growth depend on diet. Evol. Ecol. Res. 2006, 8, 703–715. [Google Scholar]
- Angilletta, J.; Michael, J.; Dunham, A.E. The temperature-size rule in ectotherms: Simple evolutionary explanations may not be general. Am. Nat. 2003, 162, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.N.; Liu, C.; Hu, G.L.; Wang, M.; Yang, L.J.; Chu, J.; Wang, J.F. Development of Aldrichina grahami (Diptera: Calliphoridae) at constant temperatures. J. Med. Entomol. 2018, 55, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Davidowitz, G.; Nijhout, H.F. The physiological basis of reaction norms: The interaction among growth rate, the duration of growth and body size. Integr. Comp. Biol. 2004, 44, 443–449. [Google Scholar] [CrossRef]
- Nijhout, H.F.; Davidowitz, G.; Roff, D. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J. Biol. 2006, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.; Hirst, A.G.; Atkinson, D. How do organisms change size with changing temperature? The importance of reproductive method and ontogenetic timing. Funct. Ecol. 2011, 25, 1024–1031. [Google Scholar] [CrossRef]
- Forster, J.; Hirst, A.G. The temperature-size rule emerges from ontogenetic differences between growth and development rates. Funct. Ecol. 2012, 26, 483–492. [Google Scholar] [CrossRef]
- Horne, C.R.; Hirst, A.G.; Atkinson, D.; Almeda, R.; Kiørboe, T. Rapid shifts in the thermal sensitivity of growth but not development rate causes temperature–size response variability during ontogeny in arthropods. Oikos 2019, 128, 823–835. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Atkinson, D.; Hoefnagel, K.N.; Hirst, A.G.; Horne, C.R.; Siepel, H. Shrinking body sizes in response to warming: Explanations for the temperature–size rule with special emphasis on the role of oxygen. Biol. Rev. 2021, 96, 247–268. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the origin of allometric scaling laws in biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. A unifying explanation for diverse metabolic scaling in animals and plants. Biol. Rev. 2010, 85, 111–138. [Google Scholar] [CrossRef]
- Xu, H.; Ye, G.Y.; Xu, Y.; Hu, C.; Zhu, G.H. Age-dependent changes in cuticular hydrocarbons of larvae in Aldrichina grahami (Aldrich) (Diptera: Calliphoridae). Forensic Sci. Int. 2014, 242, 236–241. [Google Scholar] [CrossRef]
- Fan, Z. Key to the Common Files of China; Science Press: Beijing, China, 1997. [Google Scholar]
- Davidowitz, G.; D’Amico, L.J.; Nijhout, H.F. Critical weight in the development of insect body size. Evol. Dev. 2003, 5, 188–197. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2004. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: http://www.r-project.org (accessed on 31 October 2023).
- Garcia-Robledo, C.; Baer, C.S.; Lippert, K.; Sarathy, V. Evolutionary history, not ecogeographic rules, explains size variation of tropical insects along elevational gradients. Funct. Ecol. 2020, 34, 2513–2523. [Google Scholar] [CrossRef]
- Fischer, K.; Bot, A.N.M.; Zwaan, B.J.; Brakefield, P.M. Genetic and environmental sources of egg size variation in the butterfly Bicyclus anynana. Heredity 2004, 92, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Gotthard, K.; Nylin, S.; Wiklund, C. Adaptive variation in growth rate: Life history costs and consequences in the speckled wood butterfly, Pararge aegeria. Oecologia 1994, 99, 281–289. [Google Scholar] [CrossRef] [PubMed]
- DeLong, J.P.; Brassil, C.E.; Erickson, E.K.; Forbes, V.E.; Moriyama, E.N.; Riekhof, W.R. Dynamic thermal reaction norms and body size oscillations challenge explanations of the temperature–size rule. Evol. Ecol. Res. 2017, 18, 293–303. [Google Scholar]


| Developmental Stage | Factor | df | F | p |
|---|---|---|---|---|
| Larvae | Temperature | 2, 228 | 58 | <0.001 |
| Diet | 3, 228 | 1971 | <0.001 | |
| Temperature × Diet | 6, 228 | 18 | <0.001 | |
| Adults | Temperature | 2, 468 | 66 | <0.001 |
| Diet | 3, 468 | 423 | <0.001 | |
| Temperature × Diet | 6, 468 | 5 | <0.001 |
| Diet | Temperature (°C) | AIC * | Equation | R2 | p | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| high | 20 | 465 | 412 | 413 | 413 | y = −0.00016153 x3 + 0.010228 x2 + 2.7020 x − 101.5 | 0.97 | <0.001 |
| 25 | 421 | 344 | 342 | 340 | y = −0.00040961 x3 + 0.030104 x2 + 2.8451 x − 98.4 | 0.98 | <0.001 | |
| 30 | 352 | 275 | 272 | 274 | y = 0.00070032 x3 − 0.159341 x2 + 12.3091 x − 217.1 | 0.98 | <0.001 | |
| moderate | 20 | 726 | 660 | 644 | 646 | y = −0.00010752 x3 + 0.02163815 x2 − 0.08315955 x − 15.54660964 | 0.97 | <0.001 |
| 25 | 505 | 441 | 418 | 420 | y = −0.00041413 x3 + 0.06348199 x2 − 1.15964988 x − 1.90000000 | 0.98 | <0.001 | |
| 30 | 461 | 382 | 365 | 363 | y = −0.00051627 x3 + 0.05609168 x2 + 0.23352667 x − 21.48125000 | 0.98 | <0.001 | |
| low | 20 | 644 | 637 | 619 | 620 | y = −0.00010333 x3 + 0.02784374 x2 − 1.49937023 x + 27.83584540 | 0.97 | <0.001 |
| 25 | 440 | 417 | 400 | 400 | y = −0.00030980 x3 + 0.05975379 x2 − 2.41729403 x + 32.89621212 | 0.98 | <0.001 | |
| 30 | 428 | 411 | 380 | 382 | y = −0.00037605 x3 + 0.05738587 x2 − 1.43509049 x + 12.19987374 | 0.98 | <0.001 | |
| poor | 20 | 742 | 739 | 728 | 729 | y = −0.00005192 x3 + 0.01525747 x2 − 0.81487311 x + 15.39486690 | 0.94 | <0.001 |
| 25 | 557 | 545 | 524 | 525 | y = −0.00011453 x3 + 0.02651317 x2 − 1.23866570 x + 20.98557831 | 0.96 | <0.001 | |
| 30 | 424 | 421 | 362 | 360 | y = −0.00025717 x3 + 0.04444600 x2 − 1.48207769 x + 19.16386364 | 0.99 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, G.; Li, D.; Wang, G.; Wu, L. Diet Affects the Temperature–Size Relationship in the Blowfly Aldrichina grahami. Insects 2024, 15, 246. https://doi.org/10.3390/insects15040246
Yan G, Li D, Wang G, Wu L. Diet Affects the Temperature–Size Relationship in the Blowfly Aldrichina grahami. Insects. 2024; 15(4):246. https://doi.org/10.3390/insects15040246
Chicago/Turabian StyleYan, Guanjie, Dandan Li, Guangshuai Wang, and Lingbing Wu. 2024. "Diet Affects the Temperature–Size Relationship in the Blowfly Aldrichina grahami" Insects 15, no. 4: 246. https://doi.org/10.3390/insects15040246
APA StyleYan, G., Li, D., Wang, G., & Wu, L. (2024). Diet Affects the Temperature–Size Relationship in the Blowfly Aldrichina grahami. Insects, 15(4), 246. https://doi.org/10.3390/insects15040246







